Introduction
Coronary artery disease (CAD) is the most prevalent
cause of death in patients suffering from cardiovascular diseases
(1). It is well-established that the
complications of CAD include acute coronary syndrome (ACS),
arrhythmias, heart failure and sudden death. ACS, particularly
acute myocardial infarction (AMI), is a major public health
concern, as it is the cause of ~75% of all CHD deaths and accounts
for ~50% of all CHD hospital admissions (2). Percutaneous coronary intervention (PCI)
and thrombolytic therapy are currently used in clinics as the
predominant methods of treating AMI. Dual antiplatelet therapy
(DAPT) is recommended for the treatment of coronary heart disease,
which includes aspirin and an ADP receptor blocker (3). Regardless of which therapy is used,
patients are recommended to accept anticoagulant or antiplatelet
therapies, including aspirin, clopidogrel and warfarin, in order to
prevent the onset of recurrent infarction following intervention
(4). Bleeding, including
intracranial hemorrhage and gastrointestinal hemorrhage, remains
the predominant adverse effect of antiplatelet therapy (5). However, physicians cannot terminate
antiplatelet therapy due to potential high incidence of recurrent
infarction. Therefore, the present study investigated patients
admitted to Liuzhou General Hospital (Liuzhou, Guangxi) between
2004 and 2010, who were suffering from gastrointestinal hemorrhage
following DAPT after the implantation of drug eluting stents (DES)
in order to compare the efficacy of various types of continued
antiplatelet therapy. The results of the present study may help
elucidate the most effective method of continued antiplatelet
therapy for patients who were treated with DAPT following DES
implantation and subsequently developed gastrointestinal
hemorrhage.
Materials and methods
Study participants
Patients were eligible for the present study if they
had experienced recurrent hemorrhage within one year of DES
implantation and DAPT, and had no relevant co-morbidities. A total
of 108 patients (aged 38–78 years) were recruited from our hospital
between 2004 and 2010. Exclusion criteria included i) The patient
died during PCI or within 24 h of the surgery; and ii) the patient
exhibited bleeding diathesis or was allergic to antiplatelet drugs.
The present study was approved by the Institutional Ethics
Committee, and written informed consent was obtained from all study
participants.
Grouping
Patients were randomly assigned to three groups:
Group A, 100 mg aspirin + proton pump inhibitor (PPI; n=18); group
B, 75 mg clopidogrel + PPI (n=60); and group C, DAPT + PPI (n=25).
Treatment was administered for 14 (2–25)
months. PPI treatment included esomeprazole (20 mg q.d.),
rabeprazole (20 mg q.d.), or pantoprazole (40 mg q.d.). Baseline
characteristics, including age, ethnicity, basic treatment,
condition of PCI surgery, were recorded for all three groups and no
significant heterogeneity was detected. Therapies administered
post-PCI surgery were identical in each group.
Follow up
All patients were contacted by telephone or attended
an out-patient follow up. During follow-up, the patient's state of
health and major evaluation criteria were recorded, including:
Major adverse cardiovascular events (MACE), which included heart
failure, AMI and cardiac death; net adverse clinical events (NACE),
which included bleeding stroke and MACE; and recurrent of
gastrointestinal hemorrhage. The dates of these incidents were
recorded.
Statistical analysis
Student's t-test or analysis of variance was
performed to compare continuous variables between populations.
Two-tailed Fisher's exact test or χ2 test was used to
compare categorical variables. Survival analysis was used to
compare patients' survival data and was performed using the
Log-rank, Breslow and Tarone-Ware methods (6–8). Data
were presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 15.0 software (SPSS,
Inc., Chicago, IL, USA).
