Introduction
Triptolide (TL), which is a diterpenoid, is the main
active component of the Tripterygium wilfordii plant.
Previous studies have shown that TL has potent pharmacological
activities, including anti-inflammation, anti-neoformative, immune
regulation and microcirculation improvement (1–3). TL has
been demonstrated to inhibit cancer cell proliferation in numerous
types of cancer, increase the sensitivity of tumor cells to
chemotherapy drugs and has also been used to treat various types of
immune diseases (4–16). Interleukin (IL)-1β is a
pro-inflammatory cytokine which is produced by mononuclear cells,
mast cells, smooth muscle cells and endothelial cells, and is
capable of activating various types of immune and inflammatory
cells (17). There are two types of
IL-1, IL-1α and IL-1β, and the bioactivities of IL-1 are
predominantly mediated by IL-1β (17). Previous studies have indicated that
patients with ulcerative colitis (UC) exhibit significantly
increased serum levels of IL-1β, as compared with healthy cohorts,
and the expression levels of IL-1β in the affected colonic mucosa
are also significantly increased, as compared with the non-affected
mucosa (18–23). Conversely, the mRNA expression levels
of IL-1β was not different between UC patients at non-active stage
and healthy cohorts (24–26). In the present study, TL was used to
treat a a 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS)-induced
mouse model of UC mouse model to observe the association between
alteration in IL-1β expression levels and UC severity. The present
study aimed to validate the therapeutic effect of TL on UC by
investigating its potential mechanism of inhibition of IL-1β
expression.
Materials and methods
Mice
A total of 70 female BALB/c mice, aged 4–6 weeks and
weighing 20–24 g were purchased from the Animal Care Facility of
Yangzhou University (Yangzhou, China). Mice were maintained in
cages at the Animal Care Facility of Nantong University (Nantong,
China) for two weeks at 22–24°C and 49% humidity with a 12/12 h
light/dark cycle. Mice were fed a mixed-feed formula and had ad
libitum access to distilled drinking water. Drinking water and
feed were fresh and changed daily. All animal experiments were
approved by the Ethics Committee for Animal Care and Use of the
Affiliated Hospital of Nantong University.
Establishment of the mouse model
Following acclimatization for two weeks, a
DSS-induced (MP Biomedicals, Santa Ana, CA, USA) mouse model was
established according to the method described by Stevceva et
al (27). A total of 70 BALB/c
female mice were randomly classified into seven equal groups and
raised in separate cages (10 mice/cage). Group A received no
treatment and was the blank control; group B, the DSS-induced
model, received 0.2 ml normal saline by intraperitoneal injection
once a day; group C was intraperitoneally injected with 0.2 ml
propylene glycol (20%) as a vehicle control once a day following
successful DSS induction; groups D (TL1), E (TL2) and F (TL3) were
intraperitoneally injected with TL (Zelang Medical Science and
Technology Co., Ltd. Nanjing, China) dissolved in 20% propylene
glycol at a daily dose of 0.2, 0.4 and 0.6 mg/kg, respectively,
following successful DSS induction; and group G was
intraperitoneally injected with dexamethasone dissolved in normal
saline at a daily dose of 0.1 mg/kg.
Disease activity index (DAI)
From the first day of DSS induction, the vigor,
activity, diet, body weight change, stool characteristics and
hematochezia condition of the mice were recorded each day. The DAI
was calculated to analyze the effects of TL on UC, as follows: DAI
score=(body weight loss score + stool characteristics score +
hematochezia score) / 3 (Table I)
(28).
 | Table I.Dextran sulfate sodium-induced
ulcerative colitis scale. |
Table I.
Dextran sulfate sodium-induced
ulcerative colitis scale.
| Score | Body weight loss
(%) | Stool
characteristic | Hematochezia |
|---|
| 0 | 0 | Normal | (−) |
| 1 | 1–5 | In-between |
|
| 2 | 5–10 | Loose | Occult blood (+) |
| 3 | 10–15 | In-between |
|
| 4 | >15 | Sparce | Gross blood (++) |
Gross morphology
Mice were sacrificed by cervical dislocation and the
intestinal cavity was exposed via a longitudinal incision along the
mesenteric junction. Following removal of the stools, the intestine
was washed several times in pre-cooled normal saline, and dried
using filter paper. The intestinal wall was spread and fixed with
pins for visual inspection of inflammation and ulcer formation.
Disease severity was evaluated according to the criteria outlined
by Ekstrom (29), as shown in
Table II. The intestinal segment
with lesions was subsequently cut into three symmetrical sections
(4 µm each) using a microtome (CM1950; Leica Microsystems GmbH,
Wetzlar, Germany). One section of the intestine was fixed in 4%
formaldehyde for pathological analysis with hematoxylin and eosin
(HE; Beyotime Institute of Biotechnology, Inc., Haimen, China)
staining or paraffin-embedded for immunohistochemical staining; the
second section was preserved at −80°C for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis; and the third section was used for protein extraction and
western blot analysis.
 | Table II.Gross morphology scoring. |
Table II.
Gross morphology scoring.
| Score | Description |
|---|
| 0 | No lesion |
| 1 | Mucous
hyperemia |
| 2 | Ulcer area <25%
affected area |
| 3 | Ulcer area = 25–50%
affected area |
| 4 | Ulcer area >50%
affected area |
Histology
Following conventional paraffin embedding and
segmentation using a microtome, tissue samples with prominent
lesions were subjected to pathological analysis via HE staining in
order to observe histological alterations. Using blinded analysis,
the samples were graded according to the criteria outlined by
Boirivant et al (30), as
shown in Table III. Samples were
independently graded by two pathologists, whom were blinded to the
scoring of one another, and the mean of the two histology scores
was calculated.
 | Table III.Histological scoring to evaluate the
severity of intestinal inflammation. |
Table III.
