Introduction
Adrenomedullin (ADM) was originally isolated from
human pheochromocytoma as a biologically active peptide with potent
vasodilating action (1). Human ADM
consists of 52 amino acids and has a ring structure, with one
intramolecular disulfide bond and an amidated carboxyl terminal,
similar to calcitonin gene-related peptide and amylin (2). At present, it has been demonstrated
that ADM and its receptors are expressed in several tissues,
including the heart and blood vessels, kidneys, lungs, atrium,
gastrointestinal tract, spleen and thymus, endocrine glands and
brain (3). The synthesis of ADM can
be influenced by physical factors, including shear stress,
ventricular wall stress and hypoxia and humoral factors such as
cytokines, endocrine and paracrine hormones (4). Following its release from diverse
tissues, ADM functions as an autocrine or a paracrine hormone to
regulate vascular tone and blood pressure (5). ADM exerts its biological functions
directly via cyclic adenosine 3,5-monophosphate and indirectly via
endothelial nitric oxide (6,7). The natriuretic peptide system,
including atrial and brain natriuretic peptides (ANP and BNP),
causes natriuresis, diuresis and plasma shift to increase oxygen
transport in healthy humans to counteract hypoxic conditions and
the stimulus to which the synthesis and release of natriuretic
peptides responds is the oxygen gradient among cardiocytes
(8,9). Studies using cultured cardiomyocytes
(particularly from rodents) have demonstrated that several factors,
including calcium, catecholamines, endothelins, angiotensin II and
certain cytokines, are able to regulate the expression of ANP and
BNP genes (10). Numerous
cardiovascular diseases, such as chronic heart failure, systemic
hypertension, coronary disease, endothelial dysfunction and others
are responsible for their increased secretion (11). ANP and BNP, predominantly synthesized
and released by atrial and ventricular myocytes, respectively,
exert their biological actions via an accumulation of intracellular
cyclic GMP (cGMP) (12), whereas
many of the actions of ADM are mediated by cAMP (13). It has been reported that plasma ADM
concentration was higher in pheochromocytoma patients compared with
healthy subjects, and catecholamines are able to regulate the
expression of ANP and BNP genes (10,14).
Therefore, we hypothesized that ADM, ANP and BNP may be involved in
the regulation of adrenal medulla functions in adrenal medullary
hyperplasia (AMH) patients.
To assess possible changes in plasma concentrations
of ADM, ANP and BNP and investigate their pathophysiological roles
in AMH patients, we measured the three peptides in untreated AMH
patients, EH patients and healthy control subjects. The
concentrations of ADM and catecholamines in the plasma from the
adrenal vein and the inferior vena cava (IVC) of AMH patients were
measured. In addition, we measured ADM, ANP and BNP levels after 4
weeks of effective antihypertensive therapy for EH and AMH
patients. Then laparoscopic adrenalectomy for AMH patients was
performed and the values of the three peptides were measured again
2 weeks later. Then we compared the results before and after
treatment.
Subjects and methods
Study subjects
Between January 2006 and October 2014, 20 AMH
patients (mean age, 43.0±5.5 years; age range, 34–52 years), 35 EH
patients (mean age, 43.2±5.7 years; age range, 33–55 years) and 40
healthy control subjects (mean age, 42.0±4.9 years; age range,
32–52 years) were recruited from Renmin Hospital of Wuhan
University (Wuhan, China). All subjects agreed with the aim and
provided informed consent to participate in the present study.
Diagnostic tests
Routine laboratory and radiological studies of all
patients included assays of blood routine test; urinalysis; serum
electrolytes and fasting blood glucose level; liver and kidney
function tests; serum renin activity, aldosterone, catecholamines,
cortisol, and thyroid hormones; 24-h urine vanillylmandelic acid; a
chest roentgenogram; an electrocardiogram; B-scan ultrasonography
of liver, cholecyst, pancreas, spleen, kidneys and adrenal glands.
Other radiological studies included one or more of the following
diagnostic methods: Magnetic resonance imaging, computed
tomography, positron emission tomography (PET) imaging with
fluorodeoxyglucose, dihydroxyphenylalanine-PET-computed tomography,
octreotide scan and 123I-metaiodo-benzylguanidine
scintigraphy. Furthermore, blood samples from adrenal veins were
collected in all AMH patients who clearly showed excessive
secretion of catecholamines under fluoroscopic control. The blood
was also collected for preparing the plasma samples for the
measurement of both ADM and catecholamines. Serum catecholamines
were measured by highly sensitive and specific high-performance
liquid chromatography methods using electrochemical detection
(LC-10Avp Plus; Shimadzu Co., Ltd., Kyoto, Japan).
Diagnosis of AMH
AMH patients with a familial history of MEN syndrome
were excluded from the study. Imaging examinations did not show any
abnormal masses suggestive of pheochromocytoma or other tumors
which are indicative of MEN-2. AMH, due to twelve cases of left and
eight cases of right increased adrenomedullary tissue, was
diagnosed on the basis of certain clinical manifestations and
imaging examinations mentioned above, in addition to adrenal venous
blood sample analysis. The clinical manifestations include
dizziness, flushing, tremor, diaphoresis, headache, palpitations,
sweating, anxiety, a medical history of hypertension and excessive
catecholamine excretion. However, it was further confirmed by the
postoperative histopathological results of the extirpated lesions
at surgery, which showed histomorphometric evidence of increased
adrenomedullary tissue relative to the cortex in the absence of MEN
and proliferation of cells containing normal cellular architecture
as opposed to the nests of cytologically atypical polygonal cells
that characterize pheochromocytoma. Gene detection showed no
identifiable mutation in the RET proto-oncogene. All AMH patients
were hypertensive according to WHO criteria (15) (systolic pressure, ≥140 mmHg; and/or
diastolic pressure, ≥90 mmHg). None of these patients exhibited
clinical evidence of cardiac or hepatic failure, diabetes,
pulmonary disease, angina pectoris, myocardial infarction,
Cushing's syndrome, primary aldosteronism or other diseases that
can result in secondary hypertension. All AMH patients had no
previous antihypertensive drug treatment or who had not received
any antihypertensive therapy in the prior 4 weeks. Furthermore, the
medications they used made no difference to serum concentrations of
catecholamines. Healthy controls were age- and sex-matched
normotensive subjects that had been hospitalized for a healthy
checkup.
