Introduction
Diabetic retinopathy (DR) is a leading cause of
blindness and vision loss in numerous countries (1). DR is a chronic, progressive,
sight-threatening disease associated with prolonged hyperglycemia.
Retinal pigment epithelial (RPE) cells play an important role in
the development of DR. Uncontrolled proliferation and migration of
RPE cells may form pathological membranes on both surfaces of the
neural retina, which can result in visual impairment (2).
The Dickkopf (DKK) family is one of the Wnt
antagonist families, and inhibits Wnt signaling by binding to the
lipoprotein receptor-related protein (LRP) 5/6 component of the Wnt
receptor complex (3). It consists of
four members, each of which possesses an N-terminal signal peptide
and contains two conserved cysteine-rich domains separated by a
linker region (4). Previous studies
found that DKK1 markedly suppresses tumor growth by activating
apoptosis of melanoma cells (5). In
addition, DKK1 plays a critical role in the development of
inflammatory arthritis. It has been reported that the protein
expression level of DKK1 is significantly increased in the serum of
patients with rheumatoid arthritis (6), and DKK1 regulates bone development and
accrual and maintenance of bone mass (7,8).
Recently, a study showed that plasma DKK-1 levels were
significantly lower in DR patients compared with non-diabetic
controls and non-DR (NDR) patients (9). Furthermore, DKK-1 levels were lower in
proliferative DR (PDR) patients compared with non-proliferative PDR
(NPDR) patients (9). However, there
is no information regarding the effects of DKK1 in RPE cells.
Therefore, in the present study, we investigated the effect of DKK1
on the proliferation and migration of human RPE cells, and the
signaling mechanisms underlying these processes.
Materials and methods
Cell culture
Human ARPE-19 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium and Ham's F-12
nutrient mixture (DMEM/F12; Gibco, Rockville, MD, USA) supplemented
with 10% fetal bovine serum (FBS; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), 100 U/ml penicillin and 100 U/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C in 5% CO2
and 95% humidity.
Construction of plasmids and
transfection
All recombinant adenovirus were constructed as
previously described (10). In
brief, full-length DKK1 cDNA (Sangon, Shanghai, China) was
amplified and subcloned into pAdTrack-cytomegalovirus (CMV;
Invitrogen, Carlsbad, CA, USA), and green fluorescent protein (GFP;
Sigma-Aldrich, St. Louis, MO, USA) was used as a control. Then, the
shuttle plasmids pAdTrack-CMV (Invitrogen) and pAdEasy-1 were
recombined in the Escherichia coli strain BJ5183 (Institute of
Biochemistry and Cell Biology of the Chinese Academy of Sciences,
Shanghai, China). The recombinant plasmids were transfected into
293 cells to generate recombinant adenovirus. The recombinant
adenoviruses were harvested and the titers were determined using a
p24 ELISA kit (Cell Biolabs, Inc., San Diego, CA, USA).
For in vitro transfection, ARPE-19 cells
(American Type Culture Collection, Manassas, VA, USA) were seeded
in each well of 24-well microplates, grown for 24 h to reach 50%
confluence, and transfected with Ad-DKK1 or Ad-GFP using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA), according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from ARPE-19 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. Subsequently, ~5 µg total
RNA was reverse transcribed into cDNA using M-MLV reverse
transcriptase (Clontech Laboratories, Inc., Palo Alto, CA, USA).
The following primers were used: DKK1 forward,
5′-GATCATAGCCCTTGGATGGG-3′ and reverse, 5′-GGCACAGTCTGATGACCGG-3′;
β-actin forward, 5′-CCACCCATGGCAAATTCCATGGCA-3′ and reverse,
5′-TCTAGACGGCAGGTCAGGTCCACC-3′. A total of 1.25 units GoTaq Flexi
DNA polymerase (Promega, Madison, WI, USA) was used and up to 1 µg
of total RNA was treated with two units of DNAse I (Invitrogen) in
1X DNase buffer (Invitrogen) in a total volume of 10 µl in order to
remove genomic DNA. The RT-qPCR was performed in a final volume of
20 µl, containing 2 µl of cDNA, 10 µl 2X SYBR Green I reagent, 6.25
U multi-scribe reverse transcriptase, 10 U RNase inhibitor and 0.1
mM primers. The PCR cycling protocol was as follows: 94°C for 4
min; 94°C for 20 sec, 55°C for 30 sec, and 72°C for 20 sec; 2 sec
for plate reading for 35 cycles; and melting curve from 65–95°C.
