Introduction
Pediatric asthma is a common chronic respiratory
system disease in children, with its main characteristics being
inflammation, bronchial hyperreactivity and airway remodeling
(1). Airway remodeling is an
important step in the occurrence of asthma, and is associated with
the severity of the disease (2). The
abnormal proliferation of bronchial smooth muscle cells (BSMCs) has
important roles in airway remodeling (2,3).
Previous studies reported that BSMCs in patients with bronchial
asthma secrete more cytokines than those in normal subjects,
including chemokine (C-X-C motif) ligand 10 and chemokine (C-X3-C
motif) ligand 1 (4,5). These cytokines induce the attachment of
mast cells to BSMCs, promote the survival and proliferation of mast
cells, facilitate the accumulation of inflammatory substances,
thicken smooth muscles and lead to airway hyperreactivity. Previous
investigations also demonstrated that attachment of T lymphocytes
to BSMCs induces DNA synthesis in the BSMCs, which enhances their
proliferation (6,7). Enhanced proliferation and decreased
apoptosis of BSMCs may result in airway hyperreactivity (6,8).
MicroRNA (miRNA or miR) is a type of 18–22 nt
non-encoding RNA that participates in the regulation of cell
proliferation, apoptosis and differentiation (9). Numerous miRNAs are involved in the
occurrence and development of asthma (10–12).
Feng et al (13) demonstrated
that the expression of miR-146a/b is upregulated in mouse spleen
CD4+ T cells, and is positively correlated with the
number of inflammatory cells in bronchoalveolar lavage fluid;
following treatment with dexamethasone, the expression of miR-146a
is significantly downregulated, indicating that miR-146a/b may
participate in the process of airway inflammation in asthma.
Williams et al (14)
demonstrated that the expression of miR-146a is elevated in airway
biopsies of patients with mild asthma. In addition, the expression
levels of miR-146a are increased in airway smooth muscles following
stimulation by inflammatory factors (15). These studies suggest that elevated
miR-146a levels may be associated with BSMC proliferation and
apoptosis. However, to date, changes in miR-146a expression levels
in the serum of children with asthma have yet to be reported. The
present study aimed to determine changes in the serum expression
levels of miR-146a in children with asthma, and to investigate the
effect of miR-146a on BSMCs.
Materials and methods
Patients
A total of 60 children, including 30 with asthma and
30 healthy controls, were enrolled in the present study at the
Maternal and Child Healthcare Hospital (Laiwu, China), General
Hospital of Yanzhou Mining Bureau (Jining, China), Zoucheng
People's Hospital (Zoucheng, China) and Dezhou People's Hospital
(Dezhou, China) between January 2014 and December 2014. The 30
children with asthma included 13 girls with an average age of
10.46±4.29 years and 17 boys with an average age of 10.86±3.56
years. The exclusion criteria were as follows: i) Oral intake or
intravenous injection of glucocorticoids or immunomodulators in the
previous 2 weeks; ii) first-time asthma; iii) the presence of other
immunologic diseases; and iv) cardiopulmonary failure or other
malignant diseases. The 30 children in the control group included
13 girls with an average age of 10.89±3.15 years and 17 boys with
an average age of 11.23±2.90 years. Children were enrolled in the
control group if they lacked a history of asthma, recent
respiratory tract infections or other malignant diseases. All
procedures were approved by the Ethics Committee of the Taishan
Medical College (Taian, China). Written-informed consent was
obtained from the guardians of all patients.
