Introduction
The structural foundation of the neurovascular unit
(NVU) includes neuron, blood brain barrier (BBB, including
endothelial cells, basement membrane, foot process of astrocyte and
pericyte), microglia and extracellular matrix, which maintain
completeness of brain tissues, of which BBB is a core component of
NVU (1).
Patients with cerebral arterial thrombosis
experience vasculopathy initially, followed by nervous system
lesions with corresponding symptoms. Protective treatment for
neurons only may have limited effects, resulting in possibility of
treatment being lost (2). NVU
constitutes a hot spot and has become the focus of current studies
on thrombosis. NVU involves multiple structures, multiple action
segments and complex networks of many signal channels, and an
increasing number of studies have discussed it extensively from
different aspects. Among these studies, complication occurrence,
interaction and final targets of treatment strategies are to
interfere with signal channels (3),
thus studies on signal channels may have broad and attractive
prospects. ATP-sensitive potassium channels (KATP) are
widely distributed in the central nervous system and belong to
ligand-gated voltage-independent inwardly rectifying K+
channels. The main function of KATP is to couple
cellular energy metabolism and electrophysiological activities, and
it plays important roles in the occurrence of thrombosis, ischemic
preconditioning and reperfusion injury (4). At present, studies have focused on the
regulatory roles of KATP channels in myocardial ischemic
injury, movement of smooth muscle and skeletal muscle and
pancreatic secretion (5,6). However, to the best of our knowledge,
there are few studies on thrombosis. An important mechanism of
pathological changes in thrombosis is energy metabolism dysfunction
(7). Consequently, it has been
hypothesized that KATP channels play an important role
in energy metabolism of thrombosis.
The aim of the present study was to analyze the
mechanism of cytomembrane ATP-sensitive K+ channels
(KATP) in the neurovascular unit treatment of ischemic stroke
during the recovery period.
Materials and methods
Animals
Fifteen healthy adult Wistar male rats, aged 5–8
weeks and weighing 160–200 g, were provided by the Shanghai
Laboratory Animal Research Center (Shanghai, China). The animals
were placed in an environment of room temperature 22±0.5°C, with 12
h of light/dark cycle with unlimited access to food and water.
The main reagents used were 5-hydroxydecanoate
(5-HD) and diazoxide, which were purchased from Sigma-Aldrich
Chemie GmbH, (Steinheim, Germany). A TRIzol extraction kit was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA),
and an RT-PCR kit from MBI Fermentas, Inc. (Burlington, ON,
Canada). The DNA Marker DL2000 was obtained from Shenzhen Jingmei
Engineering Biology Co., Ltd. (Shenzhen, China), and tetrazolium
chloride (TTC) from Henan Huamei Biological Engineering Co., Ltd.
(Henan, China).
The main instruments used were tissue homogenizer
and PCR amplification instrument 2400 (both from Ningbo Technology
Co., Ltd., Ningbo, China), ultraviolet spectrophotometer and
high-speed tabletop refrigerated centrifuge manufactured by Beckman
Coulter, Inc. (Brea, CA, USA), electronic analytical balance
manufactured by OHAU Company (Parsippany, NJ, USA), VDS ultraviolet
gel imaging analysis system was purchased from Pharmacia AB
(Stockholm, Sweden), THZ-C constant temperature oscillator from
Jiangsu Taijiang Experimental Equipment Factory (Jiangsu, China),
and ultra-low temperature freezer from Sanyo Electric Co., Ltd.
(Tokyo, Japan).
Preparation of middle cerebral artery
occlusion (MCAO) model
Rats were deprived of food and water for 12 and 4 h,
respectively, chloral hydrate was used to anesthetize the abdominal
cavity. The operating area was sterilized, and an incision of
approximately 8 cm was made in the middle section of neck. The skin
and muscle were bluntly dissected to expose left carotid artery,
and the internal and external carotid were separated along the
anatomic structure. The nylon mono-filament coated with
Poly-L-lysine was used to insert into the internal carotid via the
incision, and the direction of nylon thread was adjusted
continuously. When approaching bifurcation of internal and external
carotid, forward resistance was felt which meant that the origin of
middle cerebral artery occlusion was achieved, the nylon thread was
placed for 4 h and muscle and skin were sutured by layers.