β-actin was used as a control for normalizing gene expression. PCR
was performed using a Realplex Thermal Cycler (Eppendorf, Hauppage,
NY, USA). Experiments were performed independently at least three
times and the date analysed using the
R=2−[∆Ctsample-∆Ctcontrol] formula.
Western blot analysis
Total protein was extracted from ARPE-19 cells using
RIPA Cell Lysis Buffer (Bio Rad Laboratories, Inc., Hercules, CA,
USA), containing a phosphatase inhibitor and the protease inhibitor
cocktail (Sigma-Aldrich), by incubation on ice for 30 min. The
cells were then washed with ice-cold phosphate-buffered saline and
lysed with RIPA Cell Lysis Buffer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), containing a phosphatase inhibitor and the
protease inhibitor cocktail (Sigma-Aldrich), by incubation on ice
for 30 min. Lysates were collected by centrifugation at 6,000xg for
10 min at 4°C and protein concentrations were determined by the BCA
protein assay kit (BioTeke, Beijing, China). The samples (30 µg
protein/lane) were separated on 10% SDS-PAGE (Sigma-Aldrich) and
transferred onto polyvinylidene fluoride membranes. After blocking
in Tris-buffered saline buffer (50 mmol/l NaCl, 10 mmol/l Tris, pH
7.4) containing 5% nonfat milk, the blots were incubated with
primary antibodies against DKK1 (sc-374574; 1:1,500), β-catenin
(sc-53484; 1:3,000), cyclin D1 (sc-20044; 1:3,000) and β-actin
(sc-21733; 1:1,500; Invitrogen; Thermo Fisher Scientific, Inc.) at
4°C overnight. Membranes were then washed and incubated with
horseradish peroxidase-conjugated secondary antibodies (sc-395760;
1:3,000; Santa Cruz Biotechnology, Inc.). The blots were visualized
by super ECL and quantified using Quantity One software (Bio-Rad
Laboratories, Inc.).
Cell proliferation
Cell proliferation was analyzed by using cell
counting assay kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacture's protocol. Cells
transfected with Ad-GFP or Ad-DKK1 were cultured in 96-well flat
bottomed microplates and were incubated in 10% CCK-8 (Dojindo;
Kumamoto, Japan) diluted in normal cultured medium for 1 h at 37°C.
Proliferation rates were determined at 24, 48, 72 and 96 h after
transfection. The absorbance of each well was measured using a
microplate reader set at 490 nm. The experiment was repeated three
times.
Cell migration assay
RPE cells were grown to confluence in 12-well
plastic dishes and were treated with Ad-GFP or Ad-DKK1. RPE cells
in 200 µl serum-free DMEM were added to the upper compartment using
an 8-µm microporous filter (EMD Millipore, Boston, MA, USA). Then,
500 µl DMEM containing 10% FBS was added to the bottom chamber.
After 24 h incubation at 37°C, the cells on the lower surface of
the filter were fixed, stained and examined under a light
microscope. A total of ten areas were selected randomly from each
well, and the cells in three wells from each group were
quantified.
Statistical analysis
All experiments were performed independently at
least three times. Statistical significance was evaluated using
one-way analysis of variance using SPSS software version 10.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
DKK1 transfection successfully
upregulated the expression of DKK1 in ARPE-19 cells
The effect of the DKK1 transfection on the mRNA and
protein expression of DKK1 in ARPE-19 cells was determined using
RT-qPCR and western blot analyses. RT-qPCR demonstrated that the
overexpression of DKK1 obviously increased DKK1 mRNA levels in
ARPE-19 cells compared with control cells (Fig. 1A). Consistent with the results of
RT-qPCR, western blot analysis showed that the protein expression
levels of DKK1 were significantly increased in DKK1-transfected
cells compared with control cells (Fig.
1B). These results indicated that Ad-DKK1 transfection had been
successful.