Histological scoring to evaluate the
severity of intestinal inflammation.
| Score | Description |
|---|
| 0 | Normal |
| 1 | Extremely low white
cell infiltration (<10% hpf) |
| 2 | Mild white cell
infiltration (10–25% hpf) |
| 3 | Moderate white cell
infiltration (25–50% hpf) with increased vascular density and
thickening of the intestine wall |
| 4 | Massive white cell
infiltration (>50% hpf) with highly increased vascular density,
intestinal crypt deformation and distortion, intestinal wall
swelling and ulceration |
ELISA
ELISA was performed using an ELISA kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. A standard linear regression curve was
obtained by plotting a standard curve of the concentrations of the
standard samples and the corresponding optical density (OD) values.
The sample concentration was calculated according to the equation
of the standard curve.
Immunohistochemistry
An immunohistochemical assay was performed according
to the manufacturer's protocol (Abcam, Cambridge, MA, USA).
IL-1β-positive cells were stained a brown/yellow color, with the
background appearing as light blue under the light microscope
(Olympus Corporation, Tokyo, Japan). All sections were observed
using an optical microscope under identical conditions. Cells with
brown or brown/yellow granular particles in the nucleus or
cytoplasm were considered as positive for IL-1β expression, whereas
those without brown/yellow particles were considered to be
IL-1β-negative cells. At ×400 magnification, cells in ≥10 visual
fields were counted for each section and the percentage of positive
cells was calculated. Cell staining intensity was graded in four
tiers as follows: 0, negative staining; 1, weak positive staining;
2, moderate positive staining; and 3, strong positive staining.
Five scales were used to grade the number of positive cells: 0, no
positive cells; 1, 1–25% positive cells; 2, 26–50% positive cells;
3, 51–75% positive cells; and 4, 76–100% positive cells.
RT-qPCR analysis
Total RNA from each mouse colon was extracted using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). The extracted
RNA was treated with DNAse I (Roche Diagnostics, Basel,
Switzerland). Following measurement of concentration, the total RNA
was stored at −80°C. Total RNA was reverse transcribed to cDNA
using a SYBR Green Supermix kit (Tiangen Biotech (Beijing) Co.,
Ltd., Beijing, China). Forward and reverse primer sequences used to
amplify IL-1β and β-actin were synthesized by the Sangon Biotech
Co., Ltd. (Shanghai, China). The primers for IL-1β (607 bp) were as
follows: Forward, 5′-AGCTGACCCTAAACAGATGA3′, and reverse,
5′-GATCTACACTCTCCAGCTGTAGC-3′. The primers for β-actin (446 bp)
were as follows: Forward, 5′-GAGACCTTCAACACCCCAGC-3′, and reverse,
5′-CCACAGGATTCCATACCCAA-3′. The mRNA expression levels of IL-1β
were analyzed by RT-qPCR using the SYBR Green Supermix kit,
according to the manufacturer's protocol. PCR was performed using a
fluorescence quantitative PCR machine (Applied Biosystems; Thermo
Fisher Scientific, Inc.), as follows: 95°C pre-degeneration for 2
min, 95°C degeneration for 15 sec, annealing at 58°C for 25 sec,
and extension at 72°C for 35 sec for 45 cycles. The fluorescent
readout was obtained at 72°C during each cycle. In order to test
the specificity of each reaction, melting curve analysis was
performed, as follows: 95°C for 15 sec, 60°C for 60 sec and 95°C
for 15 sec. The ratio of target gene to β-actin mRNA was used to
evaluate the expression levels of the target gene. The mRNA
expression levels of the target gene were normalized to those of
β-actin using the 2−ΔΔCq method.
Western blotting
IL-1β protein expression was measured by western
blot analysis. Total protein (20 µl) was extracted from intestinal
tissue samples and protein concentration was measured by UV
spectrophotometry (Shanghai Mapada Instruments Co., Ltd., Shanghai,
China). The protein was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred onto a nitrocellulose membrane (GE Healthcare
Bio-Sciences, Pittsburg, PA, USA). Following blocking for 4 h in 5%
Tris-buffered saline with Tween-20 (TBST), the membrane was
incubated with anti-mouse IL-1β primary antibody (1:2,500; cat. no.
ab200478; Abcam) at 4°C overnight. Subsequently, the membrane was
washed five times in TBST for 10 min and incubated with secondary
antibody (1:5,000; cat. no. A28175; Pierce Biotechnology; Thermo
Fisher Scientific, Inc.) for 2 h at room temperature. Bands were
visualized by enhanced chemiluminescence. Data were processed using
ImageJ version 1.36b (National Institutes of Health, Bethesda, MA,
USA), and the normalized expression level of IL-1β was measured as
the gray scale ratio of IL-1β to β-actin bands.