Treatment
After the initial evaluation, 20 AMH patients were
started on antihypertensive therapy with phenoxybenzamine (Topfond
Pharmaceutical Co., Ltd., Zhengzhou, China) at 10 mg once or twice
daily and increased by 10–20 mg every 2–3 days for optimal blood
pressure and symptom control to be normal. Furthermore, 35 EH
patients received antihypertensive therapy with slow-release
nifedipine (Bayer AG, Leverkusen, Germany) at 10–30 mg twice daily
for optimal blood pressure control to be normal. Plasma
concentrations of ADM, ANP and BNP were determined prior to the
initiation of therapy and after 4 weeks of effective
antihypertensive treatment. After the conditions of all AMH
patients were optimized prior to surgery, unilateral laparoscopic
adrenalectomy was performed in all AMH cases. Adrenalectomy was
performed by a transperitoneal laparoscopic approach in all 20
cases. After 2 weeks, the therapeutic effect was evaluated with
normalization of catecholamine hypersecretion and complete
disappearance of symptoms, as well as the reduction or abstention
of antihypertensive therapy. The values of ADM, ANP and BNP were
measured for a third time.
Preparations of human ADM, ANP and
BNP
Blood samples were drawn from an antecubital vein
between 7:00 and 8:00 (morning) after an overnight fast and a
supine rest, and were transferred to ice-chilled tubes with l mg/ml
EDTA-2Na and 500 kallikrein inhibitory units (KIU)/ml aprotinin
(Amresco Co., Ltd., Solon, OH, USA). Plasma was obtained by
centrifugation at 600 × g for 10 min at 4°C and then immediately
frozen and stored in polypropylene tubes at −70°C until
determination.
Hormone measurements
Plasma ADM concentrations were measured by
immunoradiometric assay using a specific kit (Shionogi & Co.,
Ltd., Osaka, Japan) after extraction and purification as described
previously (16,17). Briefly, 2 ml plasma was applied to a
Sep-Pak C18 cartridge (Waters Corporation, Milford, MA, USA)
equilibrated with 5 ml saline. After the cartridge was washed with
5 ml isotonic saline and 10% acetonitrile in 0.1% trifluoroacetic
acid (TFA), the absorbed materials were eluted with 4 ml 60%
acetonitrile in 0.1% TFA, lyophilized, and stored at −70°C until
determination. The residue was dissolved in 300 µl
radioimmunoprecipitation assay (RIA) buffer (Phoenix
Pharmaceuticals, Inc., Belmont, CA, USA), 50 mmol/l sodium
phosphate buffer (pH 7.4) containing 0.5% bovine serum albumin
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), 0.5% Triton
X-100, 80 mmol/1 sodium chloride, 25 mmol/l EDTA, 0.05% sodium
azide and 500 KIU/ml aprotinin. Next, 100 µl dissolved plasma
extract was subjected to a specific RIA for human ADM (hADM) as
reported previously (16,17). According to the manufacturer's
instructions, the anti-hADM antibody (cat. no. RIN9500; Peninsula
Laboratories, Belmont, CA, USA) used in this RIA had no any
cross-reactivity with hAM- (13–52), rat ADM-(1–50), human CGRP,
calcitonin, α-human atrial natriuretic peptide-(1–28), brain
natriuretic peptide-32, or C-type natriuretic peptide-22 and
neuropeptide Y. The reproducibility of the RIA was estimated using
three plasma samples having different ADM concentrations. All
assays were performed three times and the mean of three
measurements was used. The detection limit was 0.5 pmol/l and the
working range (CV, <15%) was 1–300 pmol/l. The intra- and
interassay imprecision values were 3.2–7.5 and 5.4–8.7%,
respectively. Concentrations of ADM are expressed as pmol/l.
The plasma ANP and BNP concentrations were measured
with specific immunoradiometric assays for human ANP and BNP
(Shionogi & Co., Ltd.). The accuracies and the detailed methods
of these assays have been described previously (18).
Statistical analysis
All continuous data were expressed as the mean ±
standard deviation, and analyzed using SPSS software, version 19.0
(SPSS, Inc., Chicago, IL, USA). Comparisons between two variables
were performed with unpaired t-test. Multiple comparisons were
evaluated with analysis of variance followed by
Student-Newman-Keuls' method. The significance of differences
between paired variables was determined by paired t-test.
Categorical variables were assessed using the χ2 or
Fisher's exact tests. Stepwise multiple linear regression analysis
was conducted to evaluate the most important factor for
catecholamines or blood pressure. The correlation between two
variables was performed using linear regression analysis and the
significance was further confirmed using Spearman's rank test.