Effect of DKK1 on human RPE cell
proliferation
As levels of DKK-1 are lower in PDR patients
compared with NPDR patients, we analyzed the effect of DKK1
overexpression on cell proliferation using a CCK-8 assay. As shown
in Fig. 2, overexpression of DKK1
significantly suppressed the proliferation of ARPE-19 cells, as
compared with the control group.
Effect of DKK1 on human RPE cell
migration
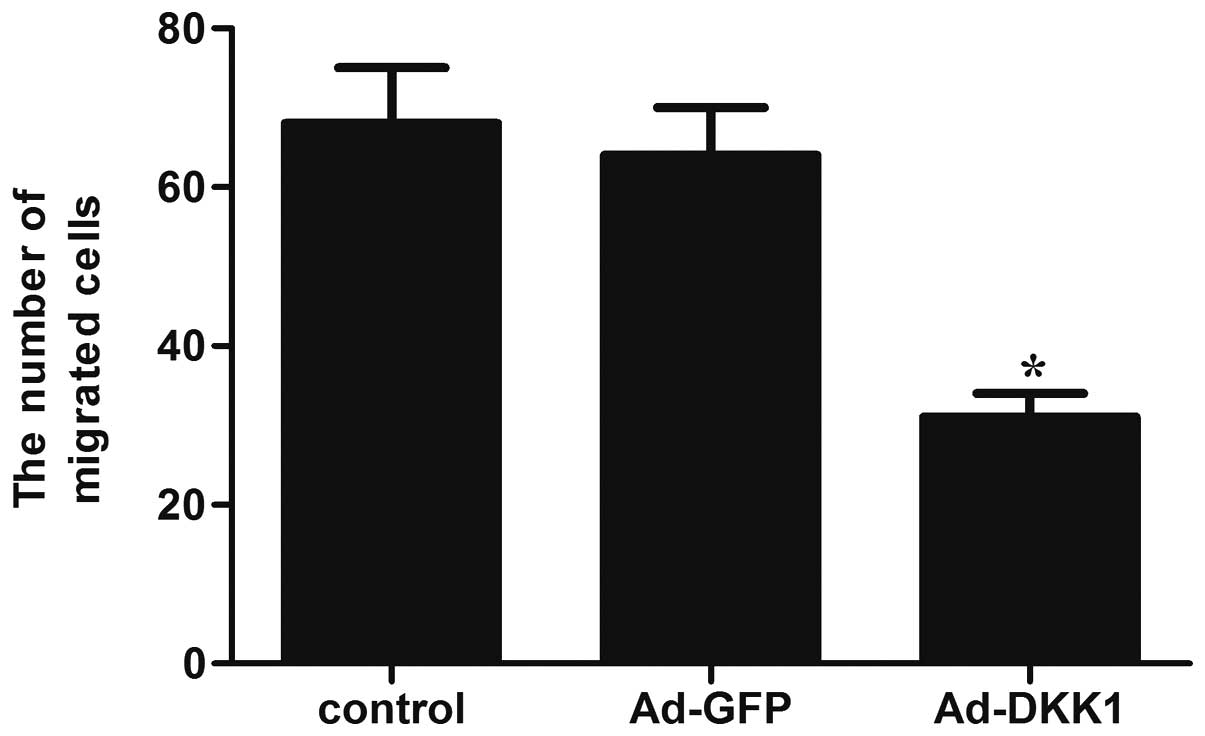
A Transwell assay was conducted to determine whether
DKK1 was involved in the regulation of migration of ARPE-19 cells.
As shown in Fig. 3, overexpression
of DKK1 significantly reduced the number of migrated cells, as
compared with the control group.