Results
DAPT and monotherapy induce similar
incidence rates of MACE, NACE and recurrent hemorrhage
The mean follow-up period for the present study was
14 months (range 2–25 months). Five patients were lost to follow-up
(changed telephone number or stopped attending out-patient
follow-up), resulting in a dropout rate of 4.6%; therefore 103
patients were postoperatively followed-up in the three groups: A
(n=18), B (n=60) and C (n=25; Table
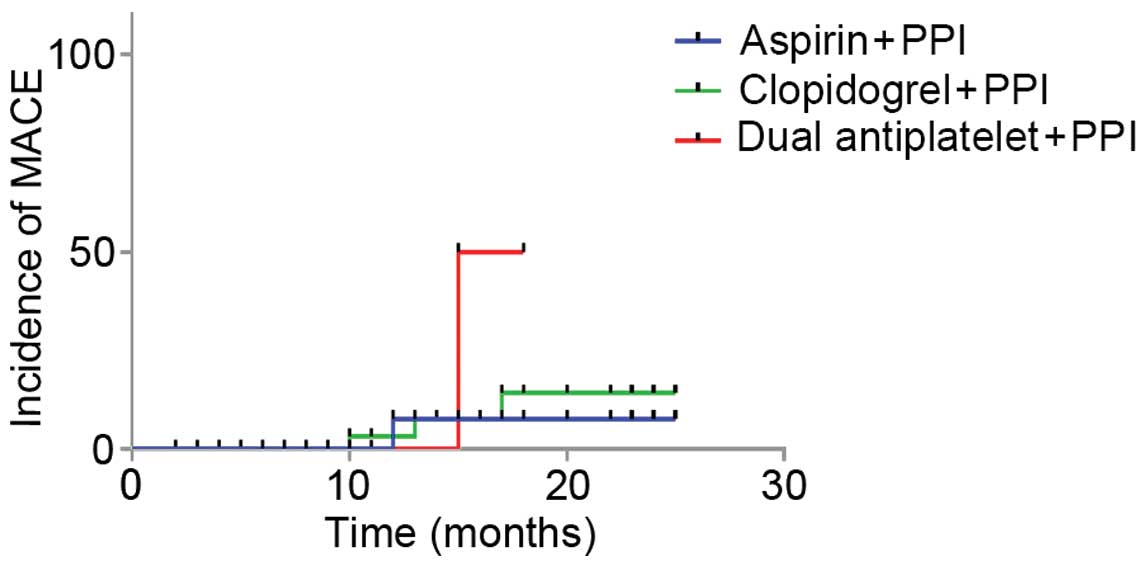
I). The incidence rates of MACE in groups A (n=1), B (n=3) and
C (n=1) were 5.6, 5.0 and 4.0%, respectively (P=0.97). Incidence
rates of NACE in groups A (n=1), B (n=4) and C (n=2) were 5.6, 6.7
and 8.0%, respectively (P=0.95) and the recurrent hemorrhage rates
in groups A (n=2), B (n=5) and C (n=4) were 11.0, 10.0 and 16.0%,
respectively (P=0.58). No significant differences in the incidence
rates of MACE, NACE and recurrent hemorrhage were detected among
the three groups (P>0.05).
 | Table I.Clinical events observed
indicators. |
Table I.
Clinical events observed
indicators.
| Variable | Group A | Group B | Group C | χ2
value | P-value |
|---|
| Patients | 18 | 60 | 25 |
|
|
| MACE, n (%) | 1 (5.6%) | 3 (5.0%) | 1 (4.0%) | 0.061 | 0.972 |
| NACE, n (%) | 1 (5.6%) | 4 (6.7%) | 2 (8.0%) | 0.103 | 0.955 |
| RH, n (%) | 2
(11.1%) | 5
(10.0%) | 4
(16.0%) | 1.092 | 0.582 |
DAPT increases gastrointestinal
rebleeding rates
Survival analysis was performed using the Log-rank,
Breslow and Tarone-Ware methods. As demonstrated in Table II, survival analysis of MACE and
NACE outcomes demonstrated that there were no significant
differences between groups A, B and C. The P-values of MACE in
groups A, B and C were 0.231, 2.763 and 3.142, respectively;
whereas the P-values of NACE were 0.157, 0.251 and 0.208,
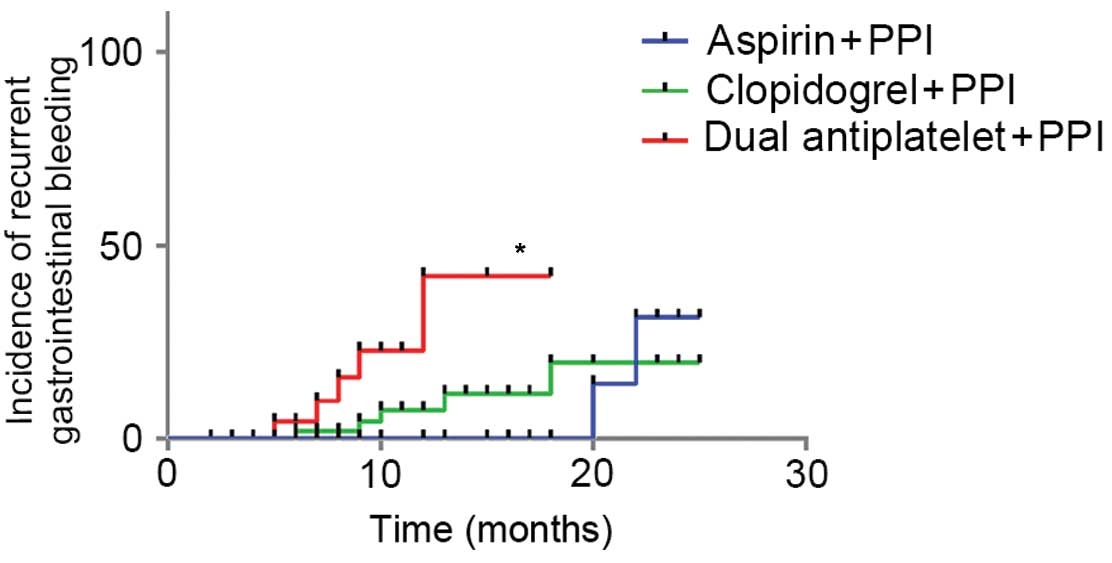
respectively. Significant differences in the frequencies of
recurrent hemorrhage were detected among the A, B and C groups
(P=0.069, 0.032 and 0.040, respectively). Furthermore, comparing
the survival times of patients among the three groups demonstrated
that there were significant differences (Table III). The Long-rank and Tarone-Ware
methods demonstrated a significant difference between groups A and
C, whereas the Breslow method presented a statistical difference
between groups A and C, and groups B and C. Taken together, these
findings indicated that the patients in group A had significantly
shorter survival times, as compared with groups B and C. Survival
curves are presented in Figs.
1–3. No statistical differences
in survival duration after MACE and NACE were detected among three
groups, whereas the DAPT group exhibited a significantly reduced
survival rate after recurrent hemorrhage.
 | Table II.Survival analysis of clinical
