Introduction
Arteriovenous malformations (AVMs) are uncommon
vascular lesions that result from multiple abnormal connections
between arteries and veins (1). The
coexistence of AVMs and aneurysms has rarely been reported since
its initial description in 1942 (2,3). A
prospective study of 678 patients reported that the rate of AVMs
associated with aneurysms was 18% (4). Stapf et al (5) demonstrated that 25% of 463 patients
with AVMs had coexisting aneurysms. Despite the disease having
severe effects on the health of sufferers, the causes and
underlying mechanisms remain unknown. There exists three hypotheses
that explain the pathogenesis of the disease; however, the
consensus is that hemodynamic mechanisms serve an important role
(6). In addition, it has been
suggested that the coexistence of these two types of vascular
disease in one patient may be a coincidence or the result of a
congenital vascular malformation (7).
Patients with an AVM and an aneurysm have been shown
to have a greater risk of experiencing an intracerebral hemorrhage,
as compared with patients with an AVM or an aneurysm alone
(8,4). Thompson et al (6) reported that the rate of intracerebral
hemorrhage in patients with AVMs and aneurysms was 27–62%.
Furthermore, Brown et al (9)
demonstrated that the incidence of intracerebral hemorrhage was 7%
per year in these patients, which was 1.7% for patients with AVM
alone.
This dual vascular disease presents a management
challenge. The present study reports the case of a young pregnant
patient with combined AVM and aneurysms who presented with a
subarachnoid hemorrhage (SAH) at the 101st Hospital of Chinese
People's Liberation Army (Wuxi, China). The authors of the present
study experienced severe challenges when attempting to deal with
the giant AVM and aneurysm in terms of the technology and
management.
Case report
A 21-year-old female was admitted to Lujiang
People's Hospital (Lujiang, China) on 6th June 2014 due to a
short-term history of a severe headache, nausea and vomiting, in
the absence of an obvious cause. The patient had no previous
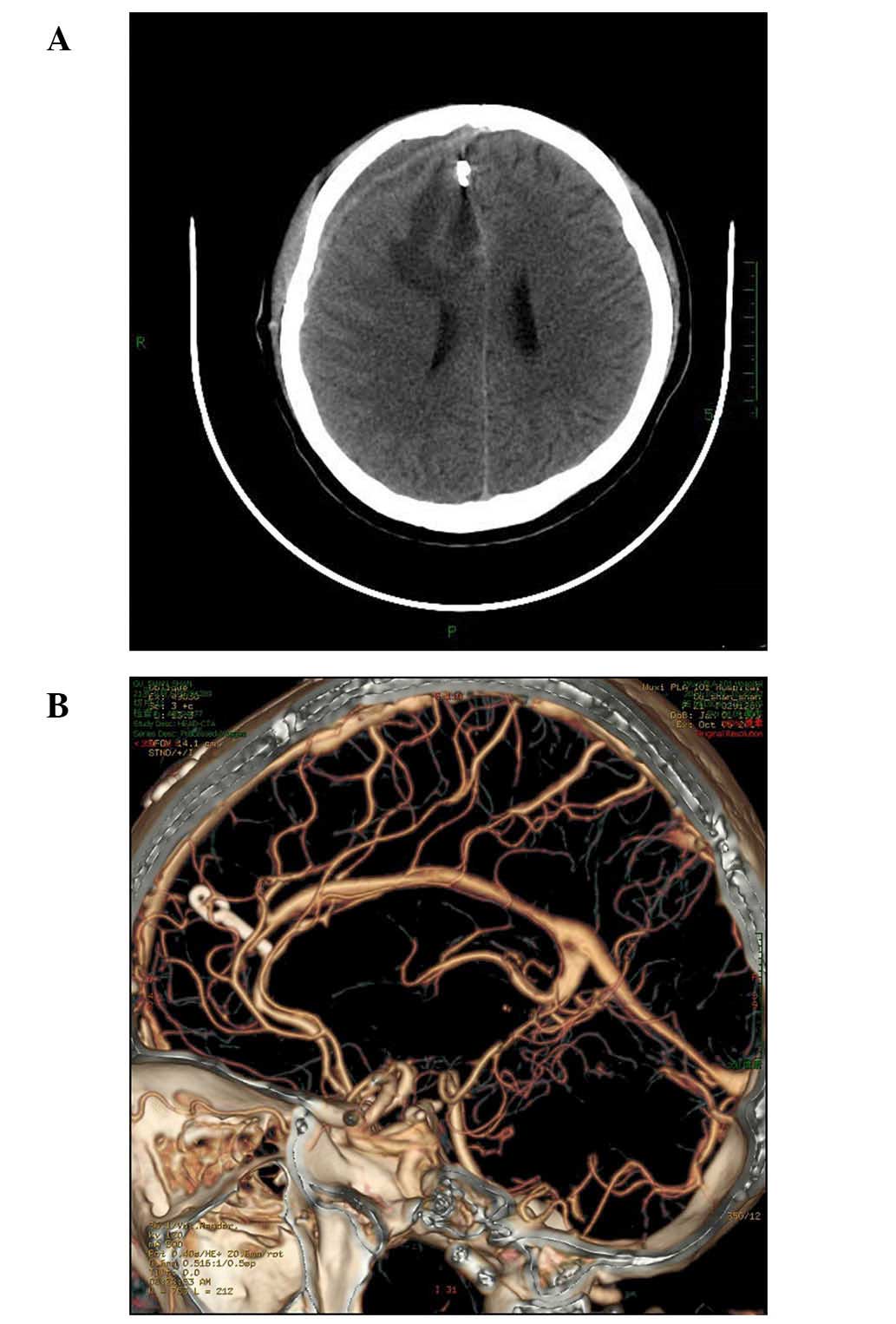
history of trauma. A computed tomography (CT) head scan revealed an
intracranial hematoma in the right frontal lobe, and a SAH
(Fig. 1). The patient was
transferred to the regional center hospital where a magnetic
resonance angiography (MRA) detected an AVM with a diameter of 5 cm
in the right corpus callosum. The nidus was fed by the branches of
the right anterior cerebral artery (ACA) and the right middle
cerebral artery (MCA). In addition, there were multiple large
cortical venous drains, one of which drained blood to the superior
sagittal sinus, and one which drained blood to the sagittal sinus.