The experiment was completed after 4 h, the nylon
thread was removed and the whole layer was sutured (Fig. 1). Headlamps were used to light rats
in the experiment and to ensure that their body temperature was at
37°C and penicillin was used to prevent infection. When rats became
awake after anaesthesia, it was observed that side limbs could not
be abducted completely, muscular tension decreased and the animals
fell to the contralateral side when standing, i.e., forelimbs. In
addition, movement decreased and the rats circled to the
contralateral side spontaneously. The contralateral limbs were
incapable of collapsing when the tails were lifted, and resistance
weakened, indicating the model was successfully created.
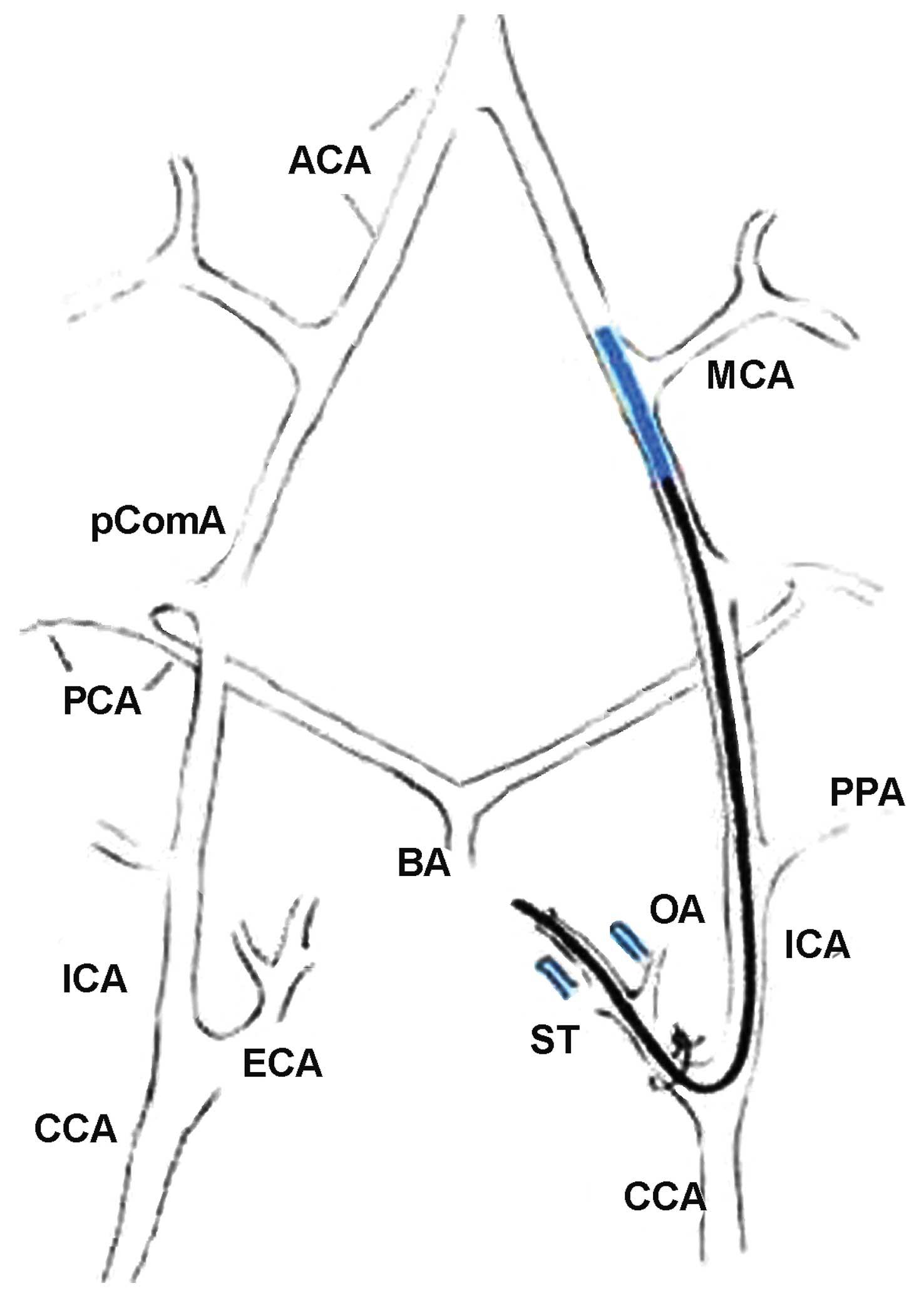 | Figure 1.Middle cerebral artery occlusion
(MCAO) model showing intra-luminal suture method.ACA, anterior
cerebral artery; BA, basilar artery; CCA, common carotid artery;
ECA, external carotid artery; ICA, internal carotid artery; MCA,
middle cerebral artery; OA, occipital artery; PCA, posterior
cerebral artery; PComA, posterior communicating artery; PPA,
pterygopalatine artery; ST, superior thyroid artery. |
Experimental groups
The animals were randomly divided into the control
(sham-operation group), MCAO, KATP blocker and
KATP opener groups. Each group included 6 rats. At 3
days after successfully establishing the models, 5-HD (40 mg/Kg)
was injected into the abdominal cavity in the KATP
blocker group, and diazoxide (40 mg/Kg) was injected into the
abdominal cavity of the opener group. Surgery was performed to
expose the carotid artery and suture by layers in the
sham-operation group, and the equivalent volume of normal saline
was injected in the sham-operaton and model groups. The animals
were mutilated and samples were collected after feeding normally
for 3 days.