events. |
Table II.
Survival analysis of clinical
events.
|
| MACE | NACE | Recurrent
hemorrhage |
|---|
|
|
|
|
|
|---|
| Method | χ2
value | P-value | χ2
value | P-value | χ2
value | P-value |
|---|
| Long-rank | 2.929 | 0.231 | 3.697 | 0.157 | 5.336 | 0.069 |
| Breslow | 1.444 | 0.486 | 2.763 | 0.251 | 6.909 | 0.032 |
| Tarone-Ware | 1.971 | 0.373 | 3.142 | 0.208 | 6.419 | 0.040 |
 | Table III.Survival analysis of recurrent
hemorrhage. |
Table III.
Survival analysis of recurrent
hemorrhage.
|
| Group A | Group B | Group C |
|---|
|
|
|
|
|
|---|
| Variable | χ2
value | P-value | χ2
value | P-value | χ2
value | P-value |
|---|
| Long-rank method |
|
| Group
A |
|
| 0.205 | 0.651 | 4.054 | 0.044 |
| Group
B | 0.205 | 0.651 |
|
| 2.897 | 0.089 |
| Group
C | 4.054 | 0.044 | 2.897 | 0.089 |
|
|
| Breslow method |
|
| Group
A |
|
| 1.215 | 0.270 | 3.882 | 0.049 |
| Group
B | 1.215 | 0.270 |
|
| 3.959 | 0.047 |
| Group
C | 3.882 | 0.049 | 3.959 | 0.047 |
|
|
| Tarone-Ware
method |
|
| Group
A |
|
|
|
|
|
| Group
B | 0.796 | 0.372 | 0.796 | 0.372 | 3.987 | 0.046 |
| Group
C | 3.987 | 0.046 | 3.605 | 0.058 | 3.605 | 0.058 |
Discussion
It has previously been demonstrated that the
administration of any of the commonly recommended basic
antiplatelet strategies (5),
including aspirin + PPI, clopidogrel + PPI; DAPT + PPI, is
associated with a significant reduction of recurrent infarction
(9). However, postoperative
antiplatelet therapies are associated with digestive tract
diseases, resulting in hemorrhage and peptic ulcers, and
cardiovascular disease, which may result in MACE (10). As demonstrated in previous large
clinical trials (11–16), as compared with aspirin monotherapy,
clopidogrel (300 mg followed by 75 mg daily in all except for one
of the trials) plus aspirin reduces the risk of composite vascular
events [absolute risk reduction, 0.9–6.7%; relative risk reduction,
8.9–41.9%]. However, this improvement was associated with an
increase in major bleeding events [absolute risk increase,
0.6–2.1%; relative risk increase, −54.5–37.0%] over variable
periods from 8 days to 12 months. The mechanism of how aspirin may
result in digestive diseases, particularly peptic ulcers, can be
summarized by the following two points: i) Aspirin directly
stimulates the phospholipid layer of gastric mucosa, which damages
the hydrophobic protection barrier of the stomach (17), in addition to the increased release
of cytotoxic substances (such as leukotrienes), which may also
damage the gastric mucosa; ii) aspirin inhibits cyclooxygenase
(COX)-1 and COX-2 in gastric mucosa (18). It is well-known that prostaglandin
(PG) synthesis requires COX in gastric mucosa, and PGs can increase
the blood flow of gastric mucosa and promote the synthesis of the
mucus-HCO3 barrier (19).