Cell line and cell culture
Human BSMCs were purchased from Sciencell Research
Laboratories (Carlsbad, CA, USA) and 5×104
BSMCs/cm2 were cultured in smooth muscle culture medium
(Sciencell Research Laboratories, Carlsbad, CA, USA), supplemented
with 5% fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 5 µg/ml insulin, 2 µg/ml human fibroblast
growth factor, 50 ng/ml gentamicin, and 50 ng/ml amphotericin B
(all Sigma-Aldrich, St. Louis, MO, USA) at 37°C in an atmosphere
containing 5% CO2.
miR-146a transfection
For transfection with miR-146a, BSMCs
(2×103/cm2) were first seeded onto culture
plates. When the cells reached 50–70% confluency, they were
transfected with 50 nM miR-146a mimics, 100 nM miR-146a inhibitor,
or 50 nM negative control using riboFECT CP (all Guangzhou RiboBio
Co., Ltd., Guangzhou, China), according to the manufacturer's
protocol. The cells were then cultured at 37°C in an atmosphere
containing 5% CO2.
Cell counting kit-8 (CCK-8) assay
For the CCK-8 assay (Beyotime Institute of
Biotechnology, Haimen, China), cells
(5×103/cm2) were seeded onto 96-well plates
in triplicate. A total of 24 h after inoculation, the cells were
transfected as described above. At 6 h post-transfection, the
transfection medium was replaced with fresh medium. At 0, 12, 24,
and 48 h, WST reagent (10 µl) was added to the cells. After 1 h
culture at 37°C and 5% CO2, the absorbance was measured
at 450 nm. For the determination of caspase-3/7 activity, the cells
were also seeded onto 96-well plates in triplicate, and transfected
as described above. At 48 h post-transfection, caspase-3/7 activity
was determined using a Caspase-Glo 3/7 kit (cat. no. G8090; Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (1 µg) was extracted from the plasma (200
µl) using an miRNeasy Serum/Plasma kit (cat. no. 217184; Qiagen
GmbH, Hilden, Germany) following the manufacturer's protocol.
Reverse transcription (5 µl RNA) was performed using a miScript II
RT kit (cat. no. 218160; Qiagen GmbH). Following dilution of the
cDNA (10 times) in RNase-free water, 2 µl cDNA was used for qPCR
using the StepOnePlus™ Real-Time PCR System (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the protocol provided by the
manufacturer of the miScript SYBR Green PCR kit (Qiagen GmbH). Each
sample was analyzed in triplicate and negative (no cDNA template)
and reverse transcriptase-minus controls were performed. The Cq
value of the plasma miR-146a was corrected using cel-miR-39 as an
external reference, and the relative quantification of the target
genes was determined using the 2−ΔΔCq method (16). The primers for miR-146a and
cel-miR-39 were purchased from Qiagen. The PCR amplification
protocol was as follows: Initial denaturation at 94°C for 15 min;
40 cycles of denaturation at 94°C for 10 sec, annealing at 55°C for
30 sec, and elongation at 70°C for 30 sec.
Western blotting
The cells were lysed using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology), followed
by centrifugation at 12,000 × g for 5 min at 4°C. The protein
concentration in the supernatant was determined by performing a
bicinchoninic acid assay. A total of 30 µg protein was separated
using 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto a nitrocellulose membrane. The membrane was
blocked with 5% skimmed milk at room temperature for 1 h, and
incubated overnight at 4°C with mouse anti-phosphorylated
(p)-signal transducer and activator of transcription 3 (stat3)
monoclonal antibody (1:1,000; cat. no. 4113; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-B-cell lymphoma 2
(Bcl-2) monoclonal antibody (1:1,000; cat. no. 2870; Cell Signaling
Technology, Inc.), rabbit anti-p-extracellular regulated protein
kinase (ERK) polyclonal antibody (1:800; cat. no. ab47339; Abcam,
Cambridge, UK), rabbit anti-p-epidermal growth factor receptor
(EGFR) monoclonal antibody (1:1,000; cat. no. 3777; Cell Signaling
Technology, Inc.), rabbit anti-EGFR monoclonal antibody (1:1,000;
cat. no. 2085; Cell Signaling Technology, Inc.) and rabbit
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal
antibody (1:5,000; cat. no. 2118; Cell Signaling Technology, Inc.).
Following 3 washes with Tris-buffered saline with Tween-20 (TBST;
Sigma-Aldrich), the membrane was incubated with the secondary
antibody (1:5,000 dilution) at room temperature for 1 h. Following
a further wash with TBST, electrochemiluminescent liquid was added.