Statistical analysis
Windows Excel 2010 software (Microsoft Corp.,
Redmond, WA, USA) was utilized to form a database of experimental
results. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Numerical data was presented as the mean
± standard deviation and analyzed by analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
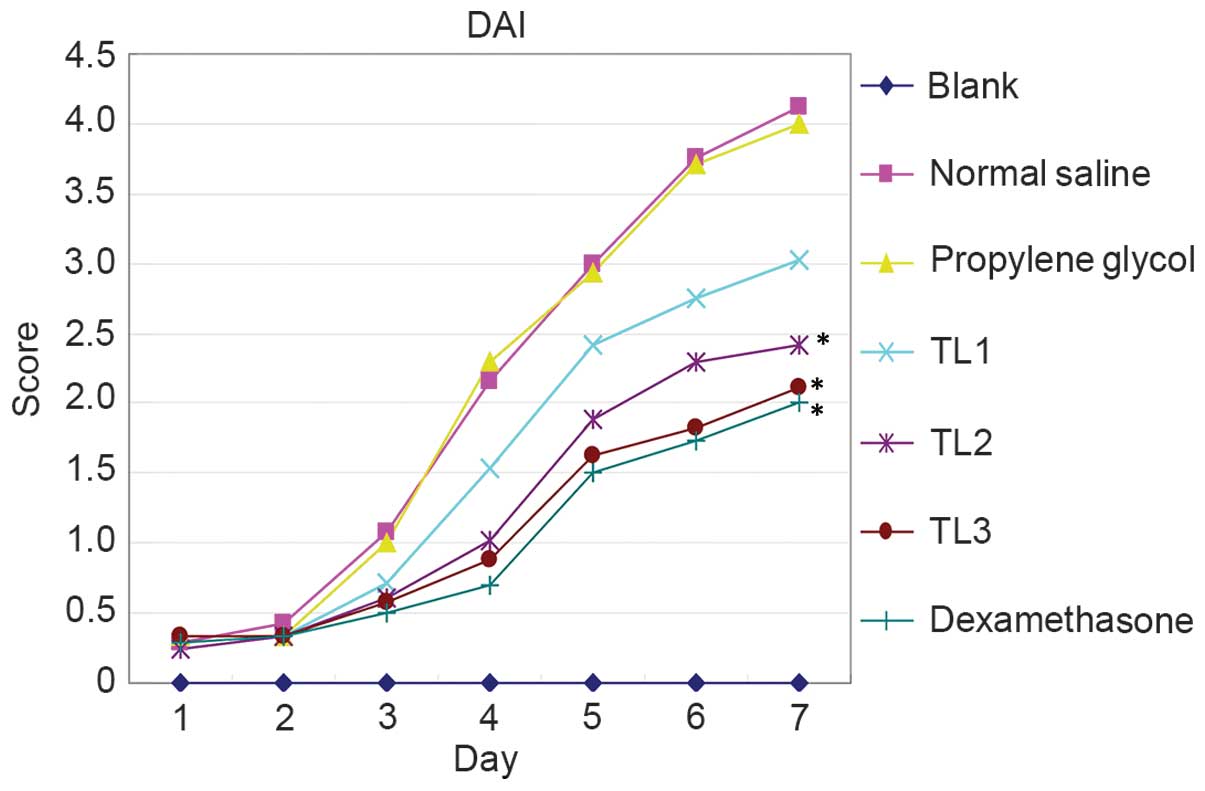
Association between DAI score and
treatment with various doses of TL
Characteristics including vigor, body weight,
activity, hair color, diet, stool property and hematochezia were
assessed from the first day of DSS induction and DAI scores were
calculated to assess the effect of TL on UC. Mice in the blank
control group exhibited shiny hair, increased body weight, normal
vigor and activity levels, and their diet and stool were normal.
From the second or third day of receiving normal saline or
propylene glycol injection, DSS-induced mice were anorexic, with
decreased activity, piloerection, darkened hair color, weight loss
and altered stool properties, including loose and soft stools.
Hematochezia or occult fecal blood were observed on the fourth day
following treatment, accompanies by prominent body weight loss and
reduced activity. One mouse in the normal saline group succumbed to
the treatment after six days. Mice in the TL2, TL3 and
dexamethasone treatment groups exhibited improved diet and body
weight, as compared with the normal saline or propylene glycol
treatment groups. In addition, the vigor and activity of mice were
improved, hair shine and color returned, stool properties returned
to normal and hematochezia and occult fecal blood were alleviated
(Fig. 1). In summary, The DAI scores
of the normal saline injection and propylene glycol injection
groups were significantly elevated as compared with the blank
control group (P<0.05); conversely, the scores for the TL and
dexamethasone treatment groups were significantly decreased
(P<0.05). The results suggest that treatment with TL and
dexamethasone may alleviate the symptoms of mouse colon ulcerative
colitis.
Gross morphology in each group
Mice in each group were scored according to the
scale outlined in Table II. The
results are presented in Table IV.
The gross morphology scores of the normal saline injection and
propylene glycol injection groups were significantly elevated as
compared with the blank control group (P<0.01), and the scores
significantly decreased in the TL and dexamethasone treatment
groups (P<0.01). No hyperemia, edema, erosion, or ulcer
formations were detected in the colonic mucosa of mice in the blank
control group. Three mice exhibited mild hyperemia of the colonic
mucosa in the distal colon. Colonic lesions were found in the anus
of mice in the normal saline and propylene glycol treatment groups,
which were gradually aggravated resulting in moderate to severe
mucosal hyperemia and edema, multiple sites of erosion and
superficial ulceration. Mild hyperemia and edema were detected in
the proximal colon. Conversely, mice that received TL or
dexamethasone treatment demonstrated markedly reduced erosion and
ulceration, and edema of the colonic mucosa was mild or moderate.
Hyperemia and edema in the proximal colon were not obvious. The
results suggest that treatment with TL and dexamethasone may reduce
gross morphology and scoring.
 | Table IV.Gross morphology score comparison
among groups. |
Table IV.