Therefore, when patients are administered aspirin as antiplatelet
therapy, COX is inhibited by aspirin and the gastric mucosa loses
the protection of the mucus-HCO3 barrier and peptic
ulcers may subsequently develop. Aspirin inhibits platelet
aggregation in a dose-dependent manner, and Patrono et al
(20) have previously demonstrated
that the risk of bleeding increases by 4–6-times when patients are
treated with higher doses of aspirin. Conversely, the antiplatelet
function of clopidogrel predominantly relies on the irreversible
inhibition of the P2Y12 subtype of adenosine (5). Although clopidogrel does not damage the
gastric mucosa directly, it may inhibit the release of platelet
derived growth factor and vascular endothelial growth factor,
resulting in the inhibition of angiogenesis and the healing of
peptic ulcers.
Following PCI treatment, various complications may
affect the patients' prognosis, particularly bleeding, which may
reduce the patients' heart function and lead to MACE. The reason
for this can be summarized by the following four points: i) Massive
hemorrhage reduces the intravascular volume and increases the heart
rate, resulting in an increase of myocardial oxygen consumption and
a decrease in myocardial perfusion; ii) in order to treat a massive
hemorrhage anticoagulation, antiplatelet and antithrombotic therapy
is terminated, which increases the risk of myocardial ischemia and
stent thrombosis; and iii) blood transfusion therapy may trigger
the release of inflammatory mediators which, in turn, may increase
the onset of stent thrombosis (21).
The most salient finding of the present study is
that neither monotherapy nor combination therapy was able to induce
statistically significant differences in MACE, NACE and recurrent
hemorrhage in patients who suffered from gastrointestinal bleeding
after PCI surgery and DAPT. These findings may deviate from the
results of previous studies; however, the present results can be
explained by the combination therapy of PPI. Yasuda et al
(22) have previously demonstrated
that combination therapy with DAPT and PPI decreases the effect of
DAPT; however, the combination therapy was shown to decrease the
risk of recurrent hemorrhage. Moreover, previous studies (23–26) have
also demonstrated that combination therapy with PPI and clopidogrel
reduced the antiplatelet effect of clopidogrel. Therefore, whether
combination therapy with clopidogrel and PPI is reasonable remains
controversial, and further clinical trials are required.
The present survival analysis results demonstrated
that there were significant differences between the aspirin + PPI,
clopidogrel + PPI and DAPT + PPI groups. The effect of aspirin +
PPI and clopidogrel + PPI combination therapy were demonstrated to
be superior to DAPT + PPI combination therapy. No significant
differences were detected between the aspirin + PPI and clopidogrel
+ PPI combination therapy groups. Therefore, we hypothesise that
antiplatelet monotherapy is suitable for patients who demonstrate a
high risk of gastrointestinal hemorrhage, as compared with DAPT.
Furthermore, aspirin is more cost-effective than clopidogrel and
patients may prefer it. A 1996 CAPRIE trial (27) demonstrated that upper digestive tract
hemorrhage was significantly reduced in patients treated with
clopidogrel, as compared with aspirin. These findings are
inconsistent with the results of the present study; this may be due
to the small number of patients in the aspirin and clopidogrel
groups. Cheung et al (28)
demonstrated that patients with AMI who are complicated by peptic
ulcer hemorrhage should not continue aspirin where viable; whereas
patients who are at low risk of peptic ulcers following PCI
treatment should regard aspirin as the preferable choice.