The images were of the membrane were subsequently captured using a
Fusion Solo 4 Chemiluminescence system (Fisher Biotec Pty, Ltd.,
Wembley, WA, Australia), and analyzed using Quantity One software,
version 4.4.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Bioinformatics analysis
Bioinformatics is a powerful tool for investigating
the functions of miRNAs. In order to elucidate the regulatory roles
of miR-146a, TargetScan 5.0 (http://www.targetscan.org) was used to predict the
target molecules of miR-146a.
Dual-luciferase reporter assay
The wild-type 3′-untranslated region (UTR) of the
EGFR mRNA that binds miR-146a and relevant mutant 3′-UTR were
cloned into a psiCHECK reporter vector (Promega Corporation) to
obtain wt-EGFR-3′-UTR vector and mut-EGFR-3′-UTR vector. The
wt-EGFR-3′-UTR vector or mut-EGFR-3′-UTR vector was transfected
into 293T cells (American Type Culture Collection, Manassas, VA,
USA) together with control plasmids, and miR-146a or negative
control mimics (Gene Copoeia, Inc., Rockville, MD, USA). The cells
were lysed using lysis buffer (Promega Corporation) and the
fluorescence intensity was determined using a GloMax 20/20
luminometer (Promega Corporation). Subsequently, a dual-luciferase
reporter assay was performed using a Dual-Luciferase Reporter Assay
system (Promega Corporation), according to the manufacturer's
protocol. Renilla fluorescence served as an internal
reference. All tests were performed in triplicate.
Statistical analysis
All analyses were performed using SPSS v19.0
software (IBM SPSS, Armonk, NY, USA). The data were presented as
means ± standard deviation (n ≥3). Differences between groups of
data were compared using t-tests. P<0.05 was considered to
indicate a statistically significant result.
Results
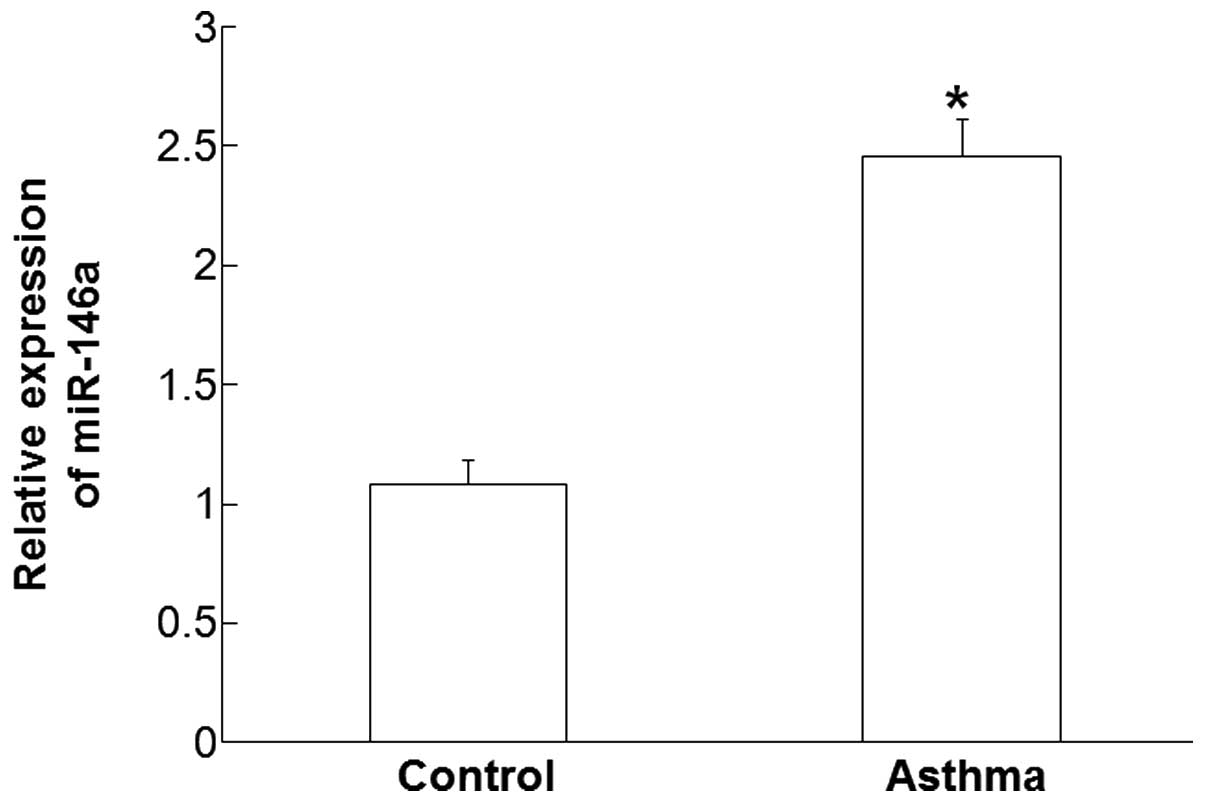