Observation indices and detection
methods
The expression level and infarct size of three
subnuits of KATP, i.e., kir6.1, sulfonylurea receptor
(SUR) 1 and SUR2 mRNA were compared in the different groups.
RT-PCR for detection of expression of
subunits
A TRIzol extraction kit was used to extract total
RNA of brain tissues according to the manufacturer's instructions.
RNA was assessed for purity and concentration using a
UV-spectrophotometer. The PCR steps consisted of primer designing
by DNAstar 7.0, which were produced by Invitrogen Life
Technologies. For reverse transcription (RT), 4 µg of total RNA was
added to 4 µl 5X RT buffer solution, 2 µl dNTP, 1 µl RNAase
inhibitor, 1 µl random primer, 1 µl M-MLV RT, DEPC-treated water to
produce the final volume as 20 µl. PCR reaction conditions were for
10 min at 25°C, 60 min at 42°C, 10 min at 70°C, and place RT
product at −20°C to preserve. For cDNA amplification, 4 µl
reverse-transcribed product was added to 12.5 µl 2X PCR buffer
solution and 1 µl specific primers, to produce the final volume of
25 µl with H2O.
Reaction conditions of β-actin were: denaturation at
94°C for 5 min, annealing at 94°C for 45 sec, at 60°C for 45 sec,
at 72°C for 45 sec, and extension at 72°C for 5 min, which
accounted for 35 cycles. Reaction conditions of Sur1 and Sur2 were:
denaturation at 95°C for 15 min, denaturation at 95°C for 30 sec,
annealing 57°C for 45 sec, extension at 72°C for 45 sec, and a
final extension at 72°C for 5 min, which accounted for 37 cycles.
For electrophoresis, PCR product was loaded on 1.5% agarose gel
electrophoresis (95 mA, 30 min) and images were captured with a gel
imaging system. Using a scanning densitometer (KS400v 3.0) (Beijing
Maisiqi High-tech Co., Ltd., Beijing, China; http://www.msdyq.cn/), the ratio of electrophoresis
strips and expression of gray level of internal control β-actin
strip were estimated. Detailed primers for RT-PCR are shown in
Table I.
 | Table I.RT-PCR primer sequences. |
Table I.
RT-PCR primer sequences.
| Gene | Sequence no. | Primer sequences | Size | Annealing | Cycles |
|---|
| Kir6.1 | D88159 |
5′-ACCAGAATTCTCTGCGGAAG-3′ | 297 bp | 60°C 45 sec | 35 |
|
|
|
5′-GCCCTGAACTGGTGATGAGT-3′ |
|
|
|
| SUR1 | L40624 |
5′-GGAGCAATCCAGACCAAGAT-3′ | 249 bp | 57°C 45 sec | 37 |
|
|
|
5′-AGCCAGCAGAATGATGACAG-3′ |
|
|
|
| SUR2 | AC108508 |
5′-CCATCATCAGTGTTCAAAAGC-3′ | 148 bp | 57°C 45 sec | 37 |
|
|
|
5′-GGCTGCTTCCTGTTTATTGGTA-3′ |
|
|
|
| β-actin | NM031144 |
5′-AAGTACCCCATTGAACACGG-3′ | 257 bp | 60°C 45 sec | 22 |
|
|
|
5′-ATCACAATGCCAGTGGTACG-3′ |
|
|
|
Calculation of infarct size in TTC
staining
After general anesthesia, the rats were decapitated
and brains were removed. The olfactory bulb and lower brain stem
were separated, and carefully removed removed. The brain was
sectioned into five 2 mm pieces after deep freezing for 20 min at
−20°C. The tissues were added to TTC buffer solution, stained for
30 min using 4% paraformaldehyde buffer solution to fix and images
were captured with high-resolution cameras. Normal brain tissue was
red or pink while the infarct areas were white. Using Photoshop
image processing software, TTC staining images were obtained and
analyzed. The corresponding measuring instruments were used to
calculate the infarct size, and the results were shown as area
percentage of the homolateral brain tissues.