The results of the present study indicated that
patients who developed gastrointestinal hemorrhage following
treatment with DAPT after DES implantation should continue to use
antiplatelet monotherapy or DAPT, as no significant differences in
the rates of clinical incidents, as determined by MACE, NACE and
recurrent hemorrhage, or the results of survival analysis, were
detected between the treatment groups. As for the prevention of
recurrent bleeding, antiplatelet monotherapy was demonstrated to be
superior to DAPT. The treatment of patients who are administered
DAPT and experience gastrointestinal hemorrhage following DES
implantation must involve an evaluation of the risk of
complications, including stent thrombosis, continuous bleeding and
recurrent hemorrhage.
Acknowledgements
The present study was supported by the Department of
Health of Guangxi Zhuang autonomous region (grant no.
z2008409).
References
|
1
|
Siogkas PK, Papafaklis MI, Sakellarios AI,
Stefanou KA, Bourantas CV, Athanasiou LM, Bellos CV, Exarchos TP,
Naka KK, Michalis LK, et al: Computational assessment of the
fractional flow reserve from intravascular ultrasound and coronary
angiography data: A pilot study. Conf Proc IEEE Eng Med Biol Soc.
2013:3885–3888. 2013.PubMed/NCBI
|
|
2
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR and White HD: Joint ESC/ACCF/AHA/WHF Task Force for
the Universal Definition of Myocardial Infarction. Katus HA,
Lindahl B, Morrow DA, et al: Third universal definition of
myocardial infarction. Circulation. 126:2020–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palmerini T, Benedetto U, Bacchi-Reggiani
L, Della Riva D, Biondi-Zoccai G, Feres F, Abizaid A, Hong MK, Kim
BK, Jang Y, et al: Mortality in patients treated with extended
duration dual antiplatelet therapy after drug-eluting stent
implantation: A pairwise and Bayesian network meta-analysis of
randomised trials. Lancet. 385:2371–2382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hallas J, Dall M, Andries A, Andersen BS,
Aalykke C, Hansen JM, Andersen M and Lassen AT: Use of single and
combined antithrombotic therapy and risk of serious upper
gastrointestinal bleeding: Population based case-control study.
BMJ. 333:7262006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lippi G, Franchini M and Cervellin G:
Diagnosis and management of ischemic heart disease. Semin Thromb
Hemost. 39:202–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flandre P, Boufassa F, Gerard L, Carre N,
Persoz A and Meyer L: The use of auxiliary events to improve the
analysis of survival for HIV-infected patients: application to the
French Prospective Multicenter Cohort (SEROCO). J Acquir Immune
Defic Syndr Hum Retrovirol. 12:174–181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sperry SM, Charlton ME and Pagedar NA:
Association of sentinel lymph node biopsy with survival for head
and neck melanoma: survival analysis using the SEER database. JAMA
Otolaryngol Head Neck Surg. 140:1101–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones MP and Crowley J: A general class of
nonparametric tests for survival analysis. Biometrics. 45:157–170.
1989. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matetzky S, Shenkman B, Guetta V, Shechter
M, Beinart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D
and Hod H: Clopidogrel resistance is associated with increased risk
of recurrent atherothrombotic events in patients with acute
myocardial infarction. Circulation. 109:3171–3175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong KS: Dual antiplatelet therapy after
noncardioembolic ischemic stroke or transient ischemic attack: Pros
and cons. J Clin Neurol. 10:189–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen ZM, Jiang LX, Chen YP, Xie JX, Pan
HC, Peto R, Collins R and Liu LS: COMMIT (ClOpidogrel and
Metoprolol in Myocardial Infarction Trial) collaborative group.
Addition of clopidogrel to aspirin in 45,852 patients with acute
myocardial infarction: Randomised placebo-controlled trial. Lancet.
366:1607–1621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehta SR, Yusuf S, Peters RJ, Bertrand ME,
Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F,
Chrolavicius S, et al: Effects of pretreatment with clopidogrel and
aspirin followed by long-term therapy in patients undergoing
percutaneous coronary intervention: The PCI-CURE study. Lancet.