Gross morphology score comparison
among groups.
| Group (n=8) | Gross score |
|---|
| Blank control |
0.20±0.08a |
| Normal saline
treatment |
3.10±0.32 |
| Propylene
glycol |
3.02±0.28 |
| TL1 (0.2
mg/kg) |
2.86±0.24 |
| TL2 (0.4
mg/kg) |
2.24±0.20a |
| TL3 (0.6
mg/kg) |
2.06±0.20a |
| Dexamethasone |
1.82±0.16a |
Histological scoring by HE
staining
Mice in each group were scored according to the
scale outlined in Table III. HE
staining demonstrated good colonic mucosa integrity in mice of the
blank control group, with well-arranged lamina propria glands of
normal morphology. Conversely, colonic mucosal lesion, loss,
erosion and ulceration were detected in mice in the normal saline
and propylene glycol treatment groups, with extensive lymphocyte
and mononuclear cell infiltration and minimal neutrophil
infiltration into the mucosa and submucosa. Lymphoid follicles were
occasionally formed, accompanied by lamina propria gland
deformation and disordered arrangement. The severity of
inflammatory cell infiltration, ulceration, and erosion was milder
in mice that received TL or dexamethasone treatment, as compared
with the normal saline or propylene glycol treatment groups
(Table V). The histological scores
of the normal saline injection and propylene glycol injection
groups were significantly elevated as compared with the blank
control group (P<0.01). The scores significantly decreased in
the TL and dexamethasone treatment groups (P<0.01). The results
suggest that treatment with TL and dexamethasone may reduce
ulceration and erosion.
 | Table V.Histology score comparison among
groups. |
Table V.
Histology score comparison among
groups.
| Group (n=8) | Histology
score |
|---|
| Blank control |
0.75±0.10a |
| Normal saline
treatment |
3.56±0.32 |
| Propylene
glycol |
3.48±0.30 |
| TL1 (0.2
mg/kg) |
2.80±0.28 |
| TL2 (0.4
mg/kg) |
2.02±0.22a |
| TL3 (0.6
mg/kg) |
1.86±0.20a |
| Dexamethasone |
1.66±0.19a |
Serum IL-1β levels in the various
groups
Serum levels of IL-1β in the blank control, normal
saline and propylene glycol treatment groups were 42.44±8.26,
96.39±10.10 and 89.53±9.88 pg/ml, respectively. Serum IL-1β levels
were significantly increased in mice in the normal saline and
propylene glycol treatment groups, as compared with those in the
blank control group (P<0.01). Serum IL-1β levels were
69.69±7.36, 61.75±7.10 and 59.26±6.22 pg/ml for mice in the TL2,
TL3 and dexamethasone treatment groups, respectively, which was
significantly decreased, as compared with the normal saline and
propylene glycol treatment groups (P<0.01). However, the serum
level of IL-1β was 84.23±8.52 pg/ml in mice that received TL1
treatment, which was not significantly different from the normal
saline and propylene glycol treatment groups. No significant
differences in serum IL-1β levels were detected between the TL2,
TL3 and dexamethasone treatment groups. The results suggest that
treatment with TL and dexamethasone may decrease the serum levels
of IL-1β.
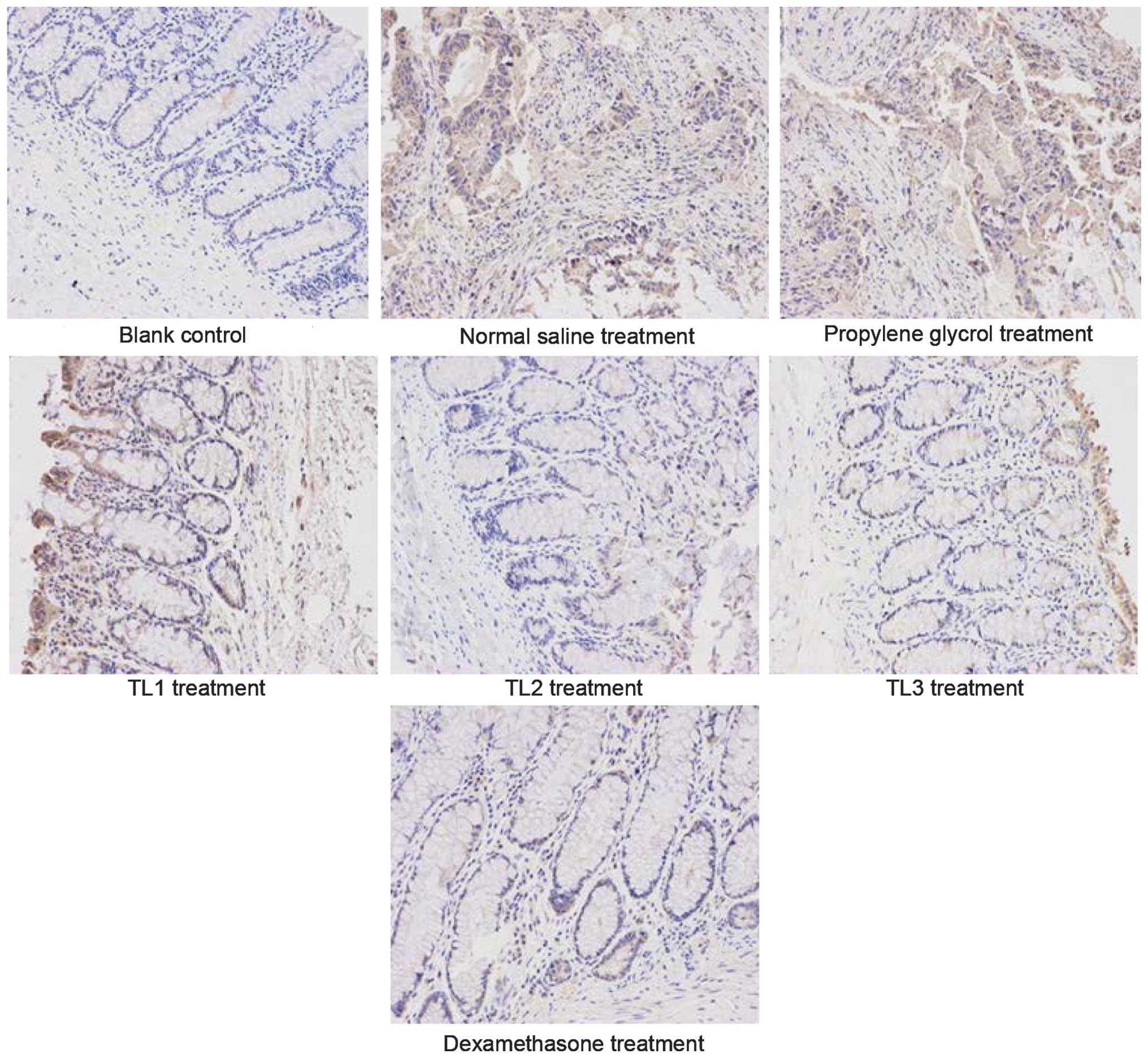
Immunohistological scores in the
various groups
Fig. 2 shows the
immunohistological images and Table
VI outlines the comparison of immunohistological scores among
the groups. As compared with the weak expression of IL-1β detected
in the colonic mucosa of mice in the blank control group, IL-1β
expression levels were significantly increased the normal saline
and propylene glycol groups (P<0.01). Furthermore, IL-1β
expression levels were significantly decreased in the TL2, TL3 and
dexamethasone treatment groups, as compared with the normal saline
and propylene glycol groups (P<0.01). No significant differences
were detected among the TL2, TL3 and dexamethasone treatment group.
