Introduction
A number of neurodegenerative disorders, including
Parkinson's disease, Alzheimer's disease (AD) and multiple
sclerosis are associated with neuroinflammation reactions involving
the activation of microglia (1).
Microglia cells are the resident macrophages in the central nervous
system (CNS), serving as the innate immune system of the CNS
(2). Exposure of microglia to
lipopolysaccharide (LPS) causes them to become activated, and they
contribute to innate and adaptive immune responses by producing
pro-inflammatory mediators, including nuclear factor (NF)-κB,
caspase-3 and heat shock protein 60 (HSP60) (3–6).
Extracellular HSP60 has been shown to increase the quantities of
pro-inflammatory factors produced in microglia, causing neuronal
death (7,8). Thus, the inhibition of HSP60 production
is an effective option for use in the treatment of
neurodegenerative disorders.
Curcumin has been the subject of extensive studies,
which have revealed a wide range of biological activities,
including anti-oxidant, anti-inflammatory, anti-infection and
anticarcinogenic activities (9–12). In
H2O2-treated BV2 microglia and glaucoma
models, curcumin was shown to improve cell viability and decrease
the intracellular levels of reactive oxygen species and apoptosis
significantly, indicating that curcumin afforded neuroprotective
effects via the inhibition of oxidative damage (13). Curcumin has also been demonstrated to
have a protective effect against HIV-1 gp120-mediated
neurotoxicity, mediated via the reduction of microglial
inflammation (14). In comparison
with other microglial inhibitors, curcumin has several advantages,
including its ability to penetrate the blood brain barrier, and few
side effects (15). Thus, it may be
considered as a new potential therapeutic option for
neuroprotection.
Thus far, despite a small number of studies
indicating that curcumin is able to attenuate inflammation caused
by microglial activation and promote neuronal survival, the
mechanisms underlying the modulatory effects of curcumin on
microglial activation remain largely unknown. Previous studies have
demonstrated that HSP60 is released extracellularly in cardiac
myocytes from rat models of heart failure and induced apoptosis
through binding to Toll-like receptor 4 (TLR-4) (16,17).
Curcumin has been found to inhibit the TLR-4 signaling pathway in
certain injury models (18–20). However, it remains unknown whether
curcumin can exert its neuroprotective effects by inhibiting the
HSP60/TLR-4 pathway. In the present study, the aim was to
investigate whether HSP60 is involved in the neuroprotective
effects of curcumin in LPS-induced inflammatory injury in BV2
microglia cells.
Materials and methods
Chemicals
Curcumin and LPS were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Antibodies against glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; ab181602) and NF-κB (ab31481) were from Abcam
(Cambridge, MA, USA). Anti-HSP60 (API-SPA-901) and anti-heat shock
factor 1 (HSF-1; ADI-SPA-806) antibodies were from Stressgen (Enzo
Life Sciences, Farmingdale, NY, USA). Anti-caspase-3 (cat. no.
9665), anti-inducible nitric oxide synthase (iNOS; cat. no. 2977),
TLR-4 (cat. no. 2219) and anti-myeloid differentiation primary
response 88 (MyD88; cat. no. 4283) antibodies were from Cell
Signaling Technology, Inc. (Beverly, MA, USA). The proteinase
inhibitor cocktails were from Merck Chemicals (Kenilworth, NJ,
USA). Interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α
enzyme-linked immunosorbent assay (ELISA) kits were from
eBioscience, Inc. (San Diego, CA, USA). Bicinchoninic acid (BCA)
and enhanced chemiluminescence (ECL) kits were from Pierce (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Dulbecco's modified
Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Gibco
(Thermo Fisher Scientific, Inc.). All additional chemicals were
purchased from ZSGB-Bio (Shanghai, China), unless otherwise
stated.
Microglial culture
Mouse BV2 microglia cells (Shanghai Honsun
Biological Technology Co., Ltd., Shanghai, China) were cultured in
DMEM supplemented with 10% FBS, penicillin (100 U/ml) and
streptomycin (100 µg/ml). Cultures were maintained at 37°C in a
humidified incubator under an atmosphere of 95% O2 and
5% CO2. Curcumin was dissolved in phosphate-buffered
saline (PBS). Following incubation of the cells with LPS (1 µg/ml)
for 30 min (LPS group), the LPS + CCM group cells were treated with
the indicated concentrations of curcumin for 24 h. Control group
cells were treated with an equivalent quantity of dimethyl
sulfoxide as a solvent control.
