Introduction
The role of positive end-expiratory pressure (PEEP)
guided by lower inflection point (LIP) in the pressure-volume (PV)
curve after recruitment maneuver (RM) in severe acute respiratory
distress syndrome (ARDS) to maintain expiratory end alveolar
recruitment is controversial. LIP may reflect the open pressure of
most alveoli at the inhalation phase, rather than alveolar closure
pressure at exhalation (1).
It was previously reported that alveolar recruitment
occurs above the inspiratory inflection point and PEEP = LIP+2–3 cm
H2O did not maintain alveolar recruitment (1–3). Point
of maximum curvature (PMC) pressure in the PV curve indicated the
initiation of alveolar closure in exhalation. Previous studies
suggested that PEEP guided by PMC was reasonable and significantly
higher than that guided by LIP, leading to significant unfavorable
impact on ventilation and blood flow as well as a higher risk of
ventilator-induced lung injury (VILI) (4–6). Airway
pressure release ventilation (APRV) involved the modification of
continuous positive airway pressure (CPAP), and had been proven to
decrease mean arterial pressure (MAP), as well as improve
hemodynamics and alveolar collapse and prevent barotrauma (7–9).
Therefore, in the present study, we established a
canine model of severe ARDS, and set high APRV pressure guided by
PMC after RM, in order to maintain stable hemodynamics and alveolar
recruitment, and improve oxygen delivery (DO2).
Materials and methods
Animal model
In total, 18 healthy dogs (8 male and 10 female)
were provided by the Laboratory Animal Center of Xuzhou Medical
College (Jiangsu, China; non-SPF). Tracheotomy was performed after
anesthesia, and intubation of trachea cannula (diameter 7.5 mm).
Volume control ventilation was performed, as well as tidal volume
(VT) 6 ml/kg, breath rate 30 times/min, inspiration
duration of 0.8 sec, inspiratory pause of 0.1 sec, fraction of
inspired oxygen (FiO2) 80%, and PEEP 5 cm H2O
(1 cm H2O=0.098 kPa).
Deep venous catheter was intubated in the right
internal jugular vein and connected with a pulse contour cardiac
output (PiCCO) instrument (Pulsion Medical Systems SE, Munich,
Germany) through a temperature detector. PiCCO arterial canal
(Pulsion Medical Systems SE) was intubated in the right femoral
artery. For the ARDS model, 0.2 ml/kg oleic acid was mixed with an
equal volume of autologous blood and pumped into vein. Arterial
blood was collected for blood gas analysis every 30 min. The dogs
with oxygenation index [partial pressure of oxygen in arterial
blood (PaO2)/FiO2] ≤100 mmHg for 30 min
constituted a successful animal model and was included in the study
(10).
The dogs received continuous intravenous injection
of 1–2 mg × kg−1 × h−1 pentobarbital sodium
for sedation. During the experiment, infusion was performed to
maintain MAP 80 mmHg (1 mmHg=0.133 kPa) and central venous pressure
(CVP) >5 cm H2O before RM.
The present study was approved by the ethics
committee of Xuzhou Medical College.
PV curve and lung RM
After the animal model became stable, a static PV
curve was plotted using a super-syringe technique, with LIP=18±1.4
cm H2O, and PMC=11±1.3 cm H2O (5). RM was performed after hemodynamics
became stable. Respiratory mode was biphasic-positive airway
pressure (BiPAP), Phigh=40 cm H2O,
Phigh=25 cm H2O, with an inspiration to
expiration ratio of 1:2, while Phigh was altered to 30
cm H2O after 90 sec (11).
Experimental groups
The dogs were randomized into 3 groups after RM:
Blip group, Plow = LIP+2 cm H2O; Bpmc group,
Plow = PMC; Apmc group, Plow = PMC. In the
APRV group, Phigh was set as PMC, with an inspiratory
duration of 4 sec, expiratory durationof 0.4 sec, and
Plow 10 cm H2O.