Non-normal distribution data were performed by Mann-Whitney U test
or Kruskal-Wallis method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics
Table I shows the
clinical profiles of the study groups. There were no significant
differences in age and sex distribution among the three groups. The
mean values of arterial systolic and diastolic blood pressure (SBP
and DBP) were significantly higher in the EH and AMH patients
compared with the controls (P<0.05). No differences in SBP and
DBP were detected between EH and AMH patients. Similar changes were
observed in blood urea nitrogen (BUN), serum creatinine (Scr), and
glomerular filtration rates (GFR) among the three groups.
 | Table I.Clinical characteristics of study
subjects. |
Table I.
Clinical characteristics of study
subjects.
| Parameter | Control (n=40) | EH (n=35) | AMH (n=20) |
|---|
| Age (years) |
42.0±4.9 |
43.2±5.7 |
43.0±5.5 |
| Gender
(male:female) |
23:17 |
20:15 | 11:9 |
| Systolic BP
(mmHg) | 120±8 |
162±12a |
168±15a |
| Diastolic BP
(mmHg) | 80±6 | 101±6a | 104±7a |
| BUN (mg/dl) | 17±3 |
20±4a |
21±5a |
| Scr (mg/dl) |
1.0±0.2 |
1.5±0.4a |
1.6±0.6a |
| GFR (ml/min) | 96±8 |
90±12a |
85±14a |
| Serum E (pg/ml) |
56±15 |
63±18 |
211±98a,b |
| Serum NE (pg/ml) | 221±
67 |
233±75 |
738±292a,b |
| Urine VMA (mg/24
h) | 4±1 | 5±2 |
16±9a,b |
| LVEF (%) | 83±5 |
79±6a |
75±7a,b |
| LVMI
(g/m2) | 115±7 |
128±10a |
140±15a,b |
As expected, the AMH patients had significantly
higher mean values of serum E, serum NE and urine VMA (P<0.05).
The mean values of left ventricular ejection fraction (LVEF) were
significantly reduced in the EH and AMH patients compared with the
controls, whereas the mean values of left ventricualr mass index
(LVMI) were significantly higher in the EH and AMH patients than in
the controls (P<0.05). A significant difference in LVEF and LVMI
was also detected between the controls and EH patients
(P<0.05).
Plasma concentrations of ADM, ANP and
BNP
The plasma concentrations of ADM, ANP and BNP in the
study groups are showed in Fig. 1.
The mean concentration of ADM was significantly higher in the AMH
group (9.61±2.78 pmol/l) compared with the EH (6.51±2.00 pmol/l)
and control (3.35±1.45 pmol/l) groups (P<0.05). A significant
difference in the mean ADM concentration was detected between the
EH patients and controls. The mean values of ANP in the controls,
EH patients and AMH patients were 2.95±1.32, 12.54±3.54 and
19.09±5.83 pmol/l, respectively, whereas the mean values of BNP in
the three groups were 4.52±1.87, 26.53±7.70 and 36.29±10.89 pmol/l,
respectively. Similar changes were observed in the mean values of
ANP and BNP among the three groups.
Concentrations of E, NE, ADM, ANP and
BNP in the IVC and adrenal vein of AMH patients
As shown in Tables
II and III, the concentrations
of ADM, ANP and BNP in the contralateral adrenal vein, along with
the concentrations of E and NE, were significantly higher than in
the infra- and supraadrenal IVCs. Moreover, there were further
increases in the AMH adrenal vein than in the contralateral adrenal
vein. There were no significant differences in the concentrations
of the three peptides between infra- and supraadrenal IVCs.
 | Table II.Concentrations of E, NE, ADM, ANP and
BNP in the IVC and adrenal vein of twelve patients with left
AMH. |
Table II.
Concentrations of E, NE, ADM, ANP and
BNP in the IVC and adrenal vein of twelve patients with left
AMH.
| Parameter | Infraadrenal
IVC | Supraadrenal
IVC | Right adrenal
vein | Left adrenal
vein |
|---|
| E (pg/ml) |
382±106 |
424±123 |
564±157a,b |
2,148±676a–c |
| NE (pg/ml) |
846±364 |
1,014±392 |
1,532±426a,b |
5,752±1684a–c |
| ADM (pmol/l) |
12.31±4.98 |
14.72±5.83 |
23.24±7.92a,b |
34.56±10.64a–c |
| ANP (pmol/l) |
18.74±6.43 |
21.32±7.71 |
30.96±9.97a,b |
41.54±11.63a–c |
| BNP (pmol/l) |
30.82±9.58 |
34.37±10.72 |
45.49±12.38a,b |
58.29±14.48a–c |
 | Table III.Concentrations of E, NE, ADM, ANP and
BNP in the IVC and adrenal vein of eight patients with right
AMH. |
Table III.
Concentrations of E, NE, ADM, ANP and
BNP in the IVC and adrenal vein of eight patients with right
AMH.
| Parameter | Infraadrenal
IVC | Supraadrenal
IVC | Left adrenal
vein | Right adrenal
vein |
|---|
| E (pg/ml) |
391±102 |
436±147 |
643±156a,b |
1,948±591a–c |
| NE (pg/ml) |
931±423 |
1,053±498 |
1,891±587a,b |
5,468±1573a–c |
| ADM (pmol/l) |
11.28±5.47 |
14.98±6.04 |
25.13±8.56a,b |
36.89±11.81a–c |
| ANP (pmol/l) |
17.92±6.91 |
20.81±8.56 |
30.95±10.14a,b |
44.13±12.84a–c |
| BNP (pmol/l) |
29.58±9.12 |
32.31±10.32 |
44.82±11.71a,b |
59.45±13.78a–c |
Association between SBP, DBP, serum E,
serum NE or urine VMA and ADM, ANP and BNP in patients with
AMH
As shown in Table
IV, ADM was the most important peptide associated with
catecholamines or blood pressure in the AMH patients. Stepwise
multiple regression analysis of independent parameters (ADM, ANP
and BNP) associated with the values of SBP, DBP, serum E, serum NE
or urine VMA was also conducted.
 | Table IV.Stepwise multiple regression analysis
of significant factors for SBP, DBP, serum E, serum NE or urine VMA
in AMH patients. |
Table IV.