Cell viability assay
A CCK-8 kit was used to evaluate cell viability
(BestBio, Shanghai, China). Cells in 100 µl solution at
5×104-1×105 cells/well were seeded into
96-well microtiter plates and treated with 1–20 µg/ml curcumin.
After 24 h, CCK-8 solution was added to each well for 2 h,
according to the manufacturer's instructions. Absorbance was
determined at 450 nm using a microplate reader.
ELISA
The culture medium was analyzed to detect the levels
of IL-6, IL-1β, HSP60 and TNF-α using ELISA kits according to the
manufacturer's protocol. Absorbance was determined at 450 nm using
a microplate reader.
Western blot analysis
Cells were washed with PBS three times and lysed
with radioimmunoprecipitation assay buffer. The protein
concentration was determined with a BCA kit according to the
manufacturer's protocol. Equal quantities of protein (10 µg) were
loaded and run on sodium dodecyl sulfate/polyacrylamide gels and
then transferred to a polyvinylidene difluoride membrane. Membranes
were blocked with 5% dried milk and incubated with primary
antibodies against GAPDH (1:1,000), NF-κB (1:1,000), HSP60
(1:1,000), HSF-1 (1:1,000), caspase-3 (1:2,000), iNOS(1:200), MyD88
(1:1,000) and TLR-4 (1:1,000), in Tris-buffered saline and Tween 20
(TBST) overnight at 4°C. After being rinsed in milk-TBST, blots
were incubated with horseradish peroxidase-conjugated secondary
antibodies goat anti-rabbit (ZB-2301; 1:5,000) and anti-mouse IgG
(ZB-2305; 1:5,000; ZSGB-BIO, Beijing, China). The target proteins
were detected using an ECL detection system and X-ray films. The
blotting results were semiquantified using Quantity One software,
version 4.6.9 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical differences were determined using
one-way analysis of variance testing. P<0.05 was considered to
indicate a statistically significant difference. Data in the text
and figures are presented as mean ± standard error of the mean; n
represents the number of experiments.
Results
Curcumin promotes the viability of BV2
microglia
To determine whether curcumin affects the viability
of LPS-stimulated BV2 cells, a CCK-8 assay was performed. The
results demonstrated that treatment of microglia with 1–20 µg/ml
curcumin for ≤24 h significantly increased the viability of
LPS-stimulated BV2 cells, compared with that cells treated with LPS
alone (data not shown). Treatment of cells with 5 µg/ml curcumin
showed the maximal viability among all the concentrations tested;
thus 5 µg/ml was chosen for the following experiments.
Curcumin inhibits HSP60 expression and
release in LPS-stimulated BV2 microglia
The expression level and release of HSP60 in
activated BV2 cells were tested. The western blotting results
showed that HSP60 expression was significantly increased following
LPS treatment and curcumin significantly inhibited this increase
(P<0.05; Fig. 1A). HSF-1 has been
found to bind with the heat shock element on the HSP60 promoter to
regulate HSP60 gene expression (21). Therefore, the HSF-1 level was
determined, and the results revealed that it was upregulated by LPS
and downregulated by curcumin, indicating that HSP60 expression was
promoted by HSF-1 (Fig. 1B). HSP60
has been reported to undergo extracellular translocation when
stressed to cause injury (17).
ELISA results indicated that HSP60 was released when BV2 was
activated and the increased extracellular HSP60 was suppressed by
curcumin (Fig. 1C).
Curcumin inhibits the expression of
proteins associated with the TLR-4/MyD88/NF-κB signaling
pathway
The effects of curcumin on TLR-4, MyD88, NF-κB and
caspase-3 expression in LPS-stimulated BV2 microglia were
investigated. Curcumin strongly inhibited TLR-4 and MyD88
expression (Fig. 2A and B). NF-κB
regulates the expression of numerous proinflammatory factors.
Therefore, the NF-κB level was measured following curcumin
treatment and it was found that levels of the p65 subunit of NF-κB
increased following LPS treatment, but the LPS-induced increase was
markedly attenuated by curcumin (Fig.
2C). Caspase-3 is an important protein of the NF-κB signaling
pathway (22). Inhibition of
caspase-3 prevents neuronal loss induced by activated microglia in
brain diseases (23). Thus, the
effects of curcumin on caspase-3 expression were evaluated, and it
was observed that caspase-3 expression was suppressed following
treatment with curcumin (Fig.
