Introduction
Vincristine is an anticancer agent of the vinca
alkaloids class, and has been used for the treatment of patients
with leukemia (1). Neurotoxicity is
a well-known complication of vincristine and manifested as
peripheral neuropathy, autonomic neuropathy and cranial neuropathy
(2). Symptoms of peripheral
neuropathy are spontaneous pain, hyperalgesia and allodynia. The
incidence of vincristine-induced peripheral neuropathy is ~60%
(3). Symptoms of vincristine-induced
neuropathy are dose-dependent, and persist for several months, even
following the discontinuation of treatment (4). Painful neuropathy is a major cause of
unwanted interruption of vincristine treatment, and limits the dose
escalation of vincristine (5,6).
Since the mechanism of vincristine-induced
neuropathy has not yet been clearly elucidated, drugs used for
various other neuropathic conditions are empirically prescribed to
treat it (7).
Theoesberiven F is a combination comprising
Melilotus extract and proxyphylline, exerting potent
anti-inflammatory, anti-edematous and analgesic effects, and
clinically prescribed for the treatment of inflammatory conditions,
edema, and cerebral and peripheral circulatory disorders (8). Melilotus extract has been
reported to have anti-inflammatory and anti-oxidative properties
and exert a suppressive effect on thermal injury in rats through
the action of phagocytic cells that accumulate in the site of
injury (9). In addition, a
coumarinic extract of Melilotus officinalis has been
demonstrated to be effective in reducing lymphedema in patients
with chronic lymphedema of the upper arm caused by lymphadenectomy
for breast cancer (10).
To the best of our knowledge, there have not yet
been any reports concerning the effect of theoesberiven F on
vincristine-induced peripheral neuropathy. Therefore, whether
theoesberiven F has anti-allodynic properties was investigated in a
rat model of vincristine-induced peripheral neuropathy in the
present study.
Materials and methods
Ethics and animal care
This study was conducted with formal approval from
the Institutional Animal Care and Use Committee in the Department
of Laboratory Animals of Bucheon St. Mary's Hospital of the
Catholic University of Korea (Bucheon City, Korea). Animals used in
this study were treated following the guidelines on the care and
use of laboratory animals as decided by this institution.
Male Sprague-Dawley rats (weight, 200–250 g) were
purchased from Nara Biotech (Seoul, Korea). The experiments were
initiated following adjustment for 7 days in a laboratory
environment. Rats were housed (2–3 animals per plastic cage) and
maintained under a 12-h light/dark cycle at 20°C, with free access
to food and water.
Vincristine-induced modeling of
peripheral neuropathy
Vincristine sulfate (Hospira Inc., Lake Forest, IL,
USA) was administered by injection to create the
vincristine-induced peripheral neuropathy model. The treatment
schedule followed was as described by Weng et al (11). Briefly, 0.1 mg/kg vincristine was
administered intraperitoneally for 5 days. Following cessation for
2 days, injection was continued for the next 5 days.
Prior to the start of the injection schedule, the
response to mechanical and cold stimulation was assessed to
establish a baseline. On day 2 after the completion of the
injections, the foot withdrawal response to von Frey filaments
using a Semmes-Weinstein von Frey aesthesiometer (Stoelting Co.,
Wood Dale, IL, USA) was measured. If a foot withdrawal response
occurred when a filament <4 g was applied to the hindpaw, it was
considered that allodynia had developed.
Drug administration
Allodynic rats were randomly allocated into a
control group and three experimental groups (TF 0.1, TF 0.25 and TF
0.5). The control group (NS, n=6) received 1 ml/kg 0.9% normal
saline. The TF 0.1 group (n=8) received 0.1 mg/kg theoesberiven F
(Theoesberiven F inj., Dai Han Pharm, Kyeongido, South Korea)
intraperitoneally. The TF 0.25 group (n=8) received 0.25 mg/kg
theoesberiven F, and the TF 0.5 group (n=8) received 0.5 mg/kg
theoesberiven F. The doses of theoesberiven F were decided by
preliminary testing (data not shown).
Pain behavioral tests
Behavioral tests were conducted in a similar manner
as in our previous study (12). The
pain behavioral tests were conducted at fixed times (1:00–6:00
p.m.) to avoid circadian rhythm errors. Following intraperitoneal
treatment, the rats were placed on metal mesh covered with a
plastic dome to measure mechanical and cold allodynia, as described
below.