Plasma levels of miR-146a are
increased in children affected by asthma
RT-qPCR was used measure the plasma levels of
miR-146a. The data demonstrated that the expression levels of
miR-146a in the plasma of patients with asthma was significantly
higher compared with those in the control group (P<0.05;
Fig. 1). These results suggest that
the plasma levels of miR-146a are increased in children with
asthma.
Enhanced miR-146a expression inhibits
the proliferation of BSMCs
To determine the expression levels of miR-146a in
BSMCs and its effect on cell proliferation, RT-qPCR and a CCK-8
assay were performed. RT-qPCR data revealed that miR-146a
expression levels in BSMCs transfected with miR-146a mimics were
968.76 times those of the control group. In addition, miR-146a
expression levels in BSMCs transfected with miR-146a inhibitor were
23.33% those of the control group, whereas the expression levels of
miR-146a in BSMCs transfected with negative control were not
significantly different from those of the control group. Moreover,
the CCK-8 assay demonstrated that the proliferation of BSMCs
transfected with mimics was inhibited after 24, 48, and 72 h
(P<0.05). However, transfection with miR-146a inhibitor had an
insignificant effect on BSMC proliferation (P>0.05; Fig. 2). These results suggest that enhanced
miR-146a expression inhibits the proliferation of BSMCs.
BSMC apoptosis is promoted by
miR-146a
To determine the effect of miR-146a on BSMC
apoptosis, caspase-3/7 activity in BSMCs was examined 48 h
post-transfection with miR-146a mimics and miR-146a inhibitor. The
data demonstrated that miR-146a mimics significantly enhanced the
activity of caspase-3/7 in BSMCs, whereas miR-146a inhibitor
significantly decreased the activity of caspase-3/7 (P<0.05;
Fig. 3). These results suggest that
miR-146a promotes BSMC apoptosis.