2D).
 | Figure 2.CCM inhibited the increased expression
of (A) TLR-4, (B) MyD88, (C) NF-κB (p65) and (D) caspase-3 in
LPS-stimulated BV2 microglia. Cells were pretreated with LPS for
0.5 h, followed by incubation with 5 µg/ml CCM for 24 h. The
lysates were probed by immunoblotting with antibodies against
TLR-4, MyD88, NF-κB and caspase-3 and GAPDH individually. Results
are the mean ± standard error. Each experiment was derived from at
least three independent cultures. *P<0.05 vs. the Ctrl group;
**P<0.05 vs. the LPS group. TLR-4, Toll-like receptor 4; MyD88,
myeloid differentiation primary response 88; NF, nuclear factor;
LPS, lipopolysaccharide; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; Ctrl, control; CCM, curcumin. |
Curcumin inhibits the production of
proinflammatory factors
Whether curcumin could reduce the release of the
proinflammatory factors TNF-α, IL-1β and IL-6 in LPS-stimulated BV2
cells was investigated by ELISA. As shown in Fig. 3, treatment of BV2 cells with curcumin
for 24 h resulted in marked increases of the aforementioned factors
in the culture media as compared with the control. The expression
of iNOS decreased notably after curcumin treatment (Fig. 3D). These results indicated that
curcumin effectively suppressed the production of neurotoxic
factors in over-activated microglia.
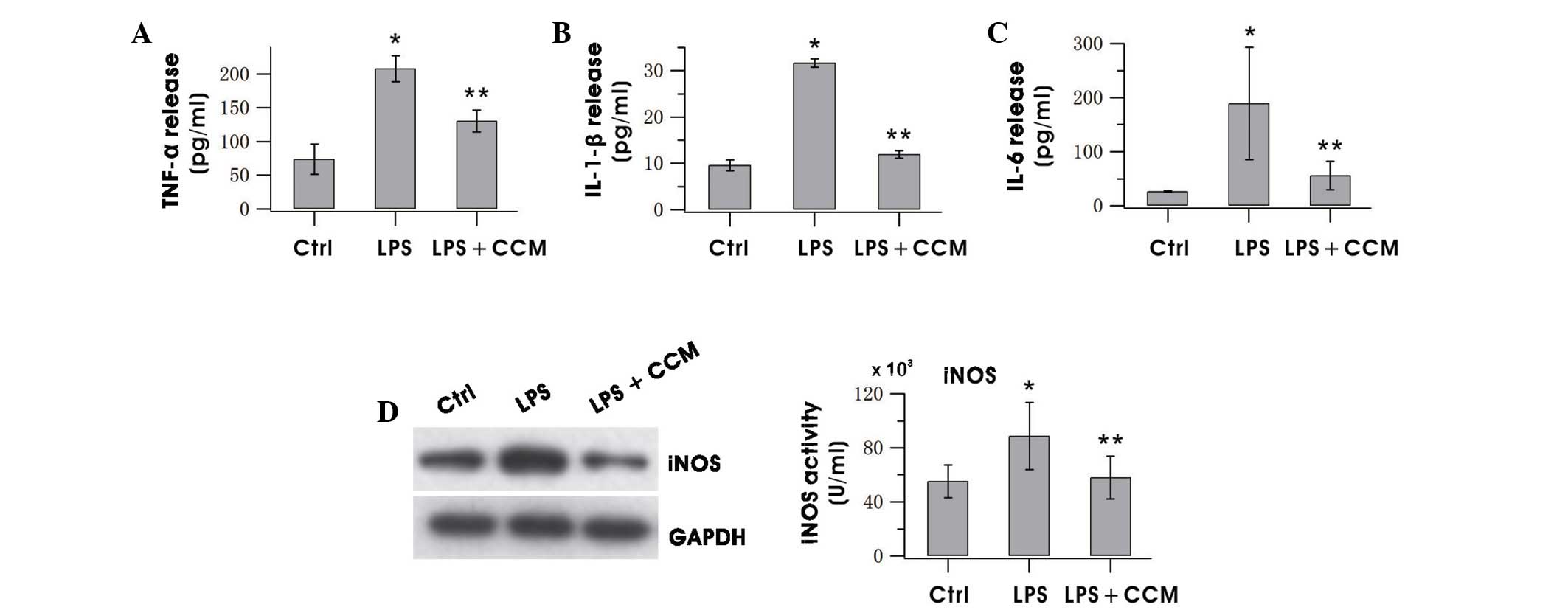 | Figure 3.CCM decreased the release of (A)
TNF-α, (B) IL-1β, (C) IL-6 and (D) iNOS in LPS-stimulated BV2
microglia. Cells were pretreated with LPS for 0.5 h, followed by
incubation with 5 µg/ml CCM for 24 h. The expression level of iNOS
was assayed by western blotting of BV2 cells and levels of TNF-α,
IL-1β and IL-6 in the culture medium were measured using ELISA. The
results are the mean ± standard error of three separate experiments
performed in triplicate. *P<0.05 vs. the Ctrl; **P<0.05 vs.
