Introduction
Cirrhosis is associated with portal hypertension.
Esophageal variceal bleeding (EVB) is is one of the most common and
dangerous complications of cirrhosis associated with portal
hypertension (1). The majority of
patients suffer from acute onset of the disease, which is
accompanied by high mortality rates due to the large amount of
bleeding that is difficult to stop (2). Approximately 50% of patients with
cirrhosis are diagnosed with esophageal varices at the outset, and
those with Child-Pugh Class B or C liver function are susceptible
to a higher incidence of esophageal varices (3), with 7% developing esophageal varices
each year (4,5). The rate of initial hemorrhage is ~12%
in the first year (6), and the
recurrence rate of hemorrhage after 1 year is ~60% (7). Mortality rates within 6 weeks of
bleeding remain high at up to 20% (8).
Since 1974, percutaneous transhepatic variceal
embolization (PTVE) has been used for the effective treatment of
EVB (9). However, its clinical
application is limited to patients with a high rate of rebleeding
(10–12). Transcatheter splenic arterial
embolization can decrease portal vein blood flow and pressure, and
improve the symptoms of hypersplenism; however, its benefits are
limited as 73% of patients experience severe complications if the
embolic volume is >70% of the vein (13). Since 2006, our group has used the
improved phased joint intervention [PTVE + staged partial splenic
embolization (PSE)] to treat portal hypertension complicated by EVB
and hypersplenism. This avoids the weaknesses of other therapeutic
methods, thus achieving satisfactory clinical efficacy. In the
current study the clinical applications of phased joint
intervention were explored by reviewing the case data. However, the
mechanism of action of this therapeutic method has yet to be
elucidated.
The central features of the occurrence and
progression of liver cirrhosis are the activation and proliferation
of hepatic stellate cells (HSCs). In vitro studies have
revealed that extracellular pressure promotes the proliferation of
HSCs and increase the expression levels of associated cytokines and
other extracellular matrix (ECM) components, such as collagen
(14,15). Phased joint intervention improves
liver hemodynamics, which has a major impact on liver restructuring
and alters the mechanical environment of the HSCs (16). Rho small G proteins have an important
role in mechanical transduction. A previous study indicated that
the aforementioned proteins are able to regulate various biological
activities of HSCs by activating crucial downstream effectors,
including Rho-associated coil protein kinase (ROCK) (17). The present study further investigated
the protein and mRNA expression levels of Rho and ROCK1/ROCK2 prior
to and following interventional embolization, and explored the
possible mechanisms underlying the effect of embolization on the
progression of liver cirrhosis.
Materials and methods
Patients and ethics statement
The present retrospective study involved 53 cases of
liver cirrhosis complicated by portal hypertension, EVB and
hypersplenism treated in Shanghai Tongren Hospital (Shanghai,
China) from October 2006 to December 2011. Portal hypertension was
predominantly due to hepatitis (35 cases), autoimmunity (10 cases)
or alcoholic cirrhosis (8 cases), with phased joint intervention
(PTVE + phased PSE) selected as the interventional therapy. The
current study included 37 men and 16 women, with an average age of
47.92±8.0 years (range, 44–71 years). The liver function of the
enrolled patients was classified as Child-Pugh class A (n=15),
class B (n=22) or class C (n=16). All 53 patients had a history of
≥1 bleeding episodes, and received emergency interventional
treatment. Ethical approval was granted by the Shanghai Changning
District Central Hospital Ethics Committee. All patients agreed to
accept the intervention, and they (or their authorized family
members) provided written informed consent. Inclusion and exclusion
criteria were performed as previously described by Wang et
al (16).
Materials
Primers of RhoA, ROCK1/ROCK2 were designed by the
current group and synthesized by Shanghai Generay Biotech Co., Ltd.
(Shanghai, China) (Table I). Rabbit
anti-ROCK1 (C8F7) monoclonal antibody (mAb; cat. no. 4035), rabbit
anti-ROCK2 (D1B1) mAb (cat. no. 9029), rabbit anti-RhoA (67B9) mAb
(cat. no. 2117), rabbit anti-myosin phosphatase target subunit 1
(MYPT1) (D6C1) mAb (cat. no. 8574) and rabbit anti-phosphorylated
(p)-MYPT1 (Thr853) polyclonal antibody (cat. no. 4563) were all
purchased from (Cell Signaling Technology, Danvers, MA, USA). Mouse
anti-β-actin mAb (cat. no. A5316), goat anti-rabbit IgG (whole
molecule)-peroxidase antibody (cat. no. A0545) and goat anti-mouse
IgG (Fc-specific)-peroxidase antibody (cat. no. A0168) were
purchased from Sigma-Aldrich, (St. Louis, MO, USA). SYBR Green
Real-Time PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan); Pierce
BCA Protein Assay kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA); Amersham ECL Plus Western Blotting Detection System (GE
Healthcare Life Sciences, Chalfont, UK); ELISA kits (cat. nos.