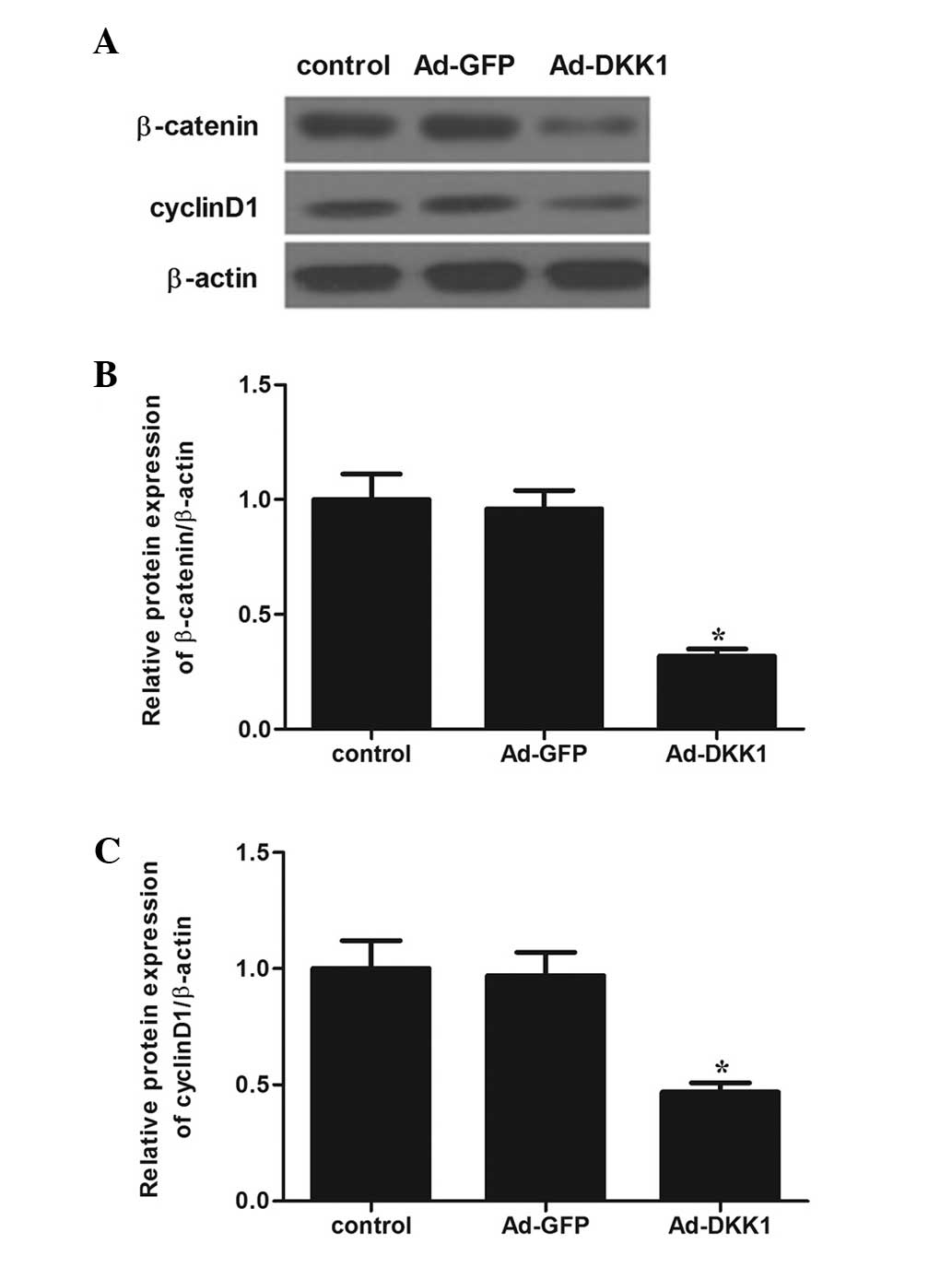
Effect of DKK1 on Wnt/β-catenin
signaling pathway
The Wnt/β-catenin signaling pathway was been
speculated to be associated with DR. Therefore, we examined the
effect of DKK1 on a number of molecules involved in the
Wnt/β-catenin signaling pathway. Western blot analysis revealed
that DKK1 overexpression markedly inhibited the protein expression
levels of β-catenin and cyclin D1 in ARPE-19 cells, as compared
with the Ad-GFP and control groups (Fig.
4).
Discussion
The results of the present study suggested that the
overexpression of DKK1 significantly inhibited the proliferation
and migration of ARPE-19 cells. In addition, the overexpression of
DKK1 markedly inhibited the expression of β-catenin and cyclin D1
in ARPE-19 cells.