Measurements
Thirty seconds after RM became stable, it was set as
0 h. The changes of hemodynamics, oxygenation and DO2
were observed at 0, 1, 2 and 4 h after RM in ARDS dogs.
Hemodynamics measurements included heart rate (HR), MAP, cardiac
index (CI) and CVP. Additionally, blood gas analysis was
PaO2 for calculation of the oxygenation index.
DO2 involved DO2=10 × CI × arterial oxygen
content (CaO2), and CaO2=1.34 × blood oxygen
saturation (SaO2) × Hb+0.003 × PaO2.
Statistical analysis
SPSS 13.0 software (Chicago, IL, USA)was used for
statistical analysis. Data were presented as mean ± SD. Analysis of
variance was used for inter-group comparison. P<0.05 indicated
statistically significant results.
Results
General
Hemothorax and pulmonary edema were identified in
specimens of ARDS dogs. Furthermore, hemorrhage in the region of
proximal spine was evident although there was no pneumothorax.
There was no significant difference in the MAP, CI
and Hb between ARDS dogs when compared to before model
establishment (P>0.05) (Table I).
For PaO2/FiO2 and static lung compliance
there was a significant decrease observed following establishment
of the ARDS model when compared to values prior to model
(P<0.05). However, the differences between the 3 groups were not
statistically significant (P>0.05).
 | Table I.Measurements at baseline and in the
ARDS models. |
Table I.
Measurements at baseline and in the
ARDS models.
| Item/group | Baseline | ARDS | t | P-value |
|---|
| MAP (mmHg) |
|
|
|
|
| Blip | 144.2±8.9 | 104.8±8.2 | 7.317 | 0.000 |
| Bpmc | 136.8±5.6 | 103.6±6.6 | 8.620 | 0.000 |
| Apmc | 137.8±6.5 | 105.8±10.3 | 5.905 | 0.000 |
| F | 0.311 | 0.551 | – | – |
|
P-value | 0.739 | 0.652 | – | – |
| CI
(l/min/cm2) |
|
|
|
|
| Blip |
4.3±0.7 |
2.6±0.4 | 4.739 | 0.000 |
| Bpmc |
3.4±0.3 |
2.5±0.4 | 4.047 | 0.001 |
| Apmc |
4.7±0.6 |
2.5±0.4 | 6.856 | 0.000 |
| F | 2.409 | 0.181 | – | – |
|
P-value | 0.132 | 0.834 | – | – |
| Hb (g/l) |
|
|
|
|
|
Blip | 142±12 | 140±14 | 0.244 | 0.847 |
|
Bpmc | 148±12 | 136±15 | 1.404 | 0.155 |
|
Apmc | 144±14 | 138±15 | 0.632 | 0.557 |
| F | 1.067 | 1.313 | – | – |
|
P-value | 0.444 | 0.305 | – | – |
| Static lung
compliance (ml/cm H2O) |
|
|
|
|
|
Blip | 31.3±1.6 |
13.9±0.8a | 4.888 | 0.000 |
|
Bpmc | 34.3±1.1 |
14.9±0.8a | 24.37 | 0.000 |
|
Apmc | 32.7±2.1 |
14.7±0.8a | 18.01 | 0.000 |
| F | 0.946 | 1.410 | – | – |
|
P-value | 0.418 | 0.29 | – | – |
|
PaO2/FiO2
(mmHg) |
|
|
|
|
|
Blip | 398±10 | 90±12a | 44.31 | 0.000 |
|
Bpmc | 424±13 | 92±9a | 47.184 | 0.000 |
|
Apmc | 414±15 | 92±10a | 40.14 | 0.000 |
| F | 1.044 | 1.073 | – | – |
|
P-value | 0.382 | 0.326 | – | – |
Hemodynamics
CI in the Bpmc group at 0, 1, 2 and 4 h was
significantly lower than that before RM (P<0.05), and in the
Blip and Apmc groups (P<0.05). CI in Blip group at 0, 1, 2 and 4
h was not significantly different from that before RM. CI in the
Apmc group at 0 h was decreased, although the difference was not
significant, and CI gradually increased (Fig. 1).