Stepwise multiple regression analysis
of significant factors for SBP, DBP, serum E, serum NE or urine VMA
in AMH patients.
|
| SBP | DBP | Serum E | Serum NE | Urine VMA |
|---|
|
|
|
|
|
|
|
|---|
| Variable | B | t | P | B | t | P | B | t | P | B | t | P | B | t | P |
|---|
| ADM | 3.287 | 2.486 | 0.024 | 1.638 |
′2.255 | 0.039 | 31.301 | 3.280 | 0.005 | 75.251 | 2.755 | 0.014 | 1.896 | 2.296 | 0.036 |
| ANP | 0.364 | 0.725 | 0.479 | 0.130 |
0.472 | 0.643 | −5.228 | −1.445 | 0.168 | −2.358 | −0.228 | 0.823 | 0.213 | 0.680 | 0.506 |
| BNP | −0.051 | −0.168 | 0.869 | 0.000 | −0.002 | 0.999 | −1.524 | −0.700 | 0.494 | 0.761 | 0.122 | 0.904 | −0.031 | −0.164 | 0.872 |
Association between plasma ADM
concentration and values of BUN, Scr, GFR, serum E, serum NE and
urine VMA
Fig. 2 shows the
association between plasma ADM concentration and BUN, Scr, GFR,
serum E, serum NE and urine VMA in the AMH group. The plasma ADM
concentration was not associated with BUN, Scr and GFR, while it
was correlated with serum E, serum NE and urine VMA
(P<0.05).
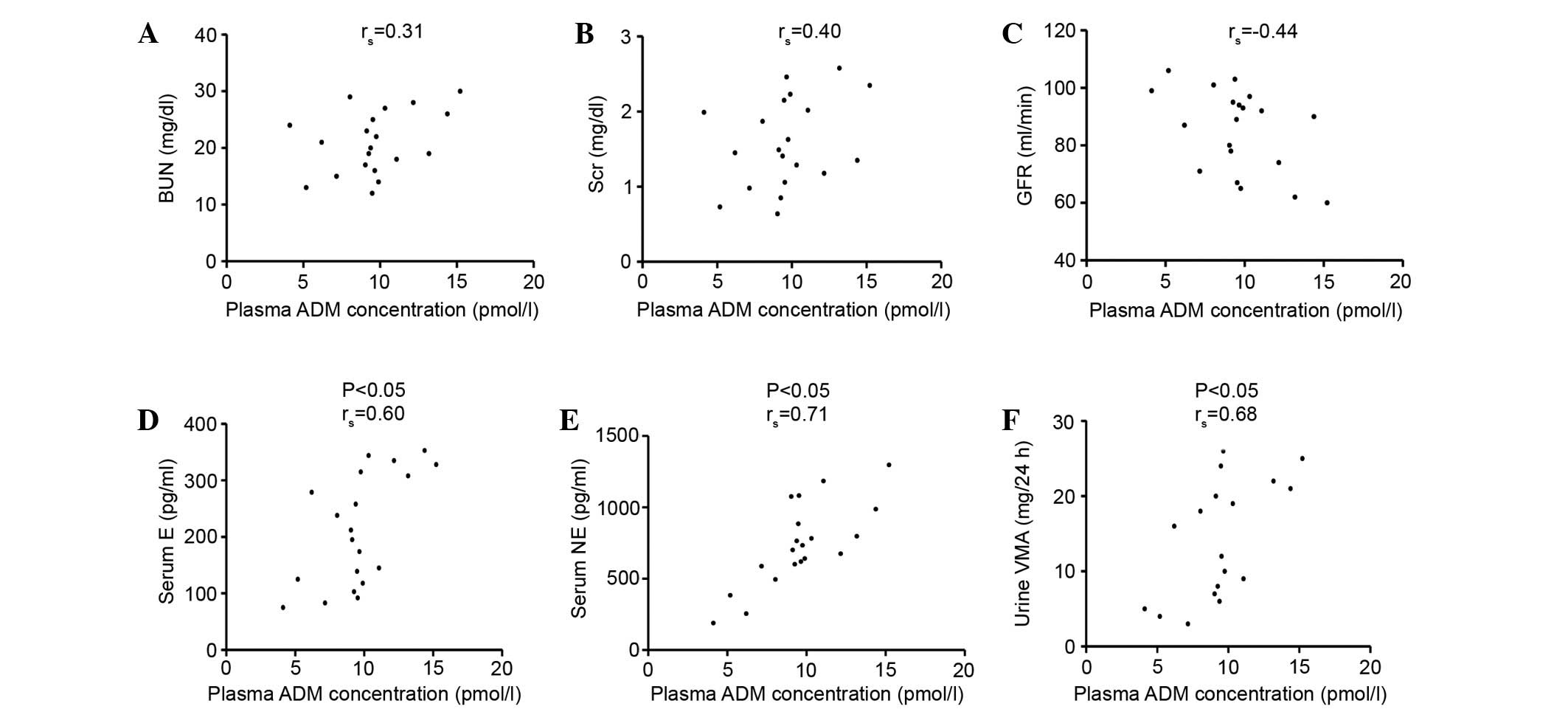 | Figure 2.Association between plasma ADM
concentration and values of (A) BUN, (B) Scr, (C) GFR, (D) serum E,
(E) serum NE and (F) urine VMA in AMH group. The plasma ADM
concentration was not associated with BUN, Scr and GFR, while it
was correlated with serum E, serum NE and urine VMA (P<0.05).