CB5683, OK0109 and OK0155; Assay Biotechnology Company Inc,
Sunnyvale, CA, USA), GE3100 flat C-DSA system; and a GE730 Color
Doppler ultrasound diagnostic apparatus, all GE Healthcare Life
Sciences).
 | Table I.Primer probe sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer probe sequences used in reverse
transcription-quantitative polymerase chain reaction.
| mRNA | Sequence (5′-3′) | Amplicon size
(bp) |
|---|
| RhoA | F:
GGAAAGCAGGTAGAGTTGGCT | 118 |
|
| R:
GGCTGTCGATGGAAAAACACAT |
|
| ROCK1 | F:
AAGTGAGGTTAGGGCGAAATG | 219 |
|
| R:
AAGGTAGTTGATTGCCAACGAA |
|
| ROCK2 | F:
TTGCTCTGGATGCAATACACTC | 223 |
|
| R:
TCTCGCCCATAGAAACCATCA |
|
| β-actin | F:
TGGAGAAAATCTGGCACCA | 189 |
|
| R:
CAGGCGTACAGGGATAGCAC |
|
Phased joint embolization
For PTVE + PSE, the PSE embolization range was
controlled between 30 and 40%. PSE was conducted again three months
later with an embolization range controlled between 30 and 40%.
PTVE
Using a 21 gauge needle (Cook Medical, Bloomington,
IN, USA), the right portal vein was percutaneously and
transhepatically punctured with following routine sterilization and
positioning of the liver. Following successful puncture, a guide
wire was inserted into the superior mesenteric vein along the
needle, and a 5 F vascular sheath (Cook Medical) was subsequently
inserted using the guide wire. The contrast agent (iohexol) was
injected to perform splenic and portal vein digital subtraction
angiography (DSA). To visualize the esophageal varices, the
catheter tip was superselectively inserted into the gastric
coronary vein. For distal embolization of the gastric coronary
vein, 5–30 ml absolute ethanol was injected according to the
radiography situation of DSA. In cases with particularly slow blood
flow, a spring ring of the appropriate specification (Cook Medical)
was utlilized for main vein thrombosis. In the event that the
angiographic image revealed complete occlusion of the gastric
coronary vein, short gastric vein thrombosis was treated according
to the same method. In cases with particularly rapid blood flow, a
steel ring (diameter, 5–10 mm) or gelatin sponge particles were
used for embolization to reduce the speed of the blood flow in the
thickened varicose branch. Absolute ethanol was gradually injected
until the varicose vein was no longer visible to the naked eye. In
order to determine the embolization effect, DSA was conducted for a
second time. During this secondary DSA, the catheter tip was once
again inserted into the splenic vein to confirm whether there were
any varicose veins. If varicose veins were detected, the same
method was applied, as previously described, for complete
embolization. Following treatment, the catheter sheath was
carefully removed from the right portal vein to reduce the risk of
bleeding. In the event of bleeding, a gelatin sponge was used to
block the needle tract with a pressure dressing to ensure no
further bleeding for 48 h.
PSE
The catheter was inserted using Seldinger's
technique via the femoral artery to reach the splenic hilum, and
its medium and lower branches via the proximal splenic artery. The
splenic vessels and blood flow were assessed by DSA, and gelatin
sponge particles were injected into the splenic artery and its
branches for embolization. Splenic arteriography was conducted, and
the puncture point was subsequently subjected to pressure dressing
once the catheter had been removed.
Postoperative treatment
Vital signs and abdominal conditions were closely
observed. Patients receiving a pressure dressing and compression
hemostasis were required to have undergone 24 h bed rest and to
have taken preventive measures, including ceftazidime injection (2
g/day; Shandong Luoxin Group Pharmaceutical, Co., Ltd., Shandong,
China), in order to prevent infection and other complications.