HR in the Bpmc group was significantly increased
compared to that before RM and also when compared to the Blip and
Apmc groups (P<0.05), whereas HR in the Blip and Apmc groups was
not significantly altered (P>0.05). MAP in the Bpmc group was
significantly lower compared to that before RM and also when
compared to the Blip and Apmc groups (P<0.05), whereas MAP in
the Blip and Apmc groups was not significantly altered (P>0.05)
(Tables II and III).
 | Table II.Effect of APRV on MAP (mmHg) after
RM. |
Table II.
Effect of APRV on MAP (mmHg) after
RM.
| Group | Before RM | 0 h | 1 h | 2 h | 4 h | F | P-value |
|---|
| Blip | 116±14 | 107±12 | 109±13 | 109±15 | 114±12 | 0.806 | 0.453 |
| Bpmc | 114±15 |
90±12a,b |
91±13a,b |
92±14a,b |
92±11a,b | 11.942 | 0.001 |
| Apmc | 114±14 | 104±12 | 102±13 | 103±11 | 106±14 | 1.395 | 0.243 |
| F | 0.045 | 8.803 | 8.823 | 8.369 | 8.419 | – | – |
| P-value | 0.956 | 0.021 | 0.021 | 0.026 | 0.023 | – | – |
 | Table III.Effect of APRV on HR (beat/min) after
RM. |
Table III.
Effect of APRV on HR (beat/min) after
RM.
| Group | Before RM | 0 h | 1 h | 2 h | 4 h | F | P-value |
|---|
| Blip | 106±14 | 102±8 | 103±12 | 102±15 | 104±12 | 0.803 | 0.452 |
| Bpmc | 104±15 |
125±9a,b |
124±12a,b |
122±14a,b |
122±13a,b | 10.256 | 0.017 |
| Apmc | 104±14 | 114±14 | 100±14 | 98±13 | 96±14 | 1.369 | 0.234 |
| F | 0.045 | 6.803 | 6.823 | 6.369 | 6.419 | – | – |
| P-value | 0.956 | 0.034 | 0.032 | 0.038 | 0.036 | – | – |
Blood gas analysis
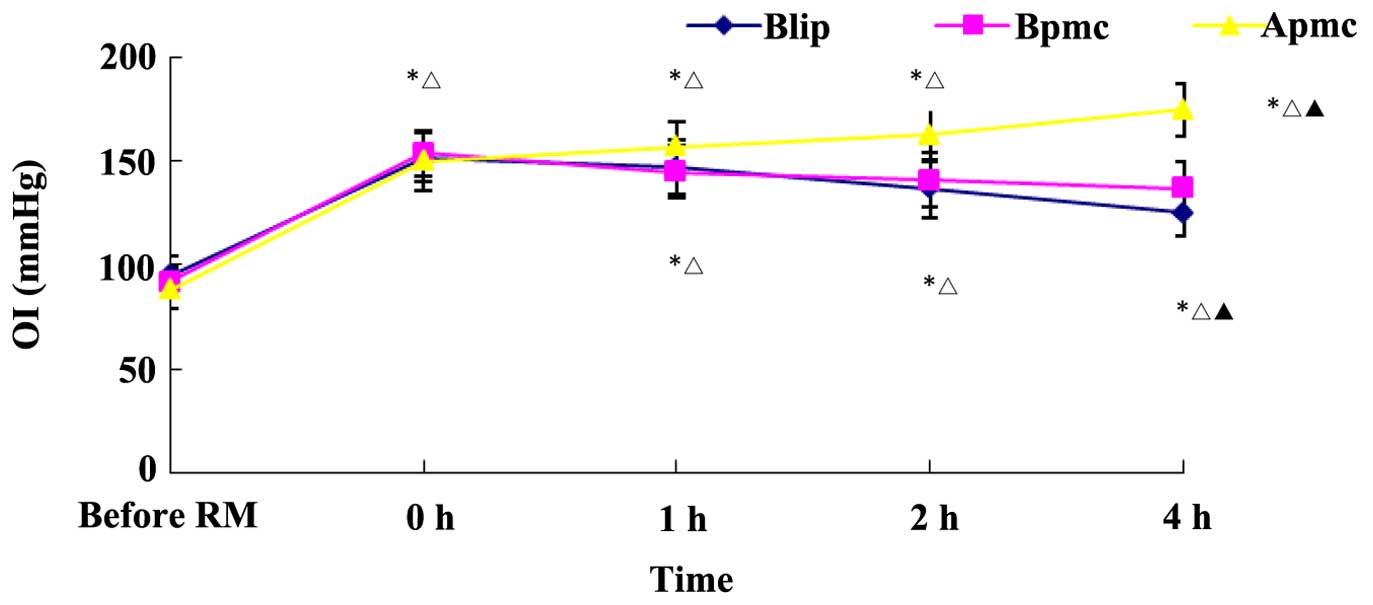
Oxygenation in the 3 groups after RM was improved
compared to before RM (Fig. 2).