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was
used to statistically analyze the data. Data were presented as mean
± standard deviation. The single-factor analysis of variance was
used for comparison of groups, and α<0.05 was taken as the
inspection level. LSD and Bonferroni tests were used for
comparisons between two groups, by taking 1/4α<0.0125 as
inspection level.
Results
mRNA expression level of three
subunits of KATP
Among the expression levels of the three subunits of
KATP, the expression level of mRNA in the opener group
was significantly higher, followed by the model and blocker groups,
with the control group being the lowest (P<0.05; Figs. 2 and 3).
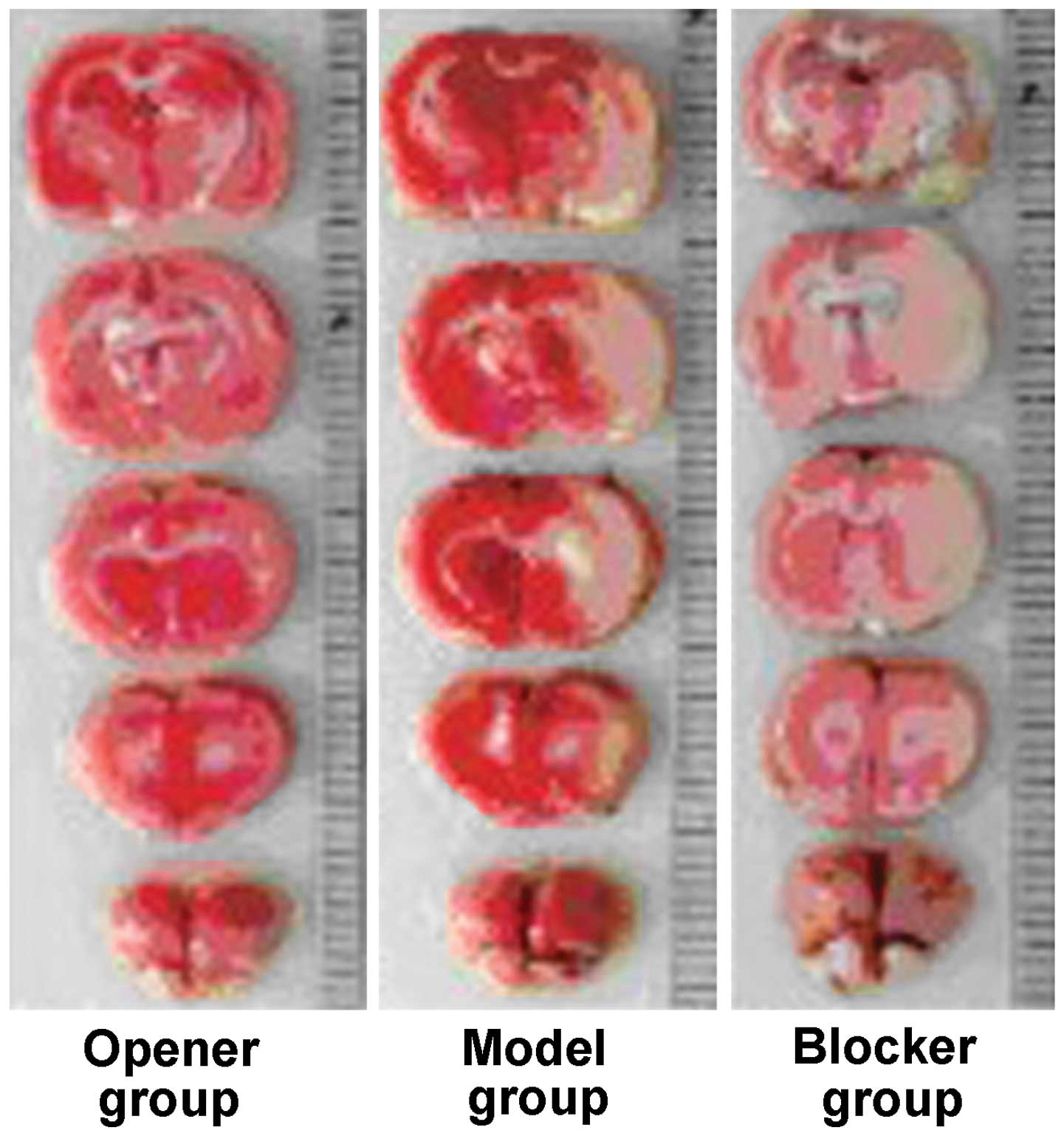
Infarct size
Infarct size in the opener group was smaller than
that in the model and blocker groups, and infarct size in the
blocker group was the largest. The difference was statistically
significant (P<0.05; Fig. 4 and
Table II).
 | Table II.Infarct size (%). |
Table II.