Assessment of efficacy
Patients underwent re-examination for routine blood
tests, liver function, gastroscopy, color Doppler ultrasound and
computed tomography. Follow-up at 6 months included routine blood
tests, and assessment of liver function, complications and
rebleeding. In the event of gastrointestinal bleeding, patients
visited the doctor immediately. Postoperative portal vein
hemodynamics were evaluated by abdominal ultrasound. Postoperative
indicators of liver function included alanine transaminase (ALT),
albumin, total bilirubin (TBIL) and prothrombin time (PT-INR);
whereas the indicators for hypersplenism improvement included white
blood cell (WBC) and platelet counts. The aforementioned indicators
were observed prior to intervention and 1, 4 (1 month after phased
PSE) and 6 months after surgery. Serum levels of transforming
growth factor (TGF)-β1, connective tissue growth factor (CTGF) and
platelet-derived growth factor (PDGF) were analyzed using ELISA
kits.
Isolation, cryopreservation and
resuscitation of peripheral blood mononuclear cells (PBMCs)
Prior to surgery and 1, 4 and 6 months after
intervention, 5 ml fresh blood was collected from the patients in
an EDTA anticoagulant tube, with 32 healthy samples used as
controls. The plasma was stored at −70°C, and the remaining cells,
resuspended in phosphate buffered saline, were carefully overlayed
onto Ficoll and centrifuged at 800 × g for 15 min at room
temperature. Finally, purified PBMCs were nitrogen-frozen in RPMI
1640 supplemented with 10% DMSO and 10% fetal serum.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from PBMCs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol, and treated with DNase (Promega
Corporation, Madison, WI, USA). The purity and concentration of the
RNA were determined by the absorbance at 260 nm and 280 nm using a
spectrophotometer. RNA (2 µg) was subsequently reverse transcribed
into cDNA using PrimeScript® 1st Strand cDNA Synthesis
kit (Takara Biotechnology Co., Ltd., Dalian, China). qPCR was
performed using the SYBR Green Real-Time PCR Master Mix in an ABI
PRISM 7900HT Sequence Detector (Thermo Fisher Scientific, Inc.).
The PCR conditions included an initial denaturation at 94°C for 5
min, followed by 40 cycles at 94°C for 30 sec, 61°C for 45 sec and
72°C for 30 sec, and a final extension step at 72°C for 5 min.
Relative mRNA expression levels were calculated using the
2−ΔΔCq method (18), with
normalization to β-actin. The primer sequences are displayed in
Table I. All experiments were at
least duplicated.
Western blotting
Total protein was extracted from PBMCs using SDS
cell lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) containing 1 mM phenylmethylsulfonyl fluoride. Protein
concentrations were measured using the Pierce BCA Protein Assay
kit. Equal quantities of proteins (100 µg) were separated by 10%
SDS-PAGE and subsequently transferred onto nitrocellulose
membranes. Membranes were blocked with 5% milk in Tris-buffered
saline supplemented with Tween-20 (TBST) for 1 h at room
temperature, then incubated with rabbit anti-ROCK1 (C8F7) mAb,
anti-ROCK2 (D1B1) mAb, anti-RhoA (67B9) mAb, anti-MYPT1 (D6C1) mAb
and anti-p-MYPT1 (Thr853) polyclonal antibody, and mouse
anti-β-actin mAb (all 1:1,000), at 4°C overnight. After washing
three times for 10 min each with TBST, the membranes were incubated
with peroxidase-conjugated goat anti-rabbit and goat anti-mouse
secondary antibodies (1:5,000) for 1 h at room temperature.
Membranes were visualized using the Amersham ECL Plus Western
Blotting Detection System and analyzed using ImageJ 1.48v software
(https://imagej.nih.gov/ij/). Relative
protein expression levels were normalized to β-actin. All
experiments were repeated in triplicate.
Statistical analysis
Data were analyzed using SPSS statistical software
(version 13.0; SPSS, Inc., Chicago, IL, USA), and expressed as mean
± standard deviation. Multiple groups were analyzed with one-way
analysis of variance; pairwise comparison was conducted via a least
significant difference t-test, and different groups were compared
using a t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Success rate of surgery
All 53 patients successfully received the joint
intervention with emergency hemostasis (Fig. 1). Bleeding ceased immediately
following surgery with an emergency hemostatic rate of 100%.