the LPS group. TNF, tumor necrosis factor; IL, interleukin; iNOS,
inducible nitric oxide synthase; LPS, lipopolysaccharide; ELISA,
enzyme-linked immunosorbent assay; Ctrl, control; CCM,
curcumin. |
Discussion
The present study demonstrates that treatment with 5
µg/ml curcumin effectively inhibits microglial activation. Curcumin
reduced the expression of TLR-4, MyD88, NF-κB, caspase-3, HSP60,
HSF-1 and iNOS in LPS-activated microglia and effectively
suppressed the release of HSP60 and the proinflammatory cytokines
TNF-α, IL-6 and IL-1β in LPS-stimulated microglia. To the best of
our knowledge, the present study is the first to report that
curcumin potentially attenuates microglia activation via the
modulation of acute neuroinflammation mediated by the
HSP60/TLR-4/MyD88/NF-κB signaling pathway.
When over-activated, microglia secrete a variety of
proinflammatory and neurotoxic factors inducing infectious
diseases, inflammation and neurodegeneration (24,25).
Since microglial activation plays an important role in
neurodegeneration, it has been suggested that inhibition of
microglial activation, in particular control of the production of
neurotoxic factors, may be an effective method for treating
neurodegenerative diseases. A number of microglia-targeted
pharmacotherapies, including protein kinase C inhibitors,
minocycline and naloxone have been suggested to inhibit microglia
and promote neuronal survival (26,27).
However, the inability to cross the blood-brain barrier and their
possible side-effects limit their clinical use.
Curcumin has been used as a spice or pigment by
humans for centuries. Curcumin has various benefits, such as a
marked safety profile with very low toxicity, numerous
pharmacological activities, ability to cross the blood-brain
barrier, widespread availability and low cost (15,28). In
addition, curcumin is potentially useful for the prevention and
treatment of various neuroinflammatory and neurodegenerative
conditions of the CNS (29).
Curcumin is a promising agent for protecting against AD and
HIV-1-assocated neurological disorders (30,31).
Studies have demonstrated that curcumin is highly pleiotropic and
interacts with numerous molecular targets; it has been shown to
inhibit the homodimerization of TLR-4, thereby attenuating
inflammatory injury via the TLR-4 pathway (18–20).
However, it remains unknown whether HSP60 is involved in the
neuroprotective effects of curcumin.
HSP60 is primarily a mitochondrial protein, but upon
stress or injury HSP60 is able to translocate to the plasma
membrane and be released extracellularly in the failing heart
(32). The present study showed that
HSP60 was present in the culture medium of LPS-activated BV2 cells,
which is consistent with the results in cardiac myocytes. This
released HSP60 may be able to act in a paracrine or autocrine
manner to activate TLR-4, for which HSP60 has been reported to be a
ligand (33). Within the TLR-4
signaling pathway, the MyD88-dependent pathway is an important
activator of NF-κB. The activation of NF-κB is a key event in the
inflammatory response caused by microglial activation as NF-κB
activation is necessary for the transcription of various
proinflammatory molecules (34,35).
Caspase-3 is an important mediator of cell apoptosis (22). When caspase 3 or 7 is inhibited,
LPS-stimulated microglia have been shown to be non-toxic to
neighboring neurons (23). The
activation of NF-κB by caspase-3 is critical in inflammation. HSP60
gene expression is regulated by the transcription factor HSF-1
binding to the HSP60 gene promoter. NF-κB has also been shown to
induce the transcription of the HSP60 stress protein gene, which
elicits a potent proinflammatory response in innate immune cells
(32). Thus, the effects of curcumin
on HSP60, TLR-4, MyD88, NF-κB and caspase-3 expression levels were
investigated in the present study. The results demonstrate that
curcumin treatment markedly attenuates HSP60, TLR-4, MyD88,
caspase-3 and the NF-κB downstream mediator p65, suggesting that
the anti-inflammatory effects of curcumin may occur via inhibition
of the HSP60/TLR-4 signaling pathway.