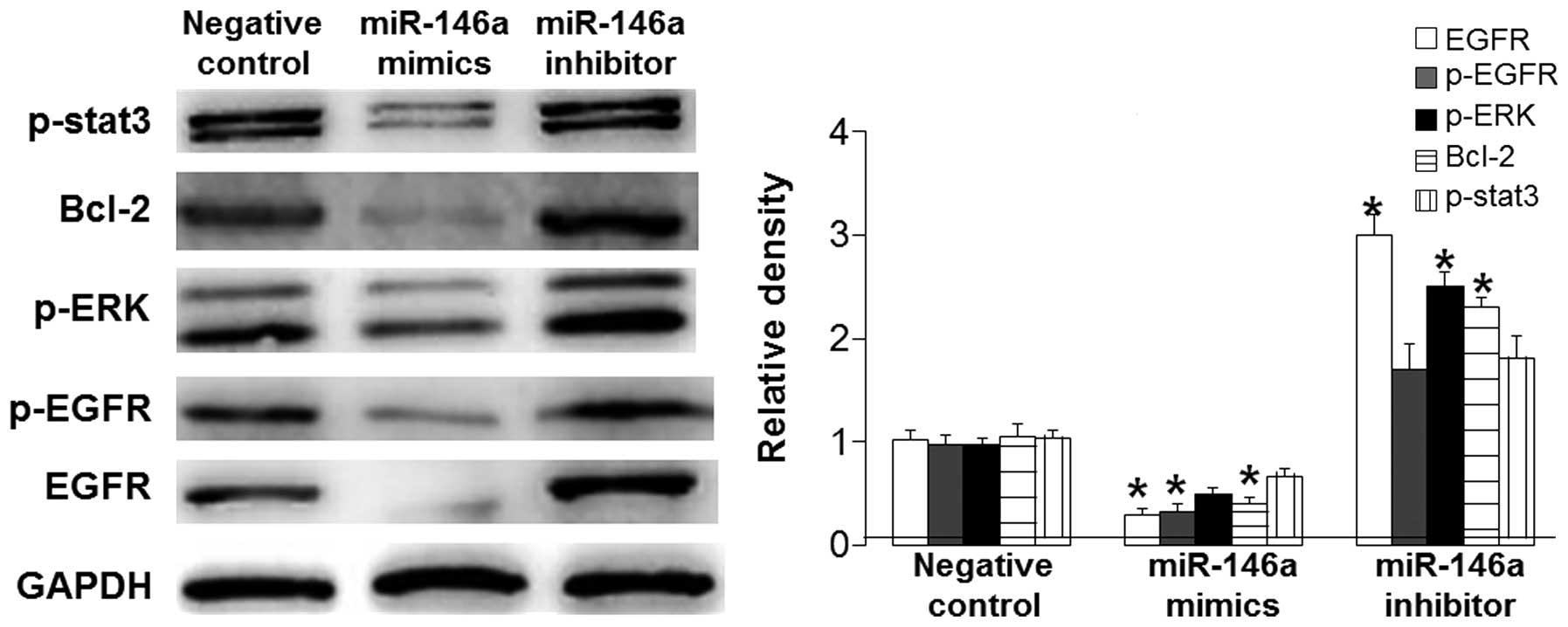
T mechanism underlying
miR-146a-induced BSMC apoptosis is its direct targeting of EGFR
that affects downstream signaling pathways
To determine the signaling pathway by which miR-146a
promotes BSMC apoptosis, western blotting, bioinformatics and a
dual-luciferase reporter assay were performed. Western blotting
data showed that miR-146a mimics significantly reduced the protein
expression levels of EGFR and p-EGFR (P<0.05), markedly
downregulated the ERK signaling pathway and p-stat3 expression
levels, and significantly decreased Bcl-2 expression levels
(P<0.05). However, these effects were reversed by miR-146a
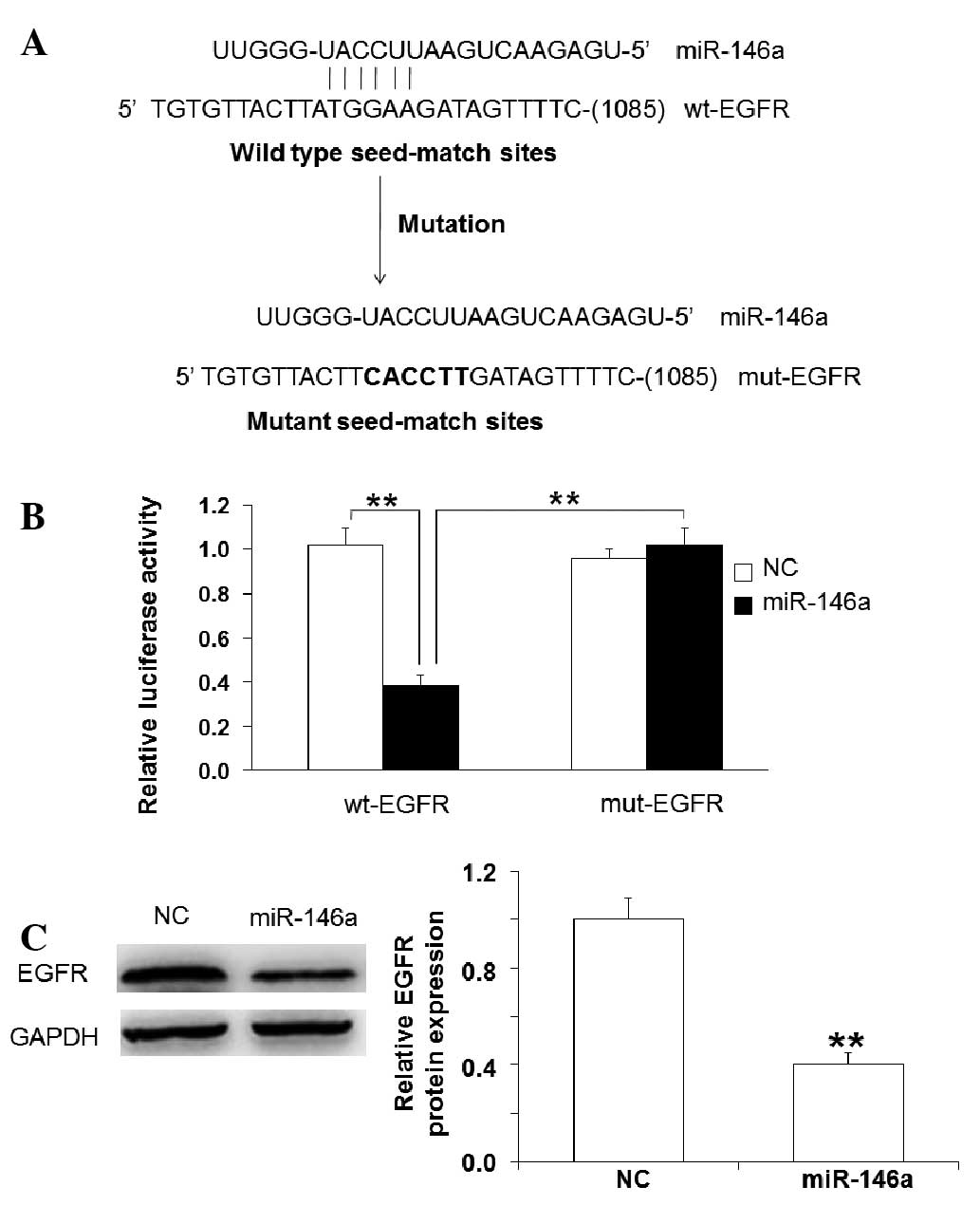
inhibitor (Fig. 4). Using
bioinformatics tools, it was demonstrated that miR-146a may bind to
the 3′-UTR of EGFR mRNA (Fig. 5A).
Dual-luciferase reporter assay demonstrated that miR-146a mimics
significantly reduced luciferase activity of wt-EGFR compared with
negative control, but had no significant effect on that of mut-EGFR
(Fig. 5B). Western blotting
demonstrated that EGFR was targeted by miR-146a (Fig. 5C). These results suggest that the
mechanism underlying miR-146a-induced promotion of BSMC apoptosis
is its direct targeting of EGFR that affects downstream signaling
pathways.
Discussion
The present study first investigated the association
between plasma miR-146a expression levels and pediatric asthma. The
results demonstrated that children with asthma had significantly
higher miR-146a expression levels compared with normal subjects,
indicating that miR-146a may have a role in pediatric asthma.
miR-146a is an important regulator of immunoreaction and
inflammatory diseases (17). A
previous study reported that the activity of T cells with knockout
of miR-146a was increased in acute and chronic inflammatory
processes (3). However, another
study reported that the expression of miR-146a was upregulated in
spleen CD4+ T cells in the ovalbumin-induced mouse
asthma model (2). Liu et al
(18) demonstrated that miR-146a
facilitates the survival of human bronchial epithelial cells by
upregulating the phosphorylation levels of B-cell lymphoma-extra
large and signal transducers and activators of transcription 3,
leading to the restoration and remodeling of tissues (18). Elevated miR-146a expression in airway
smooth muscle cells that is stimulated by inflammatory factors
negatively regulates the levels of interleukin-β (15). The results of the present study
demonstrated that miR-146a inhibited the proliferation and promoted
the apoptosis of BSMCs.