ADM, adrenomedullin; BUN, blood urea nitrogen; Scr, serum
creatinine; GFR, glomerular filtration rate; E, epinephrine; NE,
norepinephrine; VMA, vanillylmandelic acid. |
Association between plasma ADM
concentration and SBP, DBP, LVEF, LVMI, ANP and BNP
Fig. 3 shows the
association between plasma ADM concentration and SBP, DBP, LVEF,
LVMI, ANP and BNP in the AMH group. The plasma ADM concentration
was significantly associated with SBP, DBP, LVEF and LVMI, and
plasma levels of ANP and BNP (P<0.05).
 | Figure 3.Association between plasma ADM
concentration to values of (A) SBP, (B) DBP, (C) LVEF, (D) LVMI,
(E) ANP and (F) BNP in AMH group. The plasma ADM concentration was
not only associated with SBP, DBP, LVEF and LVMI, but also
correlated with plasma levels of ANP and BNP (P<0.05). ADM,
adrenomedullin; SBP, systolic blood pressure; DBP, diastolic blood
pressure; LVEF, left ventricular ejection fraction; LVMI, left
ventricular mass index; ANP, atrial natriuretic peptide; BNP, brain
natriuretic peptide. |
Concentrations of ADM, ANP and BNP in
AMH patients with or without renal dysfunction
Mean plasma concentrations of ADM, ANP and BNP in
the AMH group with or without renal dysfunction are listed in
Table V. No significant differences
were detected between the patients with (Scr ≥1.5 mg/dl or GFR ≤80
ml/min) and without (Scr <1.5 mg/dl or GFR >80 ml/min) renal
dysfunction, although the values of ADM, ANP and BNP in the
patients with or without renal dysfunction were higher compared
with the controls (P<0.05).
 | Table V.Mean concentrations of ADM, ANP and
BNP in AMH patients with or without renal dysfunction. |
Table V.
Mean concentrations of ADM, ANP and
BNP in AMH patients with or without renal dysfunction.
| Characteristic | No. | ADM (pmol/l) | ANP (pmol/l) | BNP (pmol/l) |
|---|
| AMH patients |
|
|
|
|
| Scr
≥1.5 mg/dl | 9 |
10.04±3.11a |
20.64±6.13a |
39.53±10.93a |
| Scr
<1.5 mg/dl | 11 |
9.25±2.88a |
17.82±5.54a |
33.64±10.62a |
| GFR ≤80
ml/min | 8 |
10.31±2.59a |
19.69±5.59a |
36.97±10.35a |
| GFR
>80 ml/min | 12 |
9.14±2.91a |
18.69±6.21a |
35.84±11.67a |
| Control
subjects | 40 |
3.35±1.45 |
2.95±1.32 |
4.52±1.87 |
Concentrations of ADM, ANP and BNP in
EH patients with or without renal dysfunction
Table VI shows the
plasma concentrations of ADM, ANP and BNP in EH patients with or
without renal dysfunction. There were no significant differences
between patients with renal dysfunction (Scr ≥1.5 mg/dl or GFR ≤80
ml/min) and subjects without renal dysfunction (Scr <1.5 mg/dl
or GFR >80 ml/min), although the values of ADM, ANP and BNP in
patients with or without renal dysfunction were higher compared
with the controls (P<0.05).
 | Table VI.Mean concentrations of ADM, ANP and
BNP in EH patients with or without renal dysfunction. |
Table VI.
Mean concentrations of ADM, ANP and
BNP in EH patients with or without renal dysfunction.
| Characteristic | No. | ADM (pmol/l) | ANP (pmol/l) | BNP (pmol/l) |
|---|
| EH patients |
|
|
|
|
| Scr
≥1.5 mg/dl | 11 |
7.03±2.12a |
13.63±3.63a |
27.53±8.29a |
| Scr
<1.5 mg/dl | 24 |
6.27±1.94a |
12.04±3.46a |
26.08±7.56a |
| GFR ≤80
ml/min | 9 |
7.08±2.31a |
13.51±3.86a |
28.11±8.62a |
| GFR
>80 ml/min | 26 |
6.31±1.90a |
12.21±3.44a |
25.99±7.46a |
| Control
subjects | 40 |
3.35±1.45 |
2.95±1.32 |
4.52±1.87 |
Parameters of AMH patients at
diagnosis and following drug administration and surgery
Table VII shows the
clinical parameters of AMH patients at diagnosis, after drugs and
after surgery, respectively. The SBP and DBP were normal following
drug administration and surgery (P<0.05). The BUN, Scr and GFR
were not significantly different before and after treatment. The
plasma E, plasma NE, urine VMA, LVEF and LVMI were not
significantly changed after drugs but were normal after surgery
(P<0.05).
 | Table VII.Parameters of AMH patients (n=20) at
diagnosis and following drug administration and surgery. |
Table VII.