358:527–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabatine MS, Cannon CP, Gibson CM,
López-Sendón JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill
KA, Skene AM, et al: Addition of clopidogrel to aspirin and
fibrinolytic therapy for myocardial infarction with ST-segment
elevation. N Engl J Med. 352:1179–1189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabatine MS, Cannon CP, Gibson CM,
López-Sendón JL, Montalescot G, Theroux P, Lewis BS, Murphy SA,
McCabe CH and Braunwald E: Clopidogrel as Adjunctive Reperfusion
Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28
Investigators. Effect of clopidogrel pretreatment before
percutaneous coronary intervention in patients with ST-elevation
myocardial infarction treated with fibrinolytics: The PCI-CLARITY
study. JAMA. 294:1224–1232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steinhubl SR, Berger PB, Mann JT III, Fry
ET, DeLago A, Wilmer C and Topol EJ: CREDO Investigators.
Clopidogrel for the Reduction of Events During Observation: Early
and sustained dual oral antiplatelet therapy following percutaneous
coronary intervention: A randomized controlled trial. JAMA.
288:2411–2420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yusuf S, Zhao F, Mehta SR, Chrolavicius S,
Tognoni G and Fox KK: Clopidogrel in Unstable Angina to Prevent
Recurrent Events Trial Investigators: Effects of clopidogrel in
addition to aspirin in patients with acute coronary syndromes
without ST-segment elevation. N Engl J Med. 345:494–502. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahmud T, Scott DL and Bjarnason I: A
unifying hypothesis for the mechanism of NSAID related
gastrointestinal toxicity. Ann Rheum Dis. 55:211–213. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ng SC and Chan FK: NSAID-induced
gastrointestinal and cardiovascular injury. Curr Opin
Gastroenterol. 26:611–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakumoto R, Hayashi KG and Takahashi T:
Different expression of PGE synthase, PGF receptor, TNF, Fas and
oxytocin in the bovine corpus luteum of the estrous cycle and
pregnancy. Reprod Biol. 14:115–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patrono C, Bachmann F, Baigent C, Bode C,
De Caterina R, Charbonnier B, Fitzgerald D, Hirsh J, Husted S,
Kvasnicka J, et al: Expert consensus document on the use of
antiplatelet agents. The task force on the use of antiplatelet
agents in patients with atherosclerotic cardiovascular disease of
the European society of cardiology. Eur Heart J. 25:166–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Twomley KM, Rao SV and Becker RC:
Proinflammatory, immunomodulating and prothrombotic properties of
anemia and red blood cell transfusions. J Thromb Thrombolysis.
21:167–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasuda H, Yamada M, Sawada S, Endo Y,
Inoue K, Asano F, Takeyama Y and Yoshiba M: Upper gastrointestinal
bleeding in patients receiving dual antiplatelet therapy after
coronary stenting. Intern Med. 48:1725–1730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho PM, Maddox TM, Wang L, Fihn SD, Jesse
RL, Peterson ED and Rumsfeld JS: Risk of adverse outcomes
associated with concomitant use of clopidogrel and proton pump
inhibitors following acute coronary syndrome. JAMA. 301:937–944.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Juurlink DN, Gomes T, Ko DT, Szmitko PE,
Austin PC, Tu JV, Henry DA, Kopp A and Mamdani MM: A
population-based study of the drug interaction between proton pump
inhibitors and clopidogrel. CMAJ. 180:713–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gaglia MA Jr, Torguson R, Hanna N,
Gonzalez MA, Collins SD, Syed AI, Ben-Dor I, Maluenda G, Delhaye C,
Wakabayashi K, et al: Relation of proton pump inhibitor use after
percutaneous coronary intervention with drug-eluting stents to
outcomes. Am J Cardiol. 105:833–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta E, Bansal D, Sotos J and Olden K:
Risk of adverse clinical outcomes with concomitant use of
clopidogrel and proton pump inhibitors following percutaneous
coronary intervention. Dig Dis Sci. 55:1964–1968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
CAPRIE Steering Committee: A randomised,
blinded, trial of clopidogrel versus aspirin in patients at risk of
ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet.
348:1329–1339. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheung J, Rajala J, Moroz D, Zhu Q, Stamm
M and Sandha GS: Acetylsalicylic acid use in patients with acute
myocardial infarction and peptic ulcer bleeding. Can J
Gastroenterol. 23:619–623. 2009. View Article : Google Scholar : PubMed/NCBI
|