Mechanical and cold allodynia were assessed prior to
drug administration and also at 15, 30, 60, 90, 120, 150 and 180
min after administration.
Measurement of mechanical
allodynia
von Frey filaments (1.0, 1.4, 2.0, 4.0, 6.0, 8.0,
10.0, 15.0 and 26.0 g) were used to measure the paw withdrawal
threshold for mechanical stimuli. The third metatarsal bone area of
left hind paw was stimulated with von Frey filaments at 3- to 4-sec
intervals, starting with the thinnest filament (1.0 g). The paw
withdrawal threshold was defined as the minimal pressure level [in
grams (g)] at which the withdrawal response occurred. If the
withdrawal response did not occur with the strongest hair (26 g),
then the threshold was recorded as 26.0 g.
Measurement of cold allodynia
Acetone was applied to the plantar surfaces of left
hind paw, and the number of foot withdrawal responses (lifting,
shaking, or licking) was counted. Application of acetone was
repeated 5 times with an interval of 3–5 min between each test. The
paw withdrawal frequency to acetone was defined as a percentage
response frequency as follows: number of paw withdrawals/number of
trials × 100.
Statistical analysis
SAS software, version 9.3 (SAS Institute, Cary, NC,
USA) was used to conduct the analysis. Results are expressed as the
mean ± standard error of the mean. Repeated-measures analysis of
variance was used to evaluate the differences, according to the
treatment group and time, followed by post hoc Dunn's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Mechanical allodynia
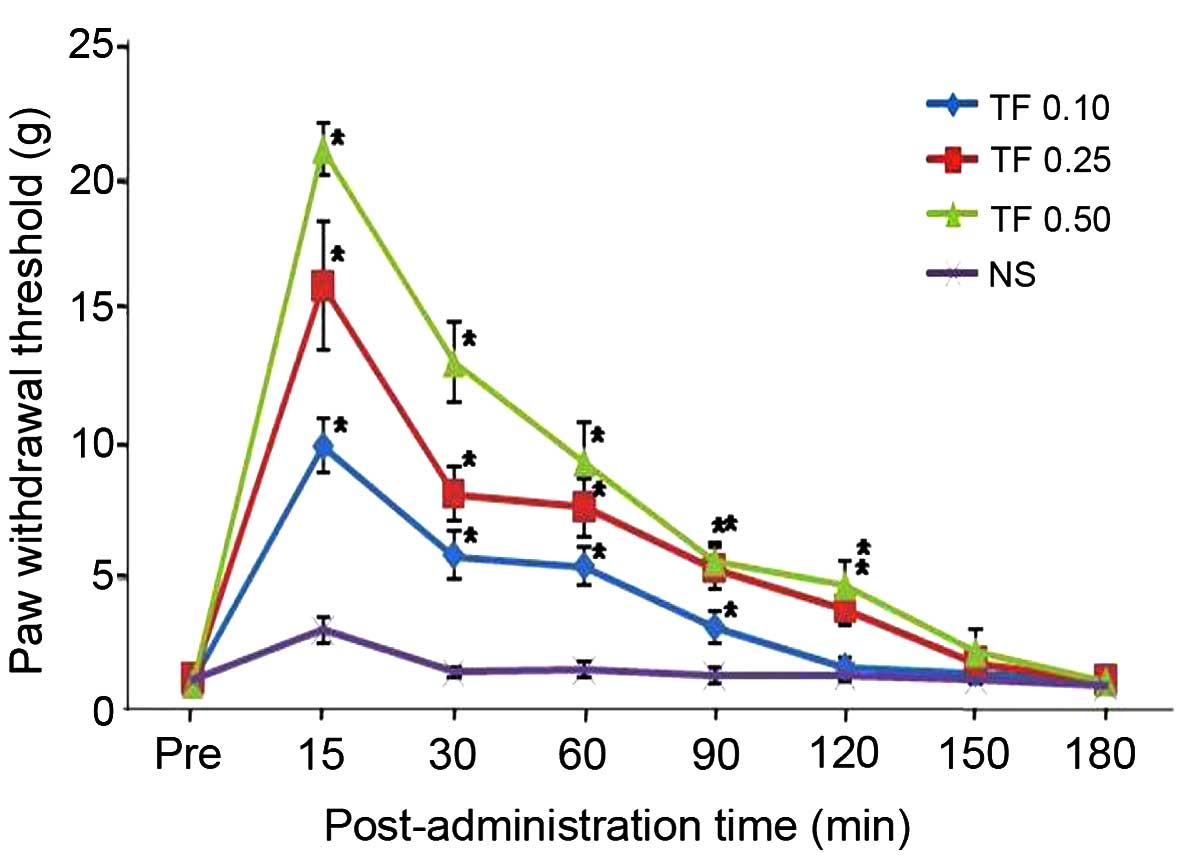
Administration of 0.1 mg/kg theoesberiven F
increased the paw withdrawal threshold significantly at 90 min
after drug administration compared with that of the control group.