EGFR is a type of receptor tyrosine kinase that is
widely distributed in human cell membranes (19). The immunoreactivity of EGFR is
enhanced in the bronchial smooth muscles of patients with asthma,
and inhibitors of EGFR are able to reduce collagen deposition and
mucus accumulation following antigen processing, suggesting that
EGFR may participate in airway remodeling (20–23). Xu
et al (24) demonstrated that
miR-146a inhibits the proliferation of prostate cancer cells by
regulating the EGFR signaling pathway. Park et al (25) reported that miR-146a affects the
apoptosis of dendritic cells by targeting tumor necrosis factor
receptor-associated factor 6 and interleukin-1 receptor-associated
kinase 1. The present study demonstrated that miR-146a
downregulated the expression of EGFR and p-EGFR, and reduced the
levels of p-ERK and p-stat3, suggesting that miR-146a altered the
proliferation and apoptosis of BSMCs via the p-ERK-dependent
signaling pathway. In addition, miR-146a increased caspase 3/7
activity and reduced Bcl-2 expression, indicating that caspases
participate in BSMC apoptosis.
In conclusion, the present study demonstrated that
an increase in miR-146a expression in patients with asthma inhibits
the proliferation and promotes the apoptosis of BSMCs. The study
provides a novel clinical marker for the diagnosis and treatment of
asthma, and an experimental basis for its molecular targeted
therapy.
Acknowledgements
The authors of the present study would like to
acknowledge Professor Xuechun Wang of Taishan Medical College for
his support and comments.
References
|
1
|
Lochte L, Nielsen KG, Petersen PE and
Platts-Mills TA: Childhood asthma and physical activity: A
systematic review with meta-analysis and Graphic Appraisal Tool for
Epidemiology assessment. BMC Pediatr. 16:502016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Boldin MP, Yu Y, Liu CS, Ea CK,
Ramakrishnan P, Taganov KD, Zhao JL and Baltimore D: miR-146a
controls the resolution of T cell responses in mice. J Exp Med.
209:1655–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omran A, Elimam D and Yin F: MicroRNAs:
New insights into chronic childhood diseases. BioMed Res Int.
2013:2918262013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsitsiou E, Williams AE, Moschos SA, Patel
K, Rossios C, Jiang X, Adams OD, Macedo P, Booton R, Gibeon D, et
al: Transcriptome analysis shows activation of circulating CD8+ T
cells in patients with severe asthma. J Allergy Clin Immunol.
129:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dileepan M, Sarver AE, Rao SP, Panettieri
RA Jr, Subramanian S and Kannan MS: MicroRNA Mediated Chemokine
Responses in Human Airway Smooth Muscle Cells. PLoS One.
11:e01508422016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bara I, Ozier A, de Tunon Lara JM, Marthan
R and Berger P: Pathophysiology of bronchial smooth muscle
remodelling in asthma. Eur Respir J. 36:1174–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roviezzo F, Sorrentino R, Bertolino A, De
Gruttola L, Terlizzi M, Pinto A, Napolitano M, Castello G,
D'Agostino B, Ianaro A, et al: S1P-induced airway smooth muscle
hyperresponsiveness and lung inflammation in vivo: Molecular and
cellular mechanisms. Br J Pharmacol. 172:1882–1893. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perry MM, Baker JE, Gibeon DS, Adcock IM
and Chung KF: Airway smooth muscle hyperproliferation is regulated
by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol.