Varicose veins were significantly reduced in portal vein
angiography after PTVE intervention, and partial spleen
embolization occurred after PSE intervention (16). During the 6 months of follow-up, all
patients survived and none reported rebleeding. Following phased
joint interventional embolization, 32 patients exhibited various
degrees of fever with body temperatures ranging from 37.5–39.4°C,
which was alleviated by symptomatic treatment. Postoperative pain
was experienced by 29 patients, and was also relieved by
symptomatic treatment. No serious complications occurred.
Quantitative determination of Rho,
ROCK1 and ROCK2 expression levels by RT-qPCR
RT-qPCR analysis demonstrated that the mRNA
expression levels of Rho, ROCK1 and ROCK2 were significantly higher
preoperatively and postoperatively, as compared with the normal
control group (P<0.01). However, the mRNA expression levels of
Rho, ROCK1 and ROCK2 were significantly lower in the postoperative
group, as compared with the preoperative group (P<0.01; Fig. 2).
 | Figure 2.Rho, ROCK1 and ROCK2 expression levels
before and after treatment, as determined by reverse
transcription-quantitative polymerase chain reaction. mRNA
expression levels of Rho, ROCK1 and ROCK2 were higher compared with
the normal control group Prior to and after intervention (Rho:
preoperative, 10.59±0.19 vs. 1.80±0.46; postoperative, 3.29±0.47
vs. 1.80±0.46; ROCK1: preoperative, 2.79±0.27 vs. 0.44±0.08;
postoperative, 1.21±0.09 vs. 0.44±0.08; and ROCK2: preoperative,
3.48±0.19 vs. 0.65±0.20; postoperative, 1.84±0.11 vs. 0.65±0.20).
mRNA expression levels of Rho, ROCK1 and ROCK2 in the postoperative
group were significantly lower compared with those of the
preoperative group (Rho: 3.29±0.47 vs. 10.59±0.19; ROCK1: 1.21±0.09
vs. 2.79±0.27; and ROCK2: 1.84±0.11 vs. 3.48±0.19). *P<0.001,
**P=0.002 and ***P=0.004 vs. the control group;
#P<0.001 vs. the preoperative group. ROCK,
Rho/Rho-associated coil protein kinase. |
Determination of Rho, ROCK1, ROCK2,
pMYPT1 and tMYPT1 protein expression levels by western
blotting
Western blotting indicated that the protein
expression levels of Rho, ROCK1 and ROCK2 were significantly higher
in the preoperative and postoperative groups, as compared with the
normal control group (P<0.05). However, the protein expression
levels of Rho, ROCK1 and ROCK2 were significantly decreased in the
postoperative group, as compared with the preoperative group
(P<0.05). The changes in protein expression levels were
consistent with those of mRNA expression levels. Postoperative
pMYPT1 protein expression levels were significantly reduced, as
compared with preoperative levels (P<0.05), although there was
no obvious change in tMYPT1 protein expression levels (Fig. 3).
 | Figure 3.Protein expression levels of Rho,
ROCK1 and ROCK2 prior to and after treatment, as determined by (A)
western blotting and (B) densitometry. Protein expression levels
were normalized to β-actin. 1, normal control group; 2,
preoperative group; 3, postoperative group. Data are presented as
the mean ± standard deviation. *P<0.05, **P<0.01 vs. the
control group; #P<0.05, ##P<0.01 vs.
the preoperative group. ROCK, Rho/Rho-associated coil protein
kinase; p phosphorylated; t, total; MYPT1, myosin phosphatase
target subunit 1. |
Determination of TGF-β1, CTGF and PDGF
in peripheral blood by ELISA
ELISA indicated that TGF-β1, CTGF and PDGF
concentrations in peripheral blood following phased joint
intervention were significantly lower than the preoperative values
(P<0.01; Fig. 4).