RPE cells serve important functions in the healthy
eye and under pathological conditions (11). The proliferation of RPE cells plays
an important role in the pathogenesis of DR (12). Cell migration is another important
process in the development of DR. Without migration, cells are
unable to access ectopic sites and form membranes (13). Therefore, the inhibition of RPE cell
proliferation and migration may offer a potential route of
investigation for developing novel treatments for DR. Previous
studies (14,15) found that DKK1 was implicated in the
control of cancer cell proliferation and migration. A recent study
showed that the overexpression of DKK1 evidently inhibited colon
cancer cell proliferation, migration and invasiveness (16). Another study reported that the
knockdown of DKK1 using small interfering RNA results in a
reduction in intrahepatic cholangiocarcinoma cell migration and
invasion (17). In the present
study, the overexpression of DKK1 significantly inhibited the
proliferation and migration of ARPE-19 cells. These dual roles of
DKK1 could attribute to organ-specific actions and different
cellular contexts. Collectively, the present results and those of
prior studies suggest that DKK1 induces the inhibition of RPE cell
proliferation and migration.
The Wnt/β-catenin signaling pathway has been widely
implicated as the regulator of proliferation and migration in
numerous cell types (18–20). β-catenin is a critical component of
the Wnt signaling pathway (21). It
has been reported that epidermal growth factor promotes RPE cell
proliferation through the β-catenin signaling pathway (22). DKK1 is a secreted antagonist of the
Wnt/β-catenin signaling pathway. Koch et al reported that
the downregulation of DKK1 increased proliferation of epithelial
cells in the large intestine, which was associated with increased
transcriptional activity of β-catenin (23). A prior study showed that FH535, a
specific inhibitor of β-catenin signaling, reduced the outgrowth of
cultured RPE sheets and suppressed dedifferentiated RPE cell
proliferation and migration (24).
Westnskow et al showed that upon β-catenin deletion, the RPE
transforms into a multilayered tissue, and the expression levels of
microphthalmia-associated transcription factor and orthodenticle
homolog are downregulated, while retina-specific gene expression is
increased (25). Consistent with
these previous studies, the present results showed that DKK1
overexpression markedly inhibited the expression of β-catenin and
cyclin D1 in ARPE-19 cells, which may lead to reduced RPE cell
proliferation and migration.
In conclusion, the present study suggests that
overexpression of DKK1 inhibits the proliferation and migration of
RPE cells by suppressing the Wnt/β-catenin signaling pathway.
Therefore, further studies are warranted to investigate the
potential for using DKK1 as a therapeutic strategy for DR.
References
|
1
|
Gardner TW, Antonetti DA, Barber AJ,
Lanoue KF and Nakamura M: New insights into the pathophysiology of
diabetic retinopathy: Potential cell-specific therapeutic targets.
Diabetes Technol Ther. 2:601–608. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pastor JC, de la Rúa ER and Martín F:
Proliferative vitreoretinopathy: Risk factors and pathobiology.
Prog Retin Eye Res. 21:127–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krupnik VE, Sharp JD, Jiang C, Robison K,
Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et
al: Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikheev AM, Mikheeva SA, Rostomily R and
Zarbl H: Dickkopf-1 activates cell death in MDA-MB435 melanoma
cells. Biochem Bioph Res Commun. 352:675–680. 2007. View Article : Google Scholar
|
|
6
|
Diarra D, Stolina M, Polzer K, Zwerina J,
Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C,
et al: Dickkopf-1 is a master regulator of joint remodeling. Nat
Med. 13:156–163. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang FS, Ko JY, Yeh DW, Ke HC and Wu HL:
Modulation of Dickkopf-1 attenuates glucocorticoid induction of
osteoblast apoptosis, adipocytic differentiation and bone mass
loss. Endocrinology. 149:1793–1801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang FS, Ko JY, Lin CL, Wu HL, Ke HJ and
Tai PJ: Knocking down dickkopf-1 alleviates estrogen deficiency
induction of bone loss. A histomorphological study in
ovariectomized rats. Bone. 40:485–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu F, He J, Zhou Y, Bai X, Wu G, Wang X
and Liu Z, Chen Y, Ma JX and Liu Z: Plasma and vitreous fluid
levels of Dickkopf-1 in patients with diabetic retinopathy. Eye
(Lond). 28:402–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Li M, Li Q, Wang CJ and Xie SQ:
DKK1 promotes hepatocellular carcinoma cell migration and invasion
through β-catenin/MMP7 signaling pathway. Mol Cancer. 12:1572013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ablonczy Z, Dahrouj M, Tang PH, Liu Y,
Sambamurti K, Marmorstein AD and Crosson CE: Human retinal pigment
epithelium cells as functional models for the RPE in vivo. Invest
Ophthalmol Vis Sci. 52:8614–8620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaczmarek R and Misiuk-Hojło M:
Patomechanisms in proliferative vitreoretinopathy. Klin Oczna.