Parameters of AMH patients (n=20) at
diagnosis and following drug administration and surgery.
| Parameter | At diagnosis | After drugs | After surgery |
|---|
| Systolic BP
(mmHg) |
168±15 |
130±6 |
127±5a,b |
| Diastolic BP
(mmHg) |
104±7 |
80±5 |
78±4a,b |
| BUN (mg/dl) |
21±5 |
20±3 |
22±5 |
| Scr (mg/dl) |
1.6±0.6 |
1.4±0.3 |
1.7±0.6 |
| GFR (ml/min) |
85±14 |
90±17 |
86±15 |
| Serum E
(pg/ml) |
211±98 |
213±99 |
88±15a,b |
| Serum NE
(pg/ml) |
738±292 |
754±303 |
306±114a,b |
| Urine VMA (mg/24
h) |
16±9 |
17±8 |
4±1a,b |
| LVEF (%) |
75±7 |
76±6 |
81±4a,b |
| LVMI
(g/m2) |
140±15 |
135±10 |
118±8a,b |
Plasma ADM, ANP, and BNP
concentrations at diagnosis and following drug administration and
surgery
The plasma ADM, ANP, and BNP concentrations
initially, 4 week after effective antihypertensive therapy and 2
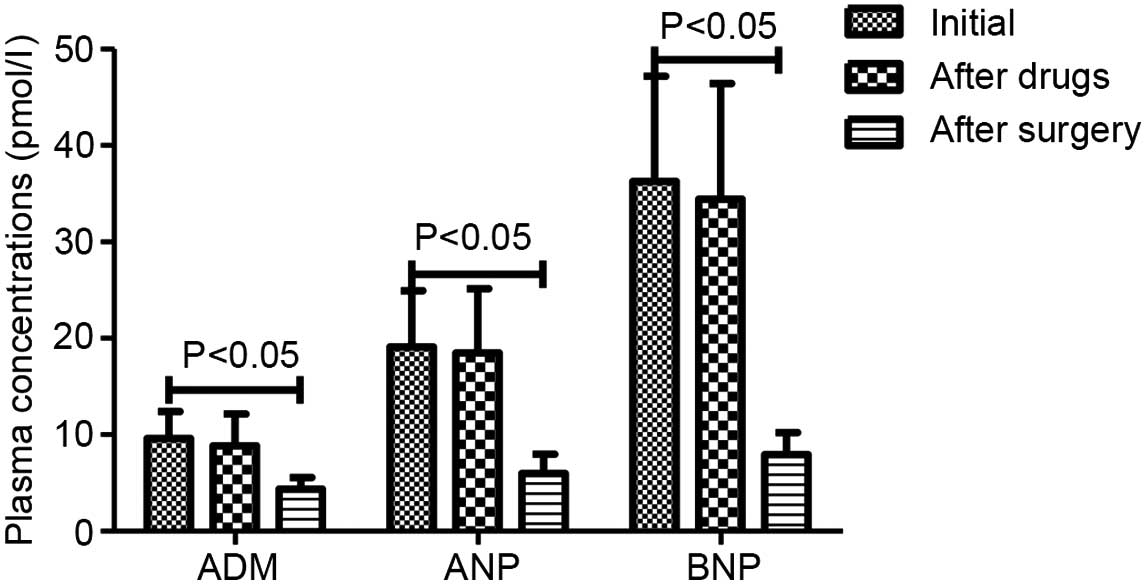
weeks after surgery in AMH group are presented in Fig. 4. Plasma concentration of ADM was not
significantly changed after drug treatment (9.61±2.78 to 8.85±3.29
pmol/l), but significantly reduced after surgery (9.61±2.78 to
4.34±1.23 pmol/l; P<0.05). The plasma ANP and BNP levels
similarly declined after drug treatment (19.09±5.83 to 18.48±6.67
and 36.29±10.89 to 34.42±12.02 pmol/l, respectively) and after
surgery (19.09±5.83 to 5.99±2.00 and 36.29±10.89 to 7.93±2.28
pmol/l, respectively; P<0.05).
The plasma concentrations of ADM, ANP and BNP were
analyzed initially and at 4 weeks after effective antihypertensive
therapy in the EH patients when the BP was normal. The
concentrations of ADM, ANP, and BNP significantly decreased after
drug treatment (6.51±2.00 to 4.05±1.43, 12.54±3.54 to 7.35±2.41,
26.53±7.70 to 18.32±4.65 pmol/l, respectively; P<0.05).
Discussion
ADM is involved in the regulation of heart and
kidney function, and inhibition of vascular smooth muscle cell
proliferation and migration and cardiac remodelling (19,20). It
has been demonstrated that this peptide is present in a variety of
organs and cells in addition to human plasma, and exerts a wide
range of physiological effects, including cardiovascular
protection, neovascularization and apoptosis suppression (21). Sporadic AMH is characterized by
excessive catecholamine excretion arising from chromaffin cells of
the adrenal medulla or extra-adrenal location, and may result in
secondary hypertension and high oxygen consumption (22). Furthermore, in addition to
catecholamines, chromaffin cells produce and secrete elevated
quantities of trophic peptides which are normally released in a
regulated manner by the normal adrenal medulla, and one of these
peptides, ADM, is particularly high (23). ANP and BNP are similar to ADM in
cardiovascular effects, including natriuresis, diuresis,
hypotensive action and anti-hypertrophic action, thereby reducing
fluid volume and increasing oxygen transport (24,25).
Previous studies have shown that plasma levels of ADM, ANP and BNP
were elevated in patients with essential hypertension (26,27).