The results suggest that treatment with TL and dexamethasone may
downregulate the expression of IL-1β in colon tissue.
 | Table VI.Immunohistological score comparison
among groups. |
Table VI.
Immunohistological score comparison
among groups.
| Group (n=7) | Immunohistological
score |
|---|
| Blank control |
1.00±0.00a |
| Normal saline
treatment |
3.46±0.36 |
| Propylene
glycol |
3.38±0.38 |
| TL1 (0.2
mg/kg) |
2.88±0.26 |
| TL2 (0.4
mg/kg) |
2.22±0.23a |
| TL3 (0.6
mg/kg) |
2.10±0.19a |
| Dexamethasone |
1.88±0.23a |
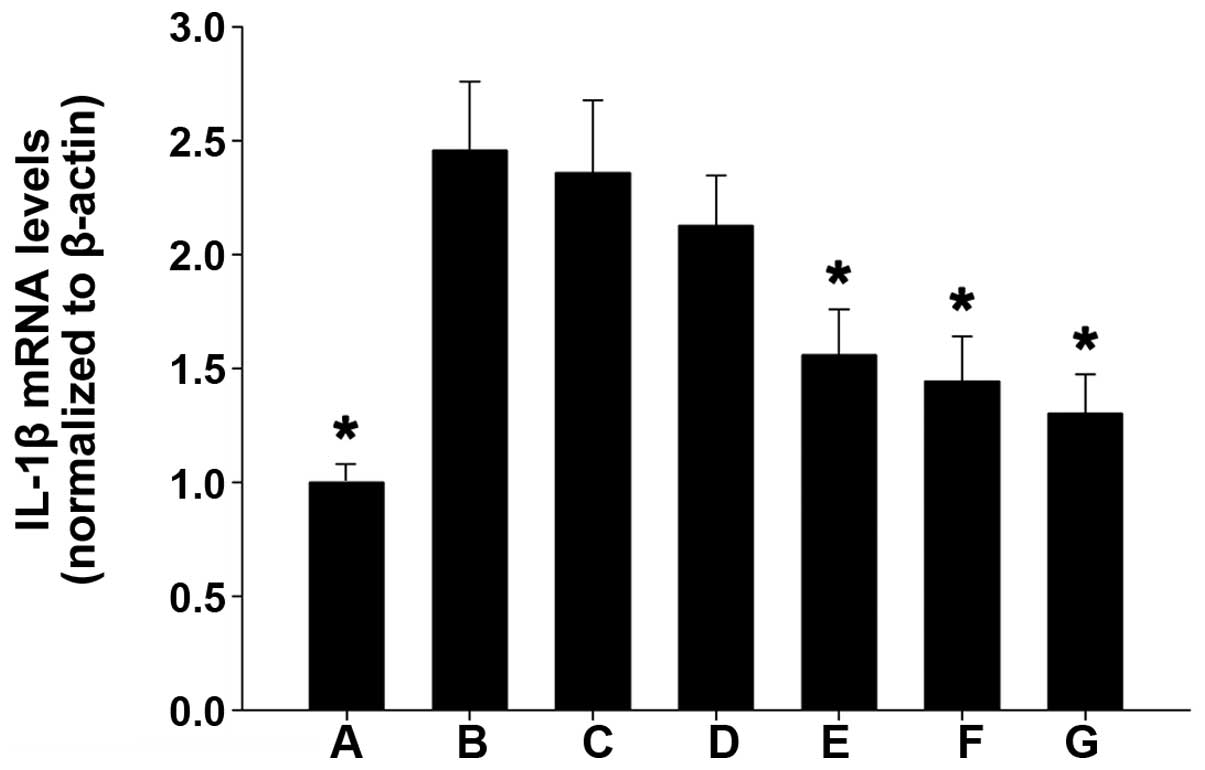
IL-1β mRNA expression levels in the
various groups
As compared with the weak IL-1β mRNA expression
detected in the colonic mucosal tissue of mice in the blank control
group, IL-1β mRNA expression was significantly increased in the
normal saline and propylene glycol treatment groups (P<0.01).
Furthermore, as compared with the blank control group, IL-1β mRNA
expression levels were markedly increased in the TL2, TL3 and
dexamethasone treatment groups, and were significantly reduced, as
compared with the normal saline and propylene glycol treatment
groups (P<0.01). No significant differences were detected among
the TL2, TL3 and dexamethasone treatment groups (Fig. 3). These results suggest that
treatment with TL and dexamethasone may inhibit IL-1β mRNA
expression in colon tissue.