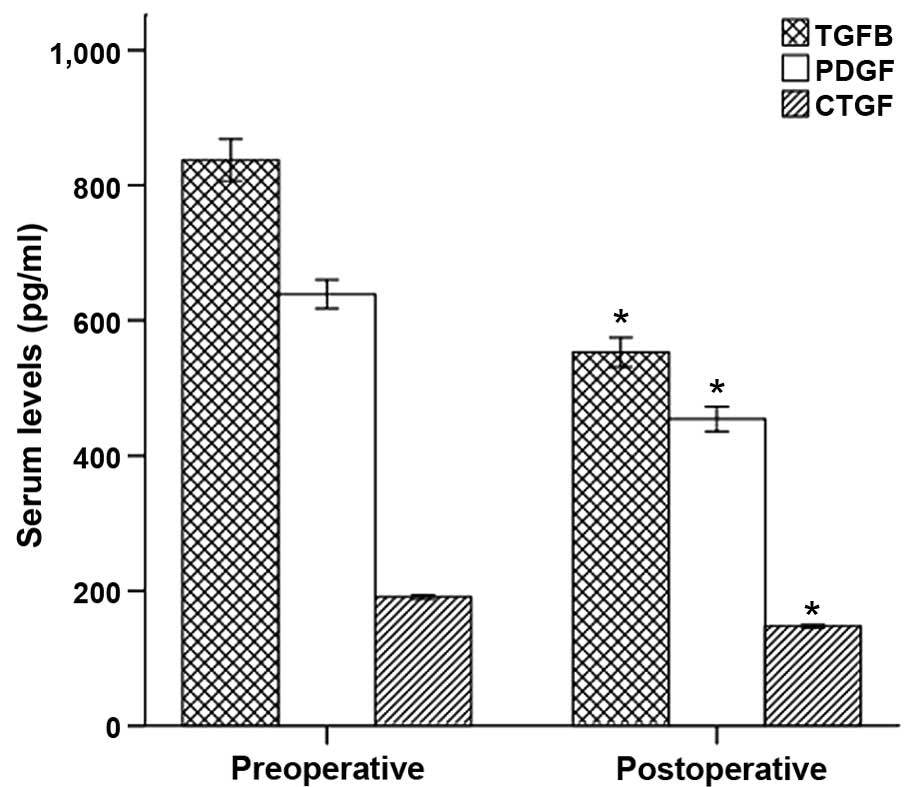 | Figure 4.TGF-β1, CTGF and PDGF concentrations
in the peripheral blood of the preoperative and postoperative
groups, determined by ELISA. TGF-β1, CTGF and PDGF concentrations
in the peripheral blood following intervention were significantly
reduced compared with the preoperative parameters (TGF-β1,
552.89±69.49 vs. 837.19±99.14; CTGF, 147.68±7.62 vs. 190.94±9.66;
and PDGF, 454.30±57.74 vs. 638.74±66.68). *P<0.001 vs. the
preoperative group. A, preoperative group; B, postoperative group;
TGF, transforming growth factor; CTGF, connective tissue growth
factor; PDGF, platelet-derived growth factor. |
Discussion
PTVE predominantly embolizes the gastric coronary
vein and short gastric vein to block the blood flow of varicose
veins and stop bleeding, which is a proven method to treat portal
hypertension and EVB (9,19). This method may increase portal vein
pressure and bleeding rate, therefore, its clinical applications
are limited. PSE intervention may decrease splenic blood flow and
return flow of the splenic vein, release the high post-hepatic
pressure, reduce portal blood flow and pressure, lower the
recurrence of esophageal and gastric variceal bleeding, and improve
symptoms of hypersplenism (20).
Previous studies have suggested that it is difficult
to return blood flow to normal levels if the splenic embolization
area is <40%, and serious adverse reactions may occur if the
embolization area is >80% (21).
Pålsson et al (22) reported
that the embolization area is associated with an increased platelet
count. In addition, Tajiri et al (23) observed that the significant increase
in postoperative red blood cell and platelet counts may be
maintained for 7.5–8 years following PSE if the degree of
embolization approaches 70%. Based upon previous results, the
present study was conducted to investigate phased joint
intervention with a small PSE area of 30–40% and a total
embolization area of 60–80%, which ensured efficacy and minimized
the incidence of complications.
In the current study, phased joint intervention led
to the immediate cessation of bleeding in 53 patients with an
emergency hemostasis rate of 100%, and led to the disappearance or
significant relief of varices. A previous study reported that
portal hemodynamics were improved and the number of WBCs and
platelets were increased significantly following the intervention
(16). The optimum range of splenic
artery embolization was determined to be 60–80%, as it did not
result in severe adverse reactions. The majority of patients
experienced low/medium fever for a short duration.
The present study consolidated upon the recent
observations regarding the efficacy of joint interventional
embolization. However, the cellular and molecular biological
mechanisms related to improvement of liver function have yet to be
elucidated.