In the present study, the EH and AMH patients had
significant higher mean BUN and Scr values and lower mean GFR
values compared with the controls. Thus, essential or secondary
hypertension resulting from AMH may lead to renal dysfunction.
These results are compatible with our previous report (unpublished
data). In addition, the BUN, Scr and GFR values in AMH patients
were not changed following drug administration and surgical
treatment. It may have been the case that certain long-standing or
elderly patients had slightly irreversible renal impairment. The EH
and AMH patients had significantly lower LVEF and higher LVMI
compared with the controls. In addition, there was a significant
difference between EH patients and controls. The LVEF and LVMI
significantly improved after drugs in EH patients (unpublished
data). However, the LVEF and LVMI remained unchanged following drug
treatment, but significantly improved along with serum E, serum NE
and urine VMA in AMH patients. Thus, the changes of LVEF and LVMI
may be due to catecholamine cardiomyopathy, resulting from
catecholamine hypersecretion which was confirmed by the association
between plasma ADM concentration to LVEF and LVMI.
Another notable result was that the plasma
concentrations of ADM, ANP and BNP were significantly higher in AMH
patients compared with EH patients and controls. Furthermore,
significant differences in mean values of the three peptides were
detected in EH patients and controls. Therefore, it may be inferred
that ADM participates alongside ANP and BNP in the compensatory and
protective mechanisms counteracting further elevation of blood
pressure in the cardiovascular system, due to their similar
physiological functions. This can be confirmed by the association
between plasma ADM concentrations and SBP and DBP. Furthermore, ADM
was identified as the most important peptide in AMH patients, which
was confirmed by stepwise multiple regression analysis of
independent parameters associated with SBP, DBP, serum E, serum NE
or urine VMA. Furthermore, the elevated levels of the three
peptides significantly decreased following drug treatment in EH
patients (unpublished data), whereas the elevated levels were only
significantly reduced after laparoscopic adrenalectomy in AMH
patients. A potential explanation is that the elevated
concentrations of the three peptides in the AMH patients were
associated with catecholamine hypersecretion. Indeed, the
concentrations of the three peptides in the contralateral adrenal
vein, along with the concentrations of E and NE, were significantly
higher compared with those of infra- and supraadrenal IVCs. The
significant increases were observed in the concentrations of the
three peptides in the AMH adrenal vein than in the contralateral
adrenal vein. These results appear to be compatible with studies by
Cotesta et al (15) and Lee
et al (28), though the study
subjects were patients with pheochromocytoma and primary
aldosteronism, respectively. However, the plasma ADM concentration
was associated with serum E, serum NE and urine VMA, in addition to
the plasma concentrations of ANP and BNP. On the basis of these
results, it may be inferred that ADM, ANP and BNP can be released
from adrenal medulla along with the catecholamine secretion and the
quantity was higher when the adrenal medulla was hyperplastic.
Therefore, ADM, ANP and BNP may be important in the regulation of
adrenal medulla functions. The specific molecular regulating
pathways for this are unclear at present and further studies will
be necessary to clarify them.
In the present study, there were no significant
differences in plasma concentrations of ADM, ANP and BNP between
patients with and without renal dysfunction in EH and AMH patients.
This was confirmed by the absence of association between plasma ADM
concentration and BUN, Scr and GFR. Therefore, elevated levels of
ADM, ANP and BNP were not associated with renal function.
As aforementioned, a number of investigations showed
increased plasma ADM, ANP and BNP levels in patients with EH, or
primary aldosteronism or pheochromocytoma (14,26,27,29–31);
however, this is the first study to assess plasma ADM, ANP and BNP
levels in patients with AMH.
Collectively, the present results indicate that ADM
may participate, along with ANP and BNP, in the mechanisms acting
against further elevation of blood pressure. They may be good
predictors of catecholamine hypersecretion and involved in the
regulation of adrenal medulla in AMH patients. However, this is a
retrospective observation based on a small number of cases due to
the low incidence of AMH, and further studies are necessary to
identify the specific pathophysiological significance of ADM, ANP
and BNP in AMH and the exact pharmacokinetics underlying their
activity in AMH patients.
Acknowledgements
This study was approved by the Ethics Committee of
Renmin Hospital Wuhan University. The authors thank the Department
of Urology in Renmin Hospital of Wuhan University. This study was
supported by grants from the National Science Fund Project of China
(grant no. 81501921) and the Doctor Research Fund Project of Wuhan
University of China (grant no. 2012302020203).
References
|
1
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: A novel
hypotensive peptide isolated from human pheochromocytoma. 1993.
Biochem Biophys Res Commun. 425:548–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheung BM, Li CY and Wong LY:
Adrenomedullin: Its role in the cardiovascular system. Semin Vasc
Med. 4:129–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minamino N: Adrenomedullin, its
distribution and regulation of production. Nihon Rinsho. 62(Suppl
9): S193–S197. 2004.
|
|
4
|
Cheung BM and Tang F: Adrenomedullin:
Exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov.
6:4–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong HK, Cheung TT and Cheung BM:
Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc Dis.
1:pii: cvd.2012.012003. 2012.PubMed/NCBI
|
|
6
|
Li Y, Jiang C, Wang X, Zhang Y, Shibahara
S and Takahashi K: Adrenomedullin is a novel adipokine:
Adrenomedullin in adipocytes and adipose tissues. Peptides.