Comparisons between the groups showed that the oxygenation index in
the 3 groups at 1 and 2 h was not significantly different than at 0
h (P>0.05). The oxygenation index at 0 h in the Blip group was
significantly lower than that in the Apmc and Bpmc groups
(P<0.05). The oxygenation index at 0 h in Apmc group was higher
than that in Blip and Bpmc groups (P<0.05). As shown in Table IV, PaO2 after RM in Bpmc
group was gradually increased (P<0.05), while PaO2
after RM in Blip and Apmc groups was not significantly increased
(P>0.05).
 | Table IV.Effect of APRV on PaCO2
(mmHg) after RM. |
Table IV.
Effect of APRV on PaCO2
(mmHg) after RM.
| Group | Before RM | 0 h | 1 h | 2 h | 4 h | F | P-value |
|---|
| Blip | 35±9 | 37±7 | 43±8 | 42±7 | 35±7 | 0.923 | 0.876 |
| Bpmc | 32±7 | 37±9 | 47±9a |
51±8a,b |
53±13a,b | 16.103 | 0.000 |
| Apmc | 33±9 | 36±8 | 39±8 | 43±7 | 42±7 | 2.494 | 0.342 |
| F | 0.666 | 0.708 | 1.554 | 3.421 | 6.788 | – | – |
| P-value | 0.519 | 0.471 | 0.316 | 0.218 | 0.036 | – | – |
DO2
Alteration of the DO2 ratio
(DO2 after RM/DO2 before RM) reflected
DO2 in different groups. DO2 in the Bpmc
group at 0, 1, and 4 h was significantly lower than that in the
Blip and Apmc groups, and not significantly improved compared to
before RM (Fig. 3). DO2
in the Blip and Apmc groups after RM was improved compared to that
before RM and in the Bpmc group. However, DO2 at 4 h in
the Blip group was significantly lower than that at 0 h and in the
Apmc group (P<0.05). DO2 at 4 h in the Apmc group was
higher than that at 0 h, in the Blip and Bpmc groups
(P<0.05).
Discussion
PEEP guided by LIP in the open lung approach after
RM in severe ARDS is a controversial intervention (1). Severe ARDS required RM to open
collapsed alveoli, especially for extra pulmonary ARDS. RM
increased lung volume, and improved oxygenation and lung
compliance. However, PEEP was set to maintain open alveoli and
prevent the re-collapse of alveoli after the open lung approach
(1,12,13).