The principal feature of hepatic fibrosis is
hypothesized to be the activation of HSCs into myofibroblasts in
the area of tissue inflammation and necrosis (24). HSCs are able to activate, synthesize
and secrete ECM components (25).
HSCs of the liver sinusoid have contractile activity similar to
that of smooth muscle cells, and have an important role in the
regulation of hepatic blood circulation (26,27). The
myofibroblast effect of activated HSCs is one of the most important
features of portal hypertension (28). We envisage that phased joint
intervention improves liver hemodynamics, thereby affecting the
biological behavior of HSCs, including their proliferation and
expression of cytokines and ECM components. The present study
further investigated the effect of Rho-ROCK expression on phased
joint interventional embolization, and explored the possible
mechanisms of the effect of embolization on liver hemodynamics.
Rho small G proteins are associated with cell
adhesion and cytoskeletal proteins, and belong to the small G
protein superfamily, serving an important role in force
transduction. Critical molecules of the Rho-ROCK signaling pathway
include Rho, ROCK and myosin phosphatase target subunit 1 (MYPT1).
ROCK receives activation signals from Rho and is phosphorylated at
multiple amino acid sites for activation (29). Activated ROCK is associated with a
number of diseases such as organ fibrosis through multiple cascade
reactions that cause biological effects such as cell contraction,
migration, adhesion, growth and division, production of stress
fibers, movement of smooth muscles, and collagen synthesis
(30,31). The specific ROCK inhibitor Y-27632 is
able to inhibit HSC activation, thus preventing rat liver fibrosis
(32,33). Y-27632 is also able to inhibit
endothelin-induced HSC contraction and formation of portal
hypertension (34).
The current study determined that mRNA expression
levels of Rho, ROCK1 and ROCK2 were higher compared with the normal
control group prior to and after phased joint embolization,
indicating that the Rho-ROCK pathway is associated with the
development of cirrhosis. mRNA expression levels of Rho, ROCK1 and
ROCK2 following embolization were lower, compared with the
preoperative levels. Protein expression levels were consistent with
the mRNA expression levels. Postoperative pMYPT1 protein expression
levels were significantly reduced, whereas tMYPT1 protein
expression levels were not significantly affected. This indicates
that phased joint embolization lowers Rho and ROCK expression
levels and affects their activation.
TGF-β1 is a critical cytokine in promoting liver
fibrosis, and is able activate and transform HSCs into
myofibroblasts, resulting in the production of ECM components and
liver fibrosis (35). CTGF is a
downstream effector of TGF-β, and mediates TGF-β to promote cell
proliferation and synthesize ECM (36,37).
Hahn et al (37) have
indicated that Rho proteins and the entire cytoskeleton have an
important role in the expression of CTGF and TGF-β in fibroblasts.
Sakata et al (14) used the
Flexercell loading unit FX-2000 to prolong the cycle of
artificially cultured human HSCs to decrease the TGF-β
concentration and mRNA expression levels. The negative mutant of
transfected Rho factor is able to inhibit stretch-induced TGF-β
synthesis, which indicates that Rho is associated with
stretch-induced TGF-β synthesis in HSCs. Mechanical force is able
to rapidly induce phosphorylation of the PDGF receptor, activation
of integrin receptors, and stretching of activated cation channels
and G proteins. The aforementioned proteins may serve as mechanical
sensors, thereby activating signal transmission pathways of growth
factors (38).
The present study revealed that TGF-β1, CTGF and
PDGF concentrations in peripheral blood after phased joint
intervention were significantly reduced in the postoperative group,
compared with preoperative levels. Furthermore, the present
findings indicated that phased joint embolization improves liver
hemodynamics, reduces mRNA expression levels of Rho and ROCK, and
inhibits the production of TGF-β1, CTGF, PDGF and other cytokines,
thus inhibiting the activation and proliferation of HSCs, thereby
improving liver function and delaying the progression of
cirrhosis.
The present preliminary study also confirmed that
joint intervention is able to treat EVB and improve hypersplenism
and liver function, effectively and safely without complications.
Phased joint embolization improves liver hemodynamics, which may
have a significant impact on the activation and proliferation of
HSCs, as well as expression levels of ECM. This effect may be
associated with the Rho-ROCK signaling pathway. However, the
present study had an insufficient number of cases to be conclusive,
with short-term follow-up and a limited assessment of the long-term
outcomes subsequent to surgery. Therefore, further studies are
required with a greater number of cases and a longer period of
follow-up.