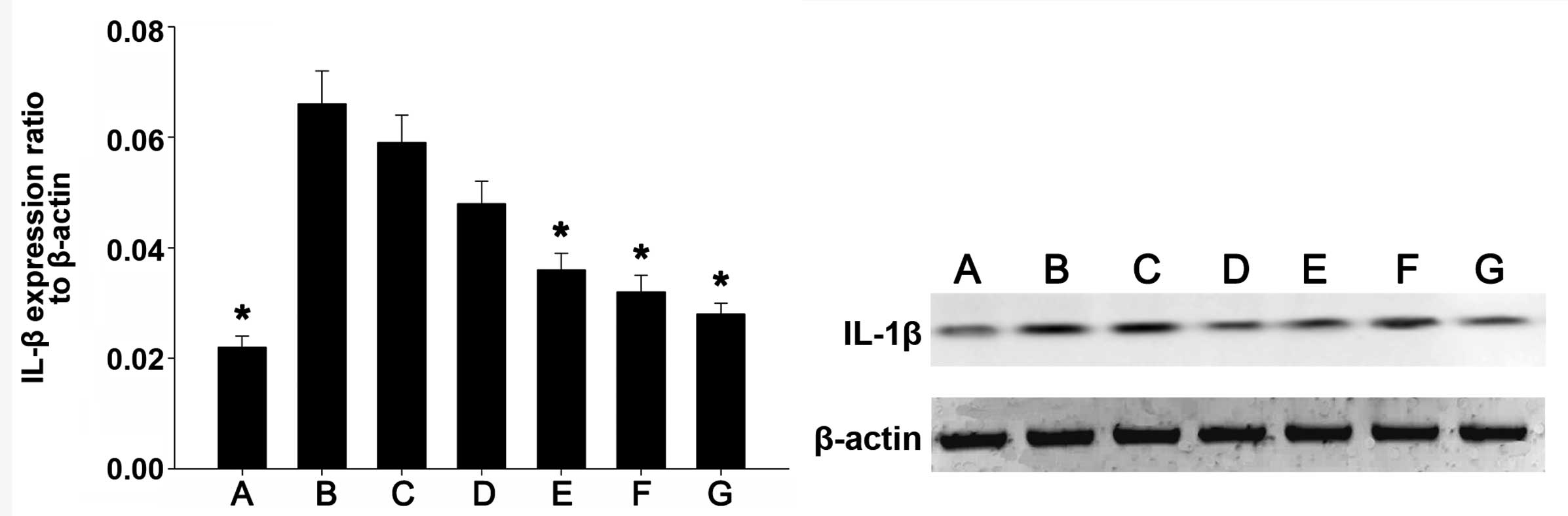
IL-1β protein expression levels in the
various groups
IL-1β protein expression was detected by western
blot analysis. Fig. 4 shows the
western blot images of IL-1β and β-actin in each group. As compared
with the weak IL-1β protein expression levels detected in the
colonic mucosa tissue of mice in the blank control group, IL-1β
protein expression levels were significantly upregulated in the
normal saline and propylene glycol treatment groups (P<0.01).
Furthermore, as compared with the blank control group, IL-1β
protein expression levels were markedly increased in the TL2, TL3
and dexamethasone treatment groups, and were significantly
decreased, as compared with the normal saline and propylene glycol
treatment groups (P<0.01). No significant differences were
detected among the TL2, TL3 and dexamethasone treatment groups. The
results suggest that treatment with TL and dexamethasone may
suppress IL-1β expression in colon tissue.
Discussion
To date, the immune regulation mechanism of TL
remains unclear; this is partially due to the broad therapeutic
profile of TL on various diseases and the complexity of the immune
system (2). Previous studies have
indicated that inhibition of T cell proliferation, induction of T
cell apoptosis, suppression of nuclear factor (NF)-κB activity,
repression of tumor angiogenesis, anti-oxidation and anti-lipid
peroxidation may be associated with the therapeutic mechanism of TL
(3–16,31).
Li et al (32)
have previously investigated the mechanism of TL attenuation of
Crohn's colitis in 10–12 week old IL-10 gene-deficient
(IL-10(−)/(−)) mice with established colitis. Following chronic TL
administration, their results indicated that TL therapy may restore
the homeostatic balance of lamina propria T cell apoptosis within
the gut, and demonstrated a novel mechanism of action for TL
therapy, which was mediated via regulation of the IL-6/signal
transducer and activator of transcription (STAT3)/suppressor of
cytokine signalling 3 signaling pathway. Another study (33) has demonstrated that TL attenuated
experimental colitis by repressing IL-17 expression via the
downregulation of the IL-6/STAT3 signaling pathway. Furthermore,
histological examination demonstrated that TL significantly reduced
the severity of colitis in a IL-10-deficient C3H/HeJBir mice.
Therefore, TL administration suppressed the IL-6/STAT3 signaling
pathway and repressed IL-17 gene expression in vivo. In
addition, treatment with 20 ng/ml TL in vitro was capable of
downregulating the IL-6/STAT3 pathway and reducing IL-17 expression
levels in cultured colonic explants from patients with Crohn's
disease (33).
In a previous study, Yu et al (34) demonstrated that TL administration
successfully ameliorated experimental colitis by inhibiting the
toll-like receptor (TLR)/NF-κB signaling pathway. TLR2 and TLR4
were upregulated in IL-10(−)/(−) mice, suggesting that TL inhibited
the TLRs/NF-κB signaling pathway in vivo. In addition, in
vitro administration of TL was able to downregulate the
TLRs/NF-κB pathway in cultured colonic explants from patients with
Crohn's disease. These results suggested that TL may have a
therapeutic effect in experimental colitis and indicated TL as a
potential therapeutic agent for the treatment of Crohn's disease.