Microglia activation is known to produce a number of
proinflammatory cytokines. This was confirmed in the present study
by measuring iNOS protein expression in BV2 cells and the levels of
TNF-α, IL-1β and IL-6 in the culture medium of LPS-stimulated BV2
cells. The iNOS gene is under the transcriptional control of a
variety of inflammatory mediators, such as cytokines and LPS
(36). TNF-α is a mediator of NF-κB
signaling and triggers increased HSP60 expression, which has is
reversed by p65 inhibition (15).
However, following curcumin treatment, the levels of TNF-α, IL-1β,
IL-6 and iNOS were clearly inhibited.
This study indicates that HSP60 may participate in
the neuroprotective effects of curcumin via inhibition of the NF-κB
signaling pathway to prevent over-activation of microglia.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81460182, 31460257,
81360196 and 31260243) and Ningxia Natural Science Foundation (no.
NZ14057). Additional funding was provided by Key Project of Science
and Technology of Ningxia (no. 2012zys239) and Ningxia Medical
University Program (no. XM2012022). Funding was provided to Dr Yin
Wang by the Program for New Century Excellent Talents in
University.
References
|
1
|
Perry VH, Nicoll JA and Holmes C:
Microglia in neurodegenerative disease. Nat Rev Neurol. 6:193–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nature Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
3
|
Hanisch UK and Kettenmann H: Microglia:
Active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gehrmann J, Matsumoto Y and Kreutzberg GW:
Microglia: Intrinsic immuneffector cell of the brain. Brain Res
Brain Res Rev. 20:269–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch MA: The multifaceted profile of
activated microglia. Mol Neurobiol. 40:139–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li YH, Teng P, Wang Y, Zhang YM, Ma CJ and
Pu J: Expression and regulation of HSP60 in activated microglia
cells. Ningxia Yixueyuan Xuebao. 8:712–714. 2011.(In Chinese).
|
|
7
|
Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J
and Gu J: Microglial LOX-1 reacts with extracellular HSP60 to
bridge neuroinflammation and neurotoxicity. Neurochem Int.
61:121–1035. 2012. View Article : Google Scholar
|
|
8
|
Lehnardt S, Schott E, Trimbuch T, Laubisch
D, Krueger C, Wulczyn G, Nitsch R and Werber JR: A vicious cycle
involving release of heat shock protein 60 from injured cells and
activation of toll-like receptor 4 mediates neurodegeneration in
the CNS. J Neurosci. 28:2320–2331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motterlini R, Foresti R, Bassi R and Green
CJ: Curcumin, an antioxidant and anti-inflammatory agent, induces
heme oxygenase-1 and protects endothelial cells against oxidative
stress. Free Radic Biol Med. 28:1303–1312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De R, Kundu P, Swarnakar S, Ramamurthy T,
Chowdhury A, Nair GB and Mukhopadhyay AK: Antimicrobial activity of
curcumin against Helicobacter pylori isolates from India and
during infections in mice. Antimicrob Agents Chemother.
53:1592–1597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piper JT, Singhal SS, Salameh MS, Torman
RT, Awasthi YC and Awasthi S: Mechanisms of anticarcinogenic
properties of curcumin: The effect of curcumin on glutathione
linked detoxification enzymes in rat liver. Int J Biochem Cell
Biol. 30:445–456. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue YK, Mo B, Zhao J, Yu YJ, Liu L, Yue CL
and Liu W: Neuroprotective effect of curcumin against oxidative
damage in BV-2 microglia and high intraocular pressure animal
model. J Ocul Pharmacol Ther. 30:657–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo L, Xing Y, Pan R, Jiang M, Gong Z, Lin
L, Wang J, Xiong G and Dong J: Curcumin protects microglia and
primary rat cortical neurons against HIV-1 gp120-mediated
inflammation and apoptosis. PLoS One. 8:e705652013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mimeault M and Batra SK: Potential
applications of curcumin and its novel synthetic analogs and
nanotechnology-based formulations in cancer prevention and therapy.
Chin Med. 6:1–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SC, Stice JP, Chen L, Jung JS, Gupta
S, Wang Y, Baumgarten G, Trial J and Knowlton AA: Extracellular
heat shock protein 60, cardiac myocytes and apoptosis. Circ Res.