Glossary
Abbreviations
Abbreviations:
|
PTVE
|
percutaneous transhepatic variceal
embolization
|
|
EVB
|
esophageal variceal bleeding
|
|
PSE
|
partial splenic embolization
|
|
HSCs
|
hepatic stellate cells
|
|
DSA
|
digital subtraction angiography
|
|
ROCK
|
Rho-associated coil protein kinase
|
|
CTGF
|
Connective tissue growth factor
|
References
|
1
|
Comar KM and Sanyal AJ: Portal
hypertensive bleeding. Gastroenterol Clin North Am. 32:1079–1105.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Augustin S, González A and Genescà J:
Acute esophageal variceal bleeding: Current strategies and new
perspectives. World J Hepatol. 2:261–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kovalak M, Lake J, Mattek N, Eisen G,
Lieberman D and Zaman A: Endoscopic screening for varices in
cirrhotic patients: Data from a national endoscopic database.
Gastrointest Endosc. 65:82–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Groszmann RJ, Garcia-Tsao G, Bosch J,
Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC,
Patch D, Matloff DS, et al: Beta-blockers to prevent
gastroesophageal varices in patients with cirrhosis. N Engl J Med.
353:2254–2261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merli M, Nicolini G, Angeloni S, Rinaldi
V, De Santis A, Merkel C, Attili AF and Riggio O: Incidence and
natural history of small esophageal varices in cirrhotic patients.
J Hepatol. 38:266–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Amico G, Pagliaro L and Bosch J:
Pharmacological treatment of portal hypertension: An evidence-based
approach. Semin Liver Dis. 19:475–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosch J and García-Pagán JC: Prevention of
variceal rebleeding. Lancet. 361:952–954. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Franchis R: Evolving consensus in
portal hypertension. Report of the Baveno IV consensus workshop on
methodology of diagnosis and therapy in portal hypertension. J
Hepatol. 43:167–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lunderquist A and Vang J: Sclerosing
injection of esophageal varices through transhepatic selective
catheterization of the gastric coronary vein. A preliminary report.
Acta Radiol Diagn (Stockh). 15:546–550. 1974.PubMed/NCBI
|
|
10
|
Benner KG, Keeffe EB, Keller FS and Rösch
J: Clinical outcome after percutaneous transhepatic obliteration of
esophageal varices. Gastroenterology. 85:146–153. 1983.PubMed/NCBI
|
|
11
|
Chikamori F, Kuniyoshi N, Shibuya S and
Takase Y: Correlation between endoscopic and angiographic findings
in patients with esophageal and isolated gastric varices. Dig Surg.
18:176–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
L'Herminé C, Chastanet P, Delemazure O,
Bonnière PL, Durieu JP and Paris JC: Percutaneous transhepatic
embolization of gastroesophageal varices: Results in 400 patients.
AJR Am J Roentgenol. 152:755–760. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koconis KG, Singh H and Soares G: Partial
splenic embolization in the treatment of patients with portal
hypertension: A review of the english language literature. J Vasc
Interv Radiol. 18:463–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakata R, Ueno T, Nakamura T, Ueno H and
Sata M: Mechanical stretch induces TGF-beta synthesis in hepatic
stellate cells. Eur J Clin Invest. 34:129–136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okada Y, Tsuzuki Y, Hokari R, Miyazaki J,
Matsuzaki K, Mataki N, Komoto S, Watanabe C, Kawaguchi A, Nagao S,
et al: Pressure loading and ethanol exposure differentially
modulate rat hepatic stellate cell activation. J Cell Physiol.
215:472–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Dong J, Meng W, Ma J, Wang N, Wei
J and Shi M: Effects of phased joint intervention on IL-35 and
IL-17 expression levels in patients with portal hypertension. Int J
Mol Med. 33:1131–1139. 2014.PubMed/NCBI
|
|
17
|
Tangkijvanich P, Tam SP and Yee HF Jr:
Wound-induced migration of rat hepatic stellate cells is modulated
by endothelin-1 through rho-kinase-mediated alterations in the
acto-myosin cytoskeleton. Hepatology. 33:74–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun A, Shi YJ, Xu ZD, Tian XG, Hu JH, Wang
GC and Zhang CQ: MDCT angiography to evaluate the therapeutic
effect of PTVE for esophageal varices. World J Gastroenterol.