Currently, most studies demonstrated that PEEP could be guided by
LIP in PV curve, PEEP=LIP+2–3 cm H2O. LIP represented
the transition component from low to high compliance in the
inspiratory PV curve, i.e., the pressure on recruitment of most
alveoli. It was reported that the point of maximum compliance in
the inspiratory PV curve was able to reflect alveolar closure
pressure indirectly. PEEP guided by LIP had the following
limitations: i) maximum compliance represented by LIP in inhale
branch indicated recruitment in most alveoli rather than end-exhale
closure; ii) PV curve in ARDS did not necessarily have LIP; and
iii) alveolar recruitment may occur above the high inflection point
of inhale branch. PEEP=LIP+2–3 cm H2O did not maintain
alveolar recruitment (14) and lung
recruitment sufficiently (1–3). In the present study, oxygenation in the
Blip group was significantly decreased 4 h after RM in comparison
to 2 h, indicating that the collapse of some recruited alveoli at 4
h after RM, leading to decreased oxygenation and
DO2.
PEEP guided by PMC was reasonable in theory. PMC in
the PV curve indicated the initiation of alveolar closure in the
exhale phase, i.e., with airway pressure decreasing, many alveoli
collapsed rapidly. PEEP set at this point may prevent alveolar
collapse in the exhale phase. However, PEEP guided by PMC was
significantly higher than that guided by LIP, leading to a more
unfavorable impact on ventilation and blood flow (15) as well as a higher risk of VILI.
Therefore, it was not applied (16,17).
Crotti et al rescued one ARDS patient with septicemia by RM,
PEEP 25 cm H2O, LIP 16–18 cm H2O (18). Xu et al showed that PEEP
guided by PMC may improve oxygenation and shunt significantly,
however, CI and even DO2 were decreased (5). In the present study, hemodynamics in
the Bpmc group was unstable, CI and MAP were decreased, and
oxygenation at 4 h after RM was decreased, although these
differences were not significant. Additionally, DO2 at
0, 1, 2 and 4 h was significantly decreased. Therefore, in the case
of PEEP simply guided by PMC, unstable hemodynamics should be
monitored to maintain DO2 and prevent increased
PCO2 or barotrauma.
APRV may recruit collapsed lung tissue, maintain
maximum and persistent alveolar recruitment as well as
hemodynamics. APRV is a modification of CPAP (7). The addition of the pressure-relief
valve in exhale branch allowed pressure control, time trigger,
pressure limit and time switch, and spontaneous breath in the
respiratory cycle (7). High pressure
maintained alveolar recruitment while low pressure facilitated
expelling CO2 and maintained open alveoli of diffusion
constant. Although MAP was increased, peak airway pressure was
decreased in order to expel CO2 and reduce barotrauma.
Transient pressure relief may decrease intrathoracic pressure and
promote right cardiac venous return. Spontaneous breath was not
only able to reduce sedative dose, but also the pressure to
intrathoracic heart and great vessels by increased airway pressure.
This may promote venous return, increase CO2 and
DO2, and improve organ perfusion (19).
In the present study, high APRV pressure in the Apmc
group was guided by PMC to avoid alveolar collapse and maintain
open lung, and further improve oxygenation; thus, oxygenation at 4
h after RM was improved. Additionally, the circulation became
stable to improve DO2. It was also able to expel
CO2 without increasing peak airway pressure.
In conclusion, high APRV pressure guided by PMC
further opened lung, improved oxygenation significantly, maintained
stable hemodynamics and improved DO2.
References
|
1
|
Richard JC, Maggiore SM, Jonson B, Mancebo
J, Lemaire F and Brochard L: Influence of tidal volume on alveolar
recruitment. Respective role of PEEP and a recruitment maneuver. Am
J Respir Crit Care Med. 163:1609–1613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cakar N, der Kloot TV, Youngblood M, Adams
A and Nahum A: Oxygenation response to a recruitment maneuver
during supine and prone positions in an oleic acid-induced lung
injury model. Am J Respir Crit Care Med. 161:1949–1956. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grasso S, Mascia L, Del Turco M, Malacarne
P, Giunta F, Brochard L, Slutsky AS and Marco Ranieri V: Effects of
recruiting maneuvers in patients with acute respiratory distress
syndrome ventilated with protective ventilatory strategy.