The individual values were 1.2±0.1, 10.0±1.0*, 5.9±0.9*, 5.5±0.7*
3.2±0.6*, 1.7±0.4, 1.5±0.3 and 1.2±0.1 g before and at 15, 30, 60,
90, 120, 150 and 180 min after 0.1 mg/kg TF administration,
respectively (*P<0.05).
Administration of 0.25 mg/kg theoesberiven F
increased the paw withdrawal threshold significantly for 120 min
after drug administration compared with that of the control group.
The individual values were 1.3±0.2, 16±2.4*, 8.2±1.0*, 7.7±1.1*,
5.4±0.8*, 3.9±0.6*, 1.9±0.3 and 1.2±0.2 g before and at 15, 30, 60,
90, 120, 150 and 180 min after 0.25 mg/kg TF administration,
respectively (*P<0.05).
Administration of 0.5 mg/kg theoesberiven F
increased the paw withdrawal threshold significantly for 120 min
after drug administration compared with that of the control group.
The individual values were 1.1±0.1, 21.1±1.0*, 13.1±1.5*, 9.4±1.5*,
5.7±0.7*, 4.8±0.9*, 2.3±0.8 and 1.2±0.1 g before and at 15, 30, 60,
90, 120, 150 and 180 min after 0.5 mg/kg TF administration,
respectively (*P<0.05) (Fig.
1).
Cold allodynia
Following the administration of 0.1 mg/kg
theoesberiven F, the withdrawal frequency did not change
significantly compared with that of the control group (85.0±5.0,
32.5±3.7, 45.0±9.8, 57.5±8.0, 62.5±4.5, 72.5±6.5 and 75.0±6.3%
before and at 15, 30, 60, 90, 120, 150 and 180 min after 0.1 mg/kg
TF administration, respectively).
The administration of theoesberiven F reduced the
withdrawal frequency to acetone application from 15 to 30 min at a
0.25 mg/kg dosage (75.0±5.0, 27.5±5.3*, 30.0±10.0*, 50.0±6.5,
57.5±11, 60.0±7.6, 72.5±8.4 and 77.5±5.9% before and at 15, 30, 60,
90, 120, 150 and 180 min after 0.25 mg/kg TF administration,
respectively) (*P<0.05). Additionally the theoesberiven F
reduced the withdrawal frequency to acetone application from 15 to
150 min for a 0.5 mg/kg dosage (80.0±8.9, 20.0±5.3*, 27.5±9.2*,
47.5±11.3*, 52.5±10.6*, 55.0±6.3*, 57.5±4.5* and 70.5±6.5% before
and at 15, 30, 60, 90, 120, 150 and 180 min after 0.5 mg/kg TF
administration, respectively) (*P<0.05), as compared with the
pre-administration values (Fig.
2).
Discussion
To the best of our knowledge, the present study is
the first to report that theoesberiven F shows an anti-allodynic
effect in a vincristine-induced neuropathic rat model.
The theoesberiven F formulation contains 1.5 ml
Melilotus extract and 240 mg proxyphylline per 2 ml.
Melilotus extract contains various components such as
melilotoside, a glycoside that releases glucose and coumaric acid,
flavones, volatile oils, resins and tannins; of these, 0.4–0.9%
coumaric acid is transformed into coumarin (13). Proxyphylline is a bronchodilator with
a methylxanthine structure, similar to theophylline (14). It has also been reported to have
vasodilatory and cardiac stimulatory effects (15). The mechanism by which theoesberiven F
exerted an anti-allodynic effect in vincristine-induced neuropathy
in the present study is unclear.