50:≈7–17. 2014.
|
|
9
|
Rebane A: microRNA and Allergy. Adv Exp
Med Biol. 888:331–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuhn AR, Schlauch K, Lao R, Halayko AJ,
Gerthoffer WT and Singer CA: MicroRNA expression in human airway
smooth muscle cells: Role of miR-25 in regulation of airway smooth
muscle phenotype. Am J Respir Cell Mol Biol. 42:506–513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu R, Pan W, Fedulov AV, Jester W, Jones
MR, Weiss ST, Panettieri RA Jr, Tantisira K and Lu Q: MicroRNA-10a
controls airway smooth muscle cell proliferation via direct
targeting of the PI3 kinase pathway. FASEB J. 28:2347–2357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pua HH and Ansel KM: MicroRNA regulation
of allergic inflammation and asthma. Curr Opin Immunol. 36:101–108.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng MJ, Shi F, Qiu C and Peng WK:
MicroRNA-181a, −146a and −146b in spleen CD4+ T lymphocytes play
proinflammatory roles in a murine model of asthma. Int
Immunopharmacol. 13:347–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams AE, Larner-Svensson H, Perry MM,
Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF and
Lindsay MA: MicroRNA expression profiling in mild asthmatic human
airways and effect of corticosteroid therapy. PloS One.
4:e58892009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Comer BS, Camoretti-Mercado B, Kogut PC,
Halayko AJ, Solway J and Gerthoffer WT: MicroRNA-146a and
microRNA-146b expression and anti-inflammatory function in human
airway smooth muscle. Am J Physiol Lung Cell Mol Physiol.
307:L727–L734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Gelfond JA, McManus LM and
Shireman PK: Reproducibility of quantitative RT-PCR array in miRNA
expression profiling and comparison with microarray analysis. BMC
Genomics. 10:4072009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saba R, Sorensen DL and Booth SA:
MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune
Response. Front Immunol. 5:5782014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Nelson A, Wang X, Kanaji N, Kim M,
Sato T, Nakanishi M, Li Y, Sun J, Michalski J, et al: MicroRNA-146a
modulates human bronchial epithelial cell survival in response to
the cytokine-induced apoptosis. Biochem Biophys Res Commun.
380:177–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan X, Lambert PF, Rapraeger AC and
Anderson RA: Stress-Induced EGFR Trafficking: Mechanisms,
Functions, and Therapeutic Implications. Trends Cell Biol.
25:352–366. 2016. View Article : Google Scholar
|
|
20
|
Wu W, Wages PA, Devlin RB, Diaz-Sanchez D,
Peden DB and Samet JM: SRC-mediated EGF receptor activation
regulates ozone-induced interleukin 8 expression in human bronchial
epithelial cells. Environ Health Perspect. 123:231–236.
2015.PubMed/NCBI
|
|
21
|
Hao Y, Kuang Z, Jing J, Miao J, Mei LY,
Lee RJ, Kim S, Choe S, Krause DC and Lau GW: Mycoplasma pneumoniae
modulates STAT3-STAT6/EGFR-FOXA2 signaling to induce overexpression
of airway mucins. Infect Immun. 82:5246–5255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Cras TD, Acciani TH, Mushaben EM,
Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U,
Ericksen M, Gibson AM, et al: Epithelial EGF receptor signaling
mediates airway hyperreactivity and remodeling in a mouse model of
chronic asthma. Am J Physiol Lung Cell Mol Physiol. 300:L414–L421.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshikawa T and Kanazawa H: Integrated
effect of EGFR and PAR-1 signaling crosstalk on airway
hyperresponsiveness. Int J Mol Med. 30:41–48. 2012.PubMed/NCBI
|
|
24
|
Xu B, Wang N, Wang X, Tong N, Shao N, Tao
J, Li P, Niu X, Feng N, Zhang L, et al: MiR-146a suppresses tumor
growth and progression by targeting EGFR pathway and in a
p-ERK-dependent manner in castration-resistant prostate cancer.
Prostate. 72:1171–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|