Wei et al (35) have
previously demonstrated that TL administration for 8 weeks results
in a significant decrease in the severity of colitis, which was
accompanied by reduced expression of TNF-α, IFN-γ and IL-4 in the
colon. Serum amyloid A levels were decreased in TL-treated mice and
IL-12 and IL-23 gene expression levels in the colon were also
downregulated following treatment. Furthermore, TL administration
markedly reduced NF-κB activation in the colonic mucosa of
IL-10(−)/(−) mice. These results suggested that the efficacy of TL
treatment for the reduction of intestinal inflammation in
IL-10(−)/(−) mice may be a result of anti-inflammatory and
immunosuppressive activity.
TL showed significant protective effect on rat
colitis induced by trinitrobenzene sulfonic acid, and both low- and
high-dose TL significantly alleviated the colon lesion and improved
the histological score by reducing mRNA expression of NF-κB p65,
IL-1β, and TNF-α (36). By comparing
the effect of TL and dexamethasone on a mouse model of DSS-induced
ulcerative colitis, the authors of the present study have
previously indicated that TL may alleviate the symptoms of UC in
the colons of mice by downregulating the expression of secondary
lymphoid tissue chemokine, implying that TL may execute its
therapeutic effect on UC by inhibiting the overexpression of
secondary lymphoid tissue chemokine (37).
The results of the present study demonstrated that,
following treatment with TL, the syndromes of mouse colon
ulcerative colitis were significantly alleviated, as indicated by
significantly reduced body weight loss, DAI and gross morphology
observation and scoring. Histological observation and scores were
also improved in the TL2, TL3 and dexamethasone treatment groups,
as compared with normal saline or propylene glycol treatment, which
was consistent with the reduced ulceration and erosion,
significantly decreased serum levels of IL-1β and the downregulated
expression of IL-1β in colon tissue. IL-1β expression levels were
upregulated in mice that received TL treatment, as compared with
blank control mice, which may be due to the DSS induction of all
TL-treated mice. The present findings also indicated that TL2 and
TL3, and dexamethasone treatment had a similar therapeutic effect,
which may be associated with the inhibition of IL-1β
overexpression; suggesting that TL treatment may be a therapeutic
option for the treatment of UC.
Although the results of the present study suggested
that TL induced a therapeutic effect on ulcerative colitis through
the inhibition of IL-1β, it remains unclear whether TL is similar
to dexamethasone in its ability to inhibit multiple types of
cytokines. Future studies should focus on whether TL has a
therapeutic effect on UC that are non-responsive to hormone or
mesalazine-like medicines by inhibiting the expression of multiple
types of cytokines, which may offer an alternative treatment option
for patients with UC.
In conclusion, the present study demonstrated the
therapeutic effect of TL on UC, which may be associated with the
inhibition of IL-1β expression. These results suggested that TL may
be a novel therapeutic target for the treatment of UC, although
further studies are required in order to elucidate the underlying
mechanism.
Acknowledgements
The present study was supported by the Social
Application Research Plans Foundation of Nantong (grant no.
2012074).
References
|
1
|
Zheng Y, Zhang WJ and Wang XM: Triptolide
with potential medicinal value for diseases of the central nervous
system. CNS Neurosci Ther. 19:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kusunoki N, Yamazaki R, Kitasato H, Beppu
M, Aoki H and Kawai S: Triptolide, an active compound identified in
a traditional Chinese herb, induces apoptosis of rheumatoid
synovial fibroblasts. BMC Pharmacol. 4:2–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou GX, Ding XL, Huang JF, Zhang H, Wu
SB, Cheng JP and Wei Q: Apoptosis of human pancreatic cancer cells
induced by Triptolide. World J Gastroenterol. 14:1504–1509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krakauer T, Chen X, Howard OM and Young
HA: Triptolide attenuates endotoxin- and staphylococcal
exotoxin-induced T-cell proliferation and production of cytokines
and chemokines. Immunopharmacol Immunotoxicol. 27:53–66. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Chen T, Chen G, Li N, Wang J, Ma P
and Cao X: Immunosuppressant triptolide inhibits dendritic
cell-mediated chemoattraction of neutrophils and T cells through
inhibiting Stat3 phosphorylation and NF-kappaB activation. Biochem
Biophys Res Commun. 345:1122–1130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shao H, Ma J, Guo T and Hu R: Triptolide
induces apoptosis of breast cancer cells via a mechanism associated
with the Wnt/β-catenin signaling pathway. Exp Ther Med. 8:505–508.
2014.PubMed/NCBI
|
|
8
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-κB signalling pathway in LPS-induced acute lung
injury in a murine model. Mol Med Rep. 10:447–452. 2014.PubMed/NCBI
|
|
10
|
Kwon HY, Kim KS, An HK, Moon HI, Kim HJ
and Lee YC: Triptolide induces apoptosis through extrinsic and
intrinsic pathways in human osteosarcoma U2OS cells. Indian J
Biochem Biophys. 50:485–491. 2013.PubMed/NCBI
|
|
11
|
Ding X, Zhang B, Pei Q, Pan J, Huang S,
Yang Y, Zhu Z, Lv Y and Zou X: Triptolide induces apoptotic cell
death of human cholangiocarcinoma cells through inhibition of
myeloid cell leukemia-1. BMC Cancer. 14:2712014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Xiao E, Yuan L and Li G: Triptolide
synergistically enhances antitumor activity of oxaliplatin in colon
carcinoma in vitro and in vivo. DNA Cell Biol. 33:418–425. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei D and Huang Z: Anti-inflammatory
effects of triptolide in LPS-induced acute lung injury in mice.
Inflammation. 37:1307–1316. 2014.g. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mujumdar N, Banerjee S, Chen Z, Sangwan V,
Chugh R, Dudeja V, Yamamoto M, Vickers SM and Saluja AK: Triptolide
activates unfolded protein response leading to chronic ER stress in
pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol.