Anesthesiology. 96:795–802. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hickling KG: Reinterpreting the
pressure-volume curve in patients with acute respiratory distress
syndrome. Curr Opin Crit Care. 8:32–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Lei Z and Niu S: Respiratory
pressure-volume curve in open lung strategy in dog with acute
respiratory distress syndrome. Chin J Respir Crit Monit. 3:198–203.
2006.(In Chinese).
|
|
6
|
Gang L, Sun XY, Xu JQ, Zhang XL, Kou LX,
Jiang ZH and Zhang L: A comparative study between inflation and
deflation pressure-volume curve in determining the optimal positive
end-expiratory pressure. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
24:74–77. 2012.(In Chinese). PubMed/NCBI
|
|
7
|
Demirkol D, Karabocuoglu M and Citak A:
Airway pressure release ventilation: an alternative ventilation
mode for pediatric acute hypoxemic respiratory failure. Indian J
Pediatr. 77:1322–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kallet RH: Patient-ventilator interaction
during acute lung injury, and the role of spontaneous breathing:
part 2: airway pressure release ventilation. Respir Care.
56:190–203; discussion 203–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamdy HA, Gaber R and Gehan HA: Study of
cardiac and hemodynamic changes with airway pressure release
ventilation and pressure control ventilation in children with acute
respiratory distress syndrome. Eur Respir J. 40:46392012.
|
|
10
|
Grasso S, Terragni P, Mascia L, Fanelli V,
Quintel M, Herrmann P, Hedenstierna G, Slutsky AS and Ranieri VM:
Airway pressure-time curve profile (stress index) detects tidal
recruitment/hyperinflation in experimental acute lung injury. Crit
Care Med. 32:1018–1027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim SC, Adams AB, Simonson DA, Dries DJ,
Broccard AF, Hotchkiss JR and Marini JJ: Intercomparison of
recruitment maneuver efficacy in three models of acute lung injury.
Crit Care Med. 32:2371–2377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frank JA, McAuley DF, Gutierrez JA, Daniel
BM, Dobbs L and Matthay MA: Differential effects of sustained
inflation recruitment maneuvers on alveolar epithelial and lung
endothelial injury. Crit Care Med. 33:181–188; discussion 254–255.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartland BL, Newell TJ and Damico N:
Alveolar recruitment maneuvers under general anesthesia: a
systematic review of the literature. Respir Care. 60:609–620. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu L, Wang SP, Zhang NX and Qin YZ:
Effects of different levels of positive end expiratory pressure on
lung recruitment and hemodynamics after sustained inflation in
acute respiratory distress syndrome in sheep. Zhongguo Wei Zhong
Bing Ji Jiu Yi Xue. 17:679–682. 2005.(In Chinese). PubMed/NCBI
|
|
15
|
Qiu HB, Xu HY, Yang Y, Zhou SX, Chen YM
and Sun HM: Effects of positive end-expiratory pressure on lung
recruited volume and oxygenation in patients with acute respiratory
distress syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
16:399–402. 2004.(In Chinese). PubMed/NCBI
|
|
16
|
Pintado MC, de Pablo R, Trascasa M,
Milicua JM, Rogero S, Daguerre M, Cambronero JA, Arribas I and
Sánchez-García M: Individualized PEEP setting in subjects with
ARDS: arandomized controlled pilot study. Respir Care.
58:1416–1423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spieth PM and de Gama Abreu M: Lung
recruitment in ARDS: We are still confused, but on a higher PEEP
level. Crit Care. 16:1082012.PubMed/NCBI
|
|
18
|
Crotti S, Mascheroni D, Caironi P, Pelosi
P, Ronzoni G, Mondino M, Marini JJ and Gattinoni L: Recruitment and
derecruitment during acute respiratory failure: a clinical study.
Am J Respir Crit Care Med. 164:131–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daoud EG, Farag HL and Chatburn RL: Airway
pressure release ventilation: what do we know? Respir Care.
57:282–292. 2012.PubMed/NCBI
|