Similar to other neuropathic pain conditions, an
inflammatory process is known to play an essential role in the
development of vincristine-induced neuropathy (16). Vincristine injection induces
macrophage infiltration and increases in the levels of
interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the
sciatic nerve section of mice, resulting in inflammation in the
peripheral nervous system (17–19). In
addition, microglial and astrocytic activation has been reported in
the lumbar spinal cord of rats following vincristine injection
(20). Kiguchi et al
(21) reported glial cell activation
and upregulation of TNF-α in the spinal cords of mice following
vincristine treatment, suggesting a potential role of
neuroinflammation in the central nervous system in
vincristine-induced neuropathy.
Tao et al (22) reported that in a cellular model
comprising lipopolysaccharide-stimulated RAW 264.7 cells, an ethyl
acetate fraction of Melilotus suaveolens Ledeb reduced the
production of pro-inflammatory cytokines, including IL-6 and TNF-α
via the suppression of nuclear factor-κB activation. Furthermore,
coumaric acid, a component of Melilotus extract, has been
reported to exert an anti-inflammatory effect by reducing the
expression of TNF-α (23). In
addition, a coumarin derivative has been shown to protect against
neurotoxicity via a improved mitochondrial function (24). Therefore, the anti-inflammatory
actions of theoesberiven F and its components might exert a
protective effect against the inflammatory process induced by
vincristine injection, decreasing the allodynia.
Oxidative stress plays an important role in the
expression of pain in various neuropathic pain models, including
sciatic nerve transection, diabetic neuropathy and spinal nerve
ligation, and the administration of anti-oxidants decreases the
pain (25–27). While oxidative stress is an important
factor in the activity of anticancer agents, it may also induce the
development of neuropathic pain (28,29). The
concentrations of thiobarbituric acid reactive substances (TBARS)
and superoxide anion have been observed to increase after
vincristine treatment (30,31). Furthermore, Melilotus extract
has been shown to inhibit the formation of TBARS, the prooxidant
H2O2 and superoxide anion in a
concentration-dependent manner (32,33).
Therefore, the anti-allodynic activity of theoesberiven F observed
in the present study may be attributed to the antioxidative effect
of Melilotus extract.
Nitric oxide (NO) is involved in synaptic
transmission in the central and peripheral nervous systems
(34), intensifying pain at a high
concentration and reducing pain at a low concentration (35). In vincristine-induced neuropathy, the
reported effects of NO and nitric oxide synthase (NOS) on the pain
are conflicting. Kamei et al (36) reported that vincristine
administration decreased the levels of NO metabolites, cGMP and
protein levels of neuronal NOS in the spinal cord, and a NOS
inhibitor reversed the analgesic effect of a NOS substrate, while
Bujalska and Gumułka reported that NOS inhibitors prevented the
development of hyperalgesia induced by vincristine administration
(37). In an inflammatory cellular
model, Melilotus extract decreased the secretion of NO and
expression of inducible NOS in a dose-dependent manner (38). The role that the effect of
Melilotus extract on NO and NOS plays in the treatment of
vincristine-induced neuropathy is unclear. Melilotus extract
exert anti-allodynic effects by rebalancing the changes of NO and
NOS induced by vincristine injection.
It is not known whether proxyphylline has an
anti-inflammatory effect or anti-oxidative properties. Therefore,
its role in the effects observed in the present study is not
clear.
In conclusion, the intraperitoneal administration of
theoesberiven F reduced mechanical and cold allodynia in a
vincristine-induced neuropathic rat model. The mechanism of
anti-allodynia of theoesberiven F is hypothesized to be a
combination of an anti-inflammatory effect, an anti-oxidative
effect and effects on NO and NOS production. Further clinical
studies are necessary before the clinical use of theoesberiven F in
patients with vincristine-induced neuropathy can be
recommended.
Acknowledgements
Statistical consultation was supported by the
Catholic Research Coordinating Center of the Korea Health 21
R&D Project (grant no. A070001), Ministry of Health &
Welfare, Republic of Korea.
References
|
1
|
Raj TA, Smith AM and Moore AS: Vincristine
sulfate liposomal injection for acute lymphoblastic leukemia. Int J
Nanomedicine. 8:4361–4369. 2013.PubMed/NCBI
|
|
2
|
Legha SS: Vincristine neurotoxicity.
Pathophysiology and management. Med Toxicol. 1:421–427. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watkins SM and Griffin JP: High incidence
of vincristine-induced neuropathy in lymphomas. Br J Med.