Infarct size (%).
| Group | Infarct size |
|---|
| Opener | 20.3±8.4 |
| Model | 46.2±13.5 |
| Blocker | 75.8±19.4 |
| F | 7.825 |
| P-value | <0.001 |
Discussion
The recovery period of cerebral arterial thrombosis
often refers to 15 days to half year of morbidity, and previous
findings have shown that this period is crucial for the restoration
of nerve function of thrombosis (8),
which is directly associated with long-term prognosis of
thrombosis. Many theories including synaptic plasticity, axonal
sprouting, denervated supersensitivity, nervus centralis bilateral
innervation on movement, study, memory, regional functional
reorganization and abundant environmental stimulus, emphasize the
important role of NYU in the recovery period of thrombosis
(9).
The KATP channels, including those from
cell membrane and mitochondrial membrane, are heteromultimers
comprising two types of subunits: Four inwardly rectifying
K+ channel subunits (Kir6.1 and Kir6.2) and four SUR
subunits, which are members of the family of ATP-binding cassette
transporter proteins. The former forms ion channels and the latter
determines KATP function (10) (Fig.
5).
At present, SUR is considered to be the main target
of drug action, which can increase the sensitivity of neuron Kir6.1
to sulfonylurea drugs and channel opener, as well as to ATP
(11). Griesemer et al showed
that there are many KATP channels in pyramidal cells and
intermediate neurons of different regions of the hippocampus
(12).
Qu et al applied diazoxide (K+
channel opener) to the MCAO rat model as a pretreatment and found
that, the cortical infarct area may be significantly reduced by
65%, and that neuronal apoptosis decreased while astrocyte
activation increased (13). The
results of the present study showed that the mRNA expression level
of the three KATP subunits in the opener group was the
highest, followed by the model and blocker groups. The results were
statistically significant as compared to the control group, which
had the lowest expression level.
Infarct size in the channel opener group was
significantly smaller than that in the model and blocker groups,
and was statistically significant, while the blocker group had the
largest infarct size. The suture method contributed to less model
injury and a higher survival rate. Under a light microscope the
pathological changes of infarction cells are similar to those of
the human body, with good model stability (14).
Current studies mainly focus on the changes of
tissues and cells during acute cerebral infarction. In the present
study, the intervention research was carried out in 3 days after
modeling during rat convalescence, and the in vitro study
was conducted 3 days after drug intervention, which basically
simulated the function of NVU target in cerebral infarction
convalescence (15).
In addition, the results have shown that many
K+ channels were active during cerebral infarction
convalescence and in the blocker group markedly reduced its
activity compared to the opener group, thereby increasing its
expression, and influencing the area of infarction, which closely
connected with the K+ channel opening degree.
The activity of KATP channel is affected
by the concentration of intracellular ATP/ADP (16). Previous findings have shown that Kir
subunit had binding sites of ATP (17), with the most effective endogenous
blocker being achieved through ATP ligand activity but not
Mg2+. ADP and other nucleoside diphosphates serve as
endogenous agonists to K+ channel (18). Therefore, the proportion of ATP and
ADP is crucial to the regulation of KATP channel
activity, and also is an essential regulatory factor to link
channel activity with cell metabolism (19). Therefore, the KATP channel
may prove an important target to improve the long-term prognosis of
stroke.
References
|
1
|
Liu Y, Wan Y, Fang Y, Yao E, Xu S, Ning Q,
Zhang G, Wang W, Huang X and Xie M: Epoxyeicosanoid signaling
provides multi-target protective effects on neurovascular unit in
rats after focal ischemia. J Mol Neurosci. 58:254–265. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Chen J, Chen C, Wang W, Wen L, Gao
K, Chen X, Xiong S, Zhao H and Li S: Wnt/β-catenin coupled with
HIF-1α/VEGF signaling pathways involved in galangin neurovascular
unit protection from focal cerebral ischemia. Sci Rep. 5:161512015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Z and Chopp M: Astrocytes, therapeutic
targets for neuroprotection and neurorestoration in ischemic
stroke. Prog Neurobiol. Oct 9–2015.(Epub ahead of print).