113:64–67. 2011.PubMed/NCBI
|
|
13
|
Kim JH, Park S, Chung H and Oh S: Wnt5a
attenuates the pathogenic effects of the Wnt/β-catenin pathway in
human retinal pigment epithelial cells via down-regulating
β-catenin and Snail. BMB Rep. 48:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koppen A, Ait-Aissa R, Hopman S, Koster J,
Haneveld F, Versteeg R and Valentijn LJ: Dickkopf-1 is
down-regulated by MYCN and inhibits neuroblastoma cell
proliferation. Cancer Lett. 256:218–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Li M, Li Q, Wang CJ and Xie SQ:
DKK1 promotes hepatocellular carcinoma cell migration and invasion
through β-catenin/MMP7 signaling pathway. Mol Cancer. 12:157–170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi L, Sun B, Liu Z, Li H, Gao J and Leng
X: Dickkopf1 inhibits epithelial-mesenchymal transition of colon
cancer cells and contributes to colon cancer suppression. Cancer
Sci. 103:828–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y,
Qiu SJ, Sun YF, Zhang X, Wang Z, Zhu K, et al: High expression of
Dickkopf-related protein 1 is related to lymphatic metastasis and
indicates poor prognosis in intrahepatic cholangiocarcinoma
patients after surgery. Cancer. 119:993–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masckauchán TN, Shawber CJ, Funahashi Y,
Li CM and Kitajewski J: Wnt/beta-catenin signaling induces
proliferation, survival and interleukin-8 in human endothelial
cells. Angiogenesis. 8:43–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin X, Zhang H, Zhou X, Wang C, Zhang H,
Zhang X and Ye L: Proliferation and migration mediated by
Dkk-1/Wnt/beta-catenin cascade in a model of hepatocellular
carcinoma cells. Transl Res. 150:281–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun MS, Kim SE, Jeon SH, Lee JS and Choi
KY: Both ERK and Wnt/beta-catenin pathways are involved in
Wnt3a-induced proliferation. J Cell Sci. 118:313–322. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de la Taille A, Rubin MA, Chen MW,
Vacherot F, de Medina SG, Burchardt M, Buttyan R and Chopin D:
Beta-Catenin-related anomalies in apoptosis-resistant and
hormone-refractory prostate cancer cells. Clin Cancer Res.
9:1801–1807. 2003.PubMed/NCBI
|
|
22
|
Krugluger W, Seidel S, Steindl K and
Binder S: Epidermal growth factor inhibits glycogen synthase
kinase-3 (GSK-3) and beta-catenin transcription in cultured ARPE-19
cells. Graefes Arch Clin Exp Ophthalmol. 245:1543–1548. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch S, Nava P, Addis C, Kim W, Denning
TL, Li L, Parkos CA and Nusrat A: The Wnt antagonist Dkk1 regulates
intestinal epithelial homeostasis and wound repair.
Gastroenterology. 141:259–268, 268.e1-e8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Umazume K, Tsukahara R, Liu L, de
Fernandez Castro JP, McDonald K, Kaplan HJ and Tamiya S: Role of
retinal pigment epithelial cell β-Catenin signaling in experimental
proliferative vitreoretinopathy. Am J Pathol. 184:1419–1428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westenskow P, Piccolo S and Fuhrmann S:
Beta-catenin controls differentiation of the retinal pigment
epithelium in the mouse optic cup by regulating Mitf and Otx2
expression. Development. 136:2505–2510. 2009. View Article : Google Scholar : PubMed/NCBI
|