1:610–612. 1978. View Article : Google Scholar
|
|
4
|
Glendenning JL, Barbachano Y, Norman AR,
Dearnaley DP, Herwich A and Huddart RA: Long-term neurologic and
peripheral vascular toxicity after chemotherapy treatment of
testicular cancer. Cancer. 116:2322–2331. 2010.PubMed/NCBI
|
|
5
|
Verstappen CC, Koeppen S, Heimans JJ,
Huijgens PC, Scheulen ME, Strumberg D, Kiburg B and Postma TJ:
Dose-related vincristine-induced peripheral neuropathy with
unexpected off-therapy worsening. Neurology. 64:1076–1077. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Postma TJ, Benard BA, Huijgens PC,
Ossenkoppele GJ and Heimans JJ: Long-term effects of vincristine on
the peripheral nervous system. J Neurooncol. 15:23–27. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lynch JJ III, Wade CL, Zhong CM, Mikusa JP
and Honore P: Attenuation of mechanical allodynia by clinically
utilized drugs in a rat chemotherapy-induced neuropathic pain
model. Pain. 110:56–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moon MS and Lee IJ: Clinical experience of
Theo-Esberiven for the patients with acute musculoskeletal injuries
and postoperative inflammation. New Med J. 9:133–137. 1985.(In
Korean).
|
|
9
|
Nishikawa M, Yamashita A, Ando K and
Mitsuhiro S: The suppressive effect of Melilotus extract on
the thermal edema of rats. Nihon Yakurigaku Zasshi. 81:193–209.
1983.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastura G, Mesiti M, Saitta M, Romeo D,
Settineri N, Maisano R, Petix M and Giudice A: Lymphedema of the
upper extremity in patients operated for carcinoma of the breast:
Clinical experience with coumarinic extract from Melilotus
officinalis. Clin Ter. 150:403–408. 1999.(In Italian).
PubMed/NCBI
|
|
11
|
Weng HR, Cordella JV and Dougherty PM:
Changes in sensory processing in the spinal dorsal horn accompany
vincristine-induced hyperalgesia and allodynia. Pain. 103:131–138.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park HJ, Lee HG, Kim YS, Lee JY, Jeon JP,
Park C and Moon DE: Ginkgo biloba extract attenuates
hyperalgesia in a rat model of vincristine-induced peripheral
neuropathy. Anesth Analg. 115:1228–1233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pleşca-Manea L, Pârvu AE, Pârvu M, Taămaş
M, Buia R and Puia M: Effects of Melilotus officinalis on
acute inflammation. Phytother Res. 16:316–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tivenius L: Comparison of drugs for
asthma. Br Med J. 2:7731971. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeda K, Katano Y, Nakagawa Y, Tsukada T,
Nakazawa M, Otorii T and Imai S: Effects of aminopylline,
proxyphylline and a proxyphylline-Melilotus extract-rutin
mixture (theoesberiven) on the heart and the coronary circulation.
Jpn J Pharmacol. 27:709–720. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kiguchi N, Maeda T, Kobayashi Y, Saika F
and Kishioka S: Involvement of inflammatory mediators in
neuropathic pain caused by vincristine. Int Rev Neurobiol.
85:179–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uçeyler N, Kobsar I, Biko L, Ulzheimer J,
Levinson SR, Martini R and Sommer C: Heterozygous P0 deficiency
protects mice from vincristine-induced polyneuropathy. J Neurosci
Res. 84:37–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kiguchi N, Maeda T, Kobayahi Y, Kondo T,
Ozaki M and Kishioka S: The critical role of invading peripheral
macrophage-derived interleukin-6 in vincristine-induced mechanical
allodynia in mice. Eur J Pharmacol. 592:87–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muthuraman A, Singh N and Jaggi AS:
Protective effect of Acorus calamus L. in rat model of
vincristine induced painful neuropathy: An evidence of
anti-inflammatory and anti-oxidative activity. Food Chemi Toxicol.