View Article : Google Scholar
|
|
4
|
Ran YH and Wang H: Iptakalim, an
ATP-sensitive potassium channel opener, confers neuroprotection
against cerebral ischemia/reperfusion injury in rats by protecting
neurovascular unit cells. J Zhejiang Univ Sci B. 12:835–845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon HJ, Park HS, Park SH, Park JH, Shin
SK, Song SE, Hwang M, Cho HC and Song DK: Evidence for
glucagon-like peptide-1 receptor signaling to activate
ATP-sensitive potassium channels in pancreatic beta cells. Biochem
Biophys Res Commun. 469:216–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu C, Liu Y, Shen Z, Miao L, Zhang K,
Wang F and Li Y: Sevoflurane Preconditioning Reduces Intestinal
Ischemia-Reperfusion Injury: Role of Protein Kinase C and
Mitochondrial ATP-Sensitive Potassium Channel. PLoS One.
10:e01414262015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong YF, Wang LX, Huang X, Cao WJ, Lu M,
Ding JH, Sun XL and Hu G: Kir6.1 knockdown aggravates cerebral
ischemia/reperfusion-induced neural injury in mice. CNS Neurosci
Ther. 19:617–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gliem M, Krammes K, Liaw L, van Rooijen N,
Hartung HP and Jander S: Macrophage-derived osteopontin induces
reactive astrocyte polarization and promotes re-establishment of
the blood brain barrier after ischemic stroke. Glia. 63:2198–2207.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing C, Hayakawa K, Lok J, Arai K and Lo
EH: Injury and repair in the neurovascular unit. Neurol Res.
34:325–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao S, Liu Y, Sun W, Zhao L, Zhang L, Liu
X and Yu T: Genome-Wide Expression Profiling of
Anoxia/Reoxygenation in Rat Cardiomyocytes Uncovers the Role of
MitoKATP in Energy Homeostasis. Oxid Med Cell Longev.
2015:7565762015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CG, Cui WY and Wang H: Sensitivity of
KATP channels to cellular metabolic disorders and the underlying
structural basis. Acta Pharmacol Sin. 37:134–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Griesemer D, Zawar C and Neumcke B:
Cell-type specific depression of neuronal excitability in rat
hippocampus by activation of ATP-sensitive potassium channels. Eur
Biophys J. 31:467–477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu YY, Yuan MY, Liu Y, Zhu YL and Xiao XJ:
Erratum to: The protective effect of epoxyeicosatrienoic acids on
cerebral ischemia/reperfusion injury is associated with PI3K/Akt
pathway and ATP-sensitive potassium channels. Neurochem Res.
40:8742015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma YZ, Li L, Song JK, Niu ZR, Liu HF, Zhou
XS, Xie FS and Du GH: A novel embolic middle cerebral artery
occlusion model induced by thrombus formed in common carotid artery
in rat. J Neurol Sci. 359:275–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sutherland BA, Neuhaus AA, Couch Y, Balami
JS, DeLuca GC, Hadley G, Harris SL, Grey AN and Buchan AM: The
transient intraluminal filament middle cerebral artery occlusion
model as a model of endovascular thrombectomy in stroke. J Cereb
Blood Flow Metab. 36:363–369. 2016.PubMed/NCBI
|
|
16
|
Umaru B, Pyriochou A, Kotsikoris V,
Papapetropoulos A and Topouzis S: ATP-sensitive potassium channel
activation induces angiogenesis in vitro and in vivo. J Pharmacol
Exp Ther. 354:79–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Makhina EN, Masia R, Hyrc KL,
Formanack ML and Nichols CG: Domain organization of the
ATP-sensitive potassium channel complex examined by fluorescence
resonance energy transfer. J Biol Chem. 288:4378–4388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ortiz D and Bryan J: Neonatal diabetes and
congenital hyperinsulinism caused by mutations in ABCC8/SUR1 are
associated with altered and opposite affinities for ATP and ADP.
Front Endocrinol (Lausanne). 6:482015.PubMed/NCBI
|
|
19
|
Zhou Q, Chen PC, Devaraneni PK, Martin GM,
Olson EM and Shyng SL: Carbamazepine inhibits ATP-sensitive
potassium channel activity by disrupting channel response to MgADP.
Channels (Austin). 8:376–382. 2014. View Article : Google Scholar : PubMed/NCBI
|