19:1563–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hadduck TA and McWilliams JP: Partial
splenic artery embolization in cirrhotic patients. World J Radiol.
6:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sangro B, Bilbao I, Herrero I, Corella C,
Longo J, Beloqui O, Ruiz J, Zozaya JM, Quiroga J and Prieto J:
Partial splenic embolization for the treatment of hypersplenism in
cirrhosis. Hepatology. 18:309–314. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pålsson B, Hallén M, Forsberg AM and
Alwmark A: Partial splenic embolization: Long-term outcome.
Langenbecks Arch Surg. 387:421–426. 2003.PubMed/NCBI
|
|
23
|
Tajiri T, Onda M, Yoshida H, Mamada Y,
Taniai N and Kumazaki T: Long-term hematological and biochemical
effects of partial splenic embolization in hepatic cirrhosis.
Hepatogastroenterology. 49:1445–1448. 2002.PubMed/NCBI
|
|
24
|
Wang Y, Gao J, Zhang D, Zhang J, Ma J and
Jiang H: New insights into the antifibrotic effects of sorafenib on
hepatic stellate cells and liver fibrosis. J Hepatol. 53:132–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parsons CJ, Bradford BU, Pan CQ, Cheung E,
Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B, et al:
Antifibrotic effects of a tissue inhibitor of metalloproteinase-1
antibody on established liver fibrosis in rats. Hepatology.
40:1106–1115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pinzani M: Hepatic stellate (ITO) cells:
Expanding roles for a liver-specific pericyte. J Hepatol.
22:700–706. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pinzani M, Failli P, Ruocco C, Casini A,
Milani S, Baldi E, Giotti A and Gentilini P: Fat-storing cells as
liver-specific pericytes. Spatial dynamics of agonist-stimulated
intracellular calcium transients. J Clin Invest. 90:642–646. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thoen LF, Guimarães EL, Dollé L, Mannaerts
I, Najimi M, Sokal E and van Grunsven LA: A role for autophagy
during hepatic stellate cell activation. J Hepatol. 55:1353–1360.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brown JH, Del Re DP and Sussman MA: The
Rac and Rho hall of fame: A decade of hypertrophic signaling hits.
Circ Res. 98:730–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hennenberg M, Biecker E, Trebicka J,
Jochem K, Zhou Q, Schmidt M, Jakobs KH, Sauerbruch T and Heller J:
Defective RhoA/Rho-kinase signaling contributes to vascular
hypocontractility and vasodilation in cirrhotic rats.
Gastroenterology. 130:838–854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riento K, Guasch RM, Garg R, Jin B and
Ridley AJ: RhoE binds to ROCK I and inhibits downstream signaling.
Mol Cell Biol. 23:4219–4229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murata T, Arii S, Mori A and Imamura M:
Therapeutic significance of y-27632, a Rho-kinase inhibitor, on the
established liver fibrosis. J Surg Res. 114:64–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klein S, Van Beuge MM, Granzow M, Beljaars
L, Schierwagen R, Kilic S, Heidari I, Huss S, Sauerbruch T,
Poelstra K and Trebicka J: HSC-specific inhibition of Rho-kinase
reduces portal pressure in cirrhotic rats without major systemic
effects. J Hepatol. 57:1220–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawada N, Seki S, Kuroki T and Kaneda K:
Rock inhibitor y-27632 attenuates stellate cell contraction and
portal pressure increase induced by endothelin-1. Biochem Biophys
Res Commun. 266:296–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bissell DM, Roulot D and George J:
Transforming growth factor beta and the liver. Hepatology.
34:859–867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boettner B and Van Aelst L: The role of
Rho GTPases in disease development. Gene. 286:155–174. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hahn A, Heusinger-Ribeiro J, Lanz T,
Zenkel S and Goppelt-Struebe M: Induction of connective tissue
growth factor by activation of heptahelical receptors. Modulation
by Rho proteins and the actin cytoskeleton. J Biol Chem.
275:37429–37435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C and Xu Q: Mechanical stress-initiated
signal transductions in vascular smooth muscle cells. Cell Signal.
12:435–445. 2000. View Article : Google Scholar : PubMed/NCBI
|


