105:1186–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin L, Kim SC, Wang Y, Gupta S, Davis B,
Simon SI, Torre-Amione G and Knowlton AA: HSP60 in heart failure:
Abnormal distribution and role in cardiac myocyte apoptosis. Am J
Physiol Heart Circ Physiol. 293:H2238–H2247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Youn HS, Saitoh SI, Miyake K and Hwang DH:
Inhibition of homodimerization of Toll-like receptor 4 by curcumin.
Biochem Pharmacol. 72:62–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng Z, Zhan L, Liao H, Chen L and Lv X:
Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27
expression via the TLR4/NF-κB signaling pathway. Planta Med.
79:102–109. 2013.PubMed/NCBI
|
|
20
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hansen JJ, Bross P, Westergaard M, Nielsen
MN, Eiberg H, Børglum AD, Mogensen J, Kristiansen K, Bolund L and
Gregersen N: Genomic structure of the human mitochondrial
chaperonin genes: HSP60 and HSP10 are localised head to head on
chromosome 2 separated by a bidirectional promoter. Hum Genet.
112:71–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soria JA, Arroyo DS, Gaviglio EA,
Rodriguez-Galan MC, Wang JM and Iribarren P: Interleukin 4 induces
the apoptosis of mouse microglial cells by a caspase-dependent
mechanism. Neurobiol Dis. 43:616–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burguillos MA, Deierborg T, Kavanagh E,
Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P,
Englund E, Venero JL and Joseph B: Caspase signalling controls
microglia activation and neurotoxicity. Nature. 472:319–324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kreutzberg GW: Microglia: A sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park E, Kim DY and Chun HS: Resveratrol
inhibits lipopolysaccharide-induced phagocytotic activity in BV2
cells. J Korean Soc Appl Biol Chem. 55:803–807. 2012. View Article : Google Scholar
|
|
26
|
Thanos S, Mey J and Wild M: Treatment of
the adult retina with microglia-suppressing factors retards
axotomy-induced neuronal degradation and enhances axonal
regeneration in vivo and in vitro. J Neurosci. 13:455–466.
1993.PubMed/NCBI
|
|
27
|
Teng P, Li YH, Cheng W, Zhou L, Shen Y and
Wang Y: Neuroprotective effects of Lycium barbarum
polysaccharides in lipopolysaccharide-induced BV2 microglial cells.
Mol Med Rep. 7:1977–1981. 2013.PubMed/NCBI
|
|
28
|
Ravindran J, Prasad S and Aggarwal BB:
Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells
Selectively? AAPS J. 11:495–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cole GM, Teter B and Frautschy SA:
Neuroprotective effects of curcumin. Adv Exp Med Biol. 595:197–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mishra S and Palanivelu K: The effect of
curcumin (turmeric) on Alzheimer's disease: An overview. Ann Indian
Acad Neurol. 11:13–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong Z, Yang L, Tang H, Pan R, Xie S, Guo
L, Wang J, Deng Q, Xiong G, Xing Y and Dong J: Protective effects
of curcumin against human immunodeficiency virus 1 gp120 V3
loop-induced neuronal injury in rats. Neural Regen Res. 7:171–175.
2012.PubMed/NCBI
|
|
32
|
Wang Y, Chen L, Hagiwara N and Knowlton
AA: Regulation of heat shock protein 60 and 72 expression in the
failing heart. J Mol Cell Cardiol. 48:360–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohashi K, Burkart V, Flohe S and Kolb H:
Cutting edge: Heat shock protein 60 is a putative endogenous ligand
of the toll-like receptor-4 complex. J Immunol. 164:558–561. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khasnavis S, Jana A, Roy A, Mazumder M,
Bhushan B, Wood T, Ghosh S, Watson R and Pahan K: Suppression of
nuclear factor-κB activation and inflammation in microglia by
physically modified saline. J Biol Chem. 287:29529–29542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu JH, Ge JB, Li M, Wu F, Zhang W and Qin
ZH: Inhibition of NF-κB activation is associated with
anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a
mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm
Sci. 47:652–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kröncke KD, Fehsel K and Kolb-Bachofen V:
Inducible nitric oxide synthase and its product nitric oxide, a
small molecule with complex biological activities. Biol Chem Hoppe
Seyler. 376:327–343. 1995.PubMed/NCBI
|

