28:1129–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishikimi T, Kuwahara K, Nakagawa Y,
Kangawa K and Nakao K: Adrenomedullin in cardiovascular disease: A
useful biomarker, its pathological roles and therapeutic
application. Curr Protein Pept Sci. 14:256–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arjamaa O: Physiology of natriuretic
peptides: The volume overload hypothesis revisited. World J
Cardiol. 6:4–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chowdhury P, Kehl D, Choudhary R and
Maisel A: The use of biomarkers in the patient with heart failure.
Curr Cardiol Rep. 15:3722013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clerico A, Giannoni A, Vittorini S and
Passino C: Thirty years of the heart as an endocrine organ:
Physiological role and clinical utility of cardiac natriuretic
hormones. Am J Physiol Heart Circ Physiol. 301:H12–H20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Federico C: Natriuretic peptide system and
cardiovascular disease. Heart Views. 11:10–15. 2010.PubMed/NCBI
|
|
12
|
Kuhn M: Endothelial actions of atrial and
b-type natriuretic peptides. Br J Pharmacol. 166:522–531. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu WW and Qi YF: Cardiovascular effects
and pathophysiological significance of adrenomedullin family
peptides. Sheng Li Ke Xue Jin Zhan. 44:177–182. 2013.(In Chinese).
PubMed/NCBI
|
|
14
|
Whitworth JA: World Health Organization,
International Society of Hypertension Writing Group: 2003 World
Health Organization (WHO)/International Society of Hypertension
(ISH) statement on management of hypertension. J Hypertens.
21:1983–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cotesta D, Caliumi C, Alò P, Petramala L,
Reale MG, Masciangelo R, Signore A, Cianci R, D'Erasmo E and
Letizia C: High plasma levels of human chromogranin a and
adrenomedullin in patients with pheochromocytoma. Tumori. 91:53–58.
2005.PubMed/NCBI
|
|
16
|
Ohta H, Tsuji T, Asai S, Tanizaki S,
Sasakura K, Teraoka H, Kitamura K and Kangawa K: A simple
immunoradiometric assay for measuring the entire molecules of
adrenomedullin in human plasma. Clin Chim Acta. 287:131–143. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohta H, Tsuji T, Asai S, Sasakura K,
Teraoka H, Kitamura K and Kangawa K: One-step direct assay for
mature-type adrenomedullin with monoclonal antibodies. Clin Chem.
45:244–251. 1999.PubMed/NCBI
|
|
18
|
Yasue H, Yoshimura M, Sumida H, Kikuta K,
Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M and Nakao
K: Localization and mechanism of secretion of b-type natriuretic
peptide in comparison with those of A-type natriuretic peptide in
normal subjects and patients with heart failure. Circulation.
90:195–203. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beltowski J and Jamroz A:
Adrenomedullin-what do we know 10 years since its discovery? Pol J
Pharmacol. 56:5–27. 2004.PubMed/NCBI
|
|
20
|
Kato J, Kitamura K and Eto T: Plasma
adrenomedullin level and development of hypertension. J Hum
Hypertens. 20:566–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia MA, Martín-Santamaría S, de
Pascual-Teresa B, Ramos A, Julián M and Martínez A: Adrenomedullin:
A new and promising target for drug discovery. Expert Opin Ther
Targets. 10:303–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marín MR, Arenas MF, Valverde FM, Garaulet
ET, Maderuelo MM, Avilés AM, Quirante FP and Blázquez AA:
Laparoscopic adrenalectomy for nonfamilial adrenal medullary
hyperplasia. JSLS. 17:433–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thouennon E, Pierre A, Yon L and Anouar Y:
Expression of trophic peptides and their receptors in chromaffin
cells and pheochromocytoma. Cell Mol Neurobiol. 30:1383–1389. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sergeeva IA and Christoffels VM:
Regulation of expression of atrial and brain natriuretic peptide,
biomarkers for heart development and disease. Biochim Biophys Acta.
1832:2403–2413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito Y: Roles of atrial natriuretic
peptide and its therapeutic use. J Cardiol. 56:262–270. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishikimi T: Adrenomedullin in essential
hypertension. Nihon Rinsho. 62(Suppl 9): S260–S263. 2004.(In
Japanese).
|
|
27
|
Soualmia H, Ayadi I, Omar S, Feki M,
Drissa H, Mebazaa A and Kaabachi N: Atrial natriuretic peptide and
brain natriuretic peptide release in human essential hypertension.
Clin Lab. 55:120–127. 2009.PubMed/NCBI
|
|
28
|
Lee YJ, Lin SR, Shin SJ, Lai YH, Lin YT
and Tsai JH: Brain natriuretic peptide is synthesized in the human
adrenal medulla and its messenger ribonucleic acid expression along
with that of atrial natriuretic peptide are enhanced in patients
with primary aldosteronism. J Clin Endocrinol Metab. 79:1476–1482.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Letizia C, De Toma G, Cerci S, Massa R,
Coassin S, Subioli S, Scuro L and De Ciocchis A: Adrenomedullin
levels are high in primary aldosteronism due to adenoma and decline
after surgical cure. Blood Press. 7:19–23. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato J, Etoh T, Kitamura K and Eto T:
Atrial and brain natriuretic peptides as markers of cardiac load
and volume retention in primary aldosteronism. Am J Hypertens.
18:354–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Letizia C, De Toma G, Caliumi C, Cerci S,
Massa R, Loria RD, Alo P, Marinoni EM, Diacinti D and D'Erasmo E:
Plasma adrenomedullin concentrations in patients with adrenal
pheochromocytoma. Horm Metab Res. 33:290–294. 2001. View Article : Google Scholar : PubMed/NCBI
|