306:G1011–G1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Liu R, Yang Y, Huang Y, Li X, Liu R
and Shen X: Triptolide-induced in vitro and in vivo
cytotoxicity in human breast cancer stem cells and primary breast
cancer cells. Oncol Rep. 31:2181–2186. 2014.PubMed/NCBI
|
|
16
|
Chen Z, Sangwan V, Banerjee S, Chugh R,
Dudeja V, Vickers SM and Saluja AK: Triptolide sensitizes
pancreatic cancer cells to TRAIL-induced activation of the death
receptor pathway. Cancer Lett. 348:156–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao R, Zhou H and Su SB: A critical role
for interleukin-1β in the progression of autoimmune diseases. Int
Immunopharmacol. 17:658–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, de Zhang K, Yi WQ, Ouyang Q, Chen YQ
and Gan HT: NF-kappaB p65 antisense oligonucleotides may serve as a
novel molecular approach for the treatment of patients with
ulcerative colitis. Arch Med Res. 39:729–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dharmani P and Chadee K: Biologic
therapies against inflammatory bowel disease: A dysregulated immune
system and the cross talk with gastrointestinal mucosa hold the
key. Curr Mol Pharmacol. 1:195–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leal RF, Coy CS, Ayrizono ML, Fagundes JJ,
Milanski M, Saad MJ, Velloso LA and Góes JR: Differential
expression of pro-inflammatory cytokines and a pro-apoptotic
protein in pelvic ileal pouches for ulcerative colitis and familial
adenomatous polyposis. Tech Coloproctol. 12:33–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang YY and Ouyang Q: Expression and
significance of mucosal beta-defensin-2, TNFalpha and IL-1beta in
ulcerative colitis. Zhonghua Neike Zazhi. 47:11–14. 2008.(In
Chinese). PubMed/NCBI
|
|
22
|
Yamamoto T, Maruyama Y, Umegae S,
Matsumoto K and Saniabadi AR: Mucosal inflammation in the terminal
ileum of ulcerative colitis patients: Endoscopic findings and
cytokine profiles. Dig Liver Dis. 40:253–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szkaradkiewicz A, Marciniak R,
Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński
T and Zwoździak B: Proinflammatory cytokines and IL-10 in
inflammatory bowel disease and colorectal cancer patients. Arch
Immunol Ther Exp (Warsz). 57:291–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Førland DT, Johnson E, Saetre L, Lyberg T,
Lygren I and Hetland G: Effect of an extract based on the medicinal
mushroom Agaricus blazei Murill on expression of cytokines
and calprotectin in patients with ulcerative colitis and Crohn's
disease. Scand J Immunol. 73:66–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Incà R, Barollo M, Scarpa M, Grillo AR,
Brun P, Vettorato MG, Castagliuolo I and Sturniolo GC: Rectal
administration of Lactobacillus casei DG modifies flora
composition and Toll-like receptor expression in colonic mucosa of
patients with mild ulcerative colitis. Dig Dis Sci. 56:1178–1187.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zimmerman NP, Vongsa RA, Wendt MK and
Dwinell MB: Chemokines and chemokine receptors in mucosal
homeostasis at the intestinal epithelial barrier in inflammatory
bowel disease. Inflamm Bowel Dis. 14:1000–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stevceva L, Pavli P, Husband AJ and Doe
WF: The inflammatory infiltrate in the acute stage of the dextran
sulphate sodium induced colitis: B cell response differs depending
on the percentage of DSS used to induce it. BMC Clin Pathol.
1:32001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murano M, Maemura K, Hirata I, Toshina K,
Nishikawa T, Hamamoto N, Sasaki S, Saitoh O and Katsu K:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ekström GM: Oxazolone-induced colitis in
rats: Effects of budesonide, cyclosporin A, and 5-aminosalicylic
acid. Scand J Gastroenterol. 33:174–179. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boirivant M, Fuss IJ, Ferroni L, De
Pascale M and Strober W: Oral administration of recombinant cholera
toxin subunit B inhibits IL-12-mediated murine experimental
(trinitrobenzene sulfonic acid) colitis. J Immunol. 166:3522–3532.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu MX, Dong J, Yang YJ, Yang XL and Xu
HB: Progress in research on triptolide. Zhongguo Zhong Yao Za Zhi.
30:170–174. 2005.(In Chinese). PubMed/NCBI
|
|
32
|
Li Y, Tian Y, Zhu W, Gong J, Zhang W, Yu
C, Gu L, Li N and Li J: Triptolide induces suppressor of cytokine
signaling-3 expression and promotes lamina propria mononuclear
cells apoptosis in Crohn's colitis. Int Immunopharmacol.
16:268–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N and
Li JS: Triptolide ameliorates IL-10-deficient mice colitis by
mechanisms involving suppression of IL-6/STAT3 signaling pathway
and down-regulation of IL-17. Mol Immunol. 47:2467–2474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu C, Shan T, Feng A, Li Y, Zhu W, Xie Y,
Li N and Li J: Triptolide ameliorates Crohn's colitis is associated
with inhibition of TLRs/NF-κB signaling pathway. Fitoterapia.
82:709–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei X, Gong J, Zhu J, Niu L, Zhu W, Li N
and Li J: Therapeutic effects of triptolide on interleukin-10
gene-deficient mice with colitis. Int Immunopharmacol. 8:1808–1812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Wu S, Chen X and Peng Y:
Protective effects of tripterine on rat colitis by trinitrobenzene
sulfonic acid. Wei Chang Bing Xue. 12:144–147. 2007.(In
Chinese).
|
|
37
|
Zhang H, Zhang X, Ding X, Cao W, Qu L and
Zhou G: Effect of secondary lymphoid tissue chemokine suppression
on experimental ulcerative colitis in mice. Genet Mol Res.
13:3337–3345. 2014. View Article : Google Scholar : PubMed/NCBI
|