49:2557–2563. 2011. View Article : Google Scholar
|
|
20
|
Sweitzer SM, Pahl JL and DeLeo JA:
Propentofylline attenuates vincristine-induced peripheral
neuropathy in the rat. Neurosci Lett. 400:258–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiguchi N, Maeda T, Kobayashi Y and
Kishioka S: Up-regulation of tumor necrosis factor-alpha in spinal
cord contributes to vincristine-induced mechanical allodynia in
mice. Neurosci Lett. 445:140–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tao JY, Zheng GH, Zhao L, Wu JG, Zhang XY,
Zhang SL, Huang ZJ, Xiong FL and Li CM: Anti-inflammatory effects
of ethyl acetate fraction from Melilotus suaveolens Ledeb on
LPS-stimulated RAW 264.7 cells. J Ethnopharmacol. 123:97–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pragasam SJ, Venkatesan V and Rasool M:
Immunomodulatory and anti-inflammatory effect of p-coumaric
acid, a common dietary polyphenol on experimental inflammation in
rats. Inflammation. 36:169–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song XY, Hu JF, Sun MN, Li ZP, Wu DH, Ji
HJ, Yuan YH, Zhu ZX, Han N, Liu G and Chen NH: IMM-H004, a novel
coumarin derivative compound, protects against amyloid beta-induced
neurotoxicity through a mitochondrial-dependent pathway.
Neuroscience. 242:28–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scheid T, Bosco LD, Guedes RP, Pavanato
MA, Belló-Klein A and Partata WA: Sciatic nerve transcetion
modulates oxidative parameters in spinal and supraspinal regions.
Neurochem Res. 38:935–942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YP, Eber A, Yuan Y, Yang Z,
Rodriguez Y, Levitt RC, Takacs P and Candiotti KA: Prophylactic and
antinociceptive effects of coenzyme Q10 on diabetic neuropathic
pain in a mouse model of type 1 diabetes. Anesthesiology.
118:945–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yowtak J, Lee KY, Kim HY, Wang J, Kim HK,
Chung K and Chung JM: Reactive oxygen species contributes to
neuropathic pain by reducing spinal GABA release. Pain.
152:844–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Huang L, Mackenzie GG and Rigas B:
Oxidative stress mediates through apoptosis the anticancer effect
of phospho-nonsteroidal anti-inflammatory drugs: implications for
the role of oxidative stress in the action of anticancer agents. J
Pharmacol Exp Ther. 338:775–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carozzi VA, Canta A and Chiorazzi A:
Chemotherapy-induced peripheral neuropathy: What do we know about
mechanisms? Neurosci Lett. 596:90–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wozniak A, Drewa G, Woźniak B,
Schachtschabel DO, Mila-Kierzenkowska C, Drewa T, Olszewska-Słonina
D and Sopońska M: The effect of antitumor drugs on oxidative stress
in B16 and S91 melanoma cells in vitro. Med Sci Monit.
11:BR22–BR29. 2005.PubMed/NCBI
|
|
31
|
Muthuraman A and Singh N: Attenuating
effect of hydroalcoholic extract of Acorus calamus in
vincristine-induced painful neuropathy in rats. J Nat Med.
65:480–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fiorentino A, D'Abrosca B, Pacifico S,
Golino A, Mastellone C, Oriano P and Monaco P: Reactive oxygen
species scavenging activity of flavone gylcosides from Melilotus
neapolitana. Molecules. 12:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Braga PC, Sasso MD, Lattuada N, Marabini
L, Calò R, Antonacci R, Bertelli A, Falch M and Verducci P:
Antioxidant activity of Melilotus officinalis extract
investigated by means of the radical scavenging activity, the
chemiluminescence of human neutrophil bursts and lipoperoxidation
assay. J Med Plants Res. 7:358–365. 2013.
|
|
34
|
Schuman EM and Madison DV: Nitric oxide
and synaptic function. Annu Rev Neurosci. 17:153–183. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prado WA, Schiavon VF and Cunha FQ: Dual
effect of local application of nitric oxide donors in a model of
incision pain in rats. Eur J Pharmacol. 441:57–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamei J, Tamura N and Saitoh A: Possible
involvement of the spinal nitric oxide/cGMP pathway in
vincristine-induced painful neuropathy in mice. Pain. 117:112–120.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bujalska M and Gumułka SW: Effect of
cyclooxygenase and nitric oxide synthase inhibitors on vincristine
induced hyperalgesia in rats. Pharmacol Rep. 60:735–741.
2008.PubMed/NCBI
|
|
38
|
Zhao L, Tao JY, Zhang SL, Pang R, Jin F,
Dong JH and Guo YJ: Inner anti-inflammatory mechanisms of petroleum
ether extract from Melilotus suaveolens Ledeb. Inflammation.
30:213–223. 2007. View Article : Google Scholar : PubMed/NCBI
|