Furthermore, a 3.0-mm enlarged blood vessel corresponding to an
aneurysm was observed, although its existence could not be
confirmed by an MRA (Fig. 2).
Digital subtraction angiography (DSA) confirmed the
existence of a large AVM located in the right corpus callosum with
multiple feeders from all branches of the ACA and MCA, and with
drainage into the cavernous sinus and transverse sinus via deep
temporal cortical veins. Furthermore, DSA clearly demonstrated an
ACA aneurysm with a diameter of 3.0 mm adjoining the AVM (Fig. 3). Since the aneurysm was adjoining
the AVM, it was difficult to evaluate the reason for the hemorrhage
using CT scans. Therefore, the AVM and aneurysm were treated
simultaneously to avoid re-bleeding. The hospital determined that
the operation was too risky and the patient was admitted to the
101st Hospital of Chinese People's Liberation Army on day 15
following initial admission. A neurological examination involving
use of the Glasgow Coma Scale (GCS) (10) detected no abnormalities (GCS score
=15), and a CT reexamination showed that the SAH and intracranial
hemorrhage blood had been absorbed.
A discussion involving neurology, neurosurgery and
interventional radiology doctors determined that interventional
vascular treatment was too risky, whereas microsurgery was
considered a relatively safe method for treatment of the patient.
The following day, the patient underwent a right pterional
craniotomy, during which the bilateral A2 segment of the ACA was
investigated using a longitudinal fissure approach to temporarily
occlude the blood flow, while protecting the venous drainage
system. The aneurysm was clipped completely after the parent
arteries, aneurysm and main feeders of the AVM had been thoroughly
explored. Subsequently, the right A3 segment was temporarily
occluded (for 15 min, followed by release for 10 min) to explore
the AVM, after which the part of the left A3 branches feeding the
AVM were also clipped. Finally, the AVM was clipped integrally.
A pathological analysis (Fig. 4), performed as previously described
(11), showed that the AVM was 5×4×4
cm (Spetzler-Martin grade 4) (12).
Postoperative control of the patient's blood pressure was important
and it was maintained below the normal (10–20 mmH2g)
(normal systolic pressure, 120–140 mmHg; normal diastolic pressure,
70–90 mmHg). The patient was discharged from the Neonatal Intensive
Care Unit on 20th June 2014 after 3 days with a GCS score of 15/15.
No nerve dysfunction was observed. A CT and CT angiography
(Fig. 5) was performed and
demonstrated that the surgery had been successful, that the
aneurysm had been clipped completely, that the AVM had been
entirely removed and that no venous drain had been injured. At the
6 month follow-up, the Glasgow Outcome Scale (13) score was 5. Informed consent was
obtained from the patient.
Discussion
The coexistence of AVMs and cerebral aneurysms has
rarely been reported. The annual incidence of spontaneous SAH is
10/100,000 worldwide, of which 75% are caused by intracranial
aneurysms (14,15). The incidence of AVM is 1.3/100,000
per year (16), and the reported
incidence of AVM coexisting with intracranial aneurysm is 18%
(4). Therefore, the incidence of
aneurysms in patients with AVMs is higher, as compared with general
patients. Furthermore, the risk of bleeding and re-bleeding are
higher in patients with AVM and aneurysm, as compared with patients
with either alone (8,4). In addition, in these patients, the risk
of experiencing a hemorrhage increases over time (4). Marks et al (17) reported that aneurysms are an
independent risk factor for re-bleeding. Therefore, it is important
that microsurgery or endovascular treatment are performed
early.
There are a number of systems used for the
classification of AVMs associated with aneurysms; however, all
systems are based on the location of the AVM relative to the
aneurysm. The most commonly used classification system at present
is that proposed by Perata et al (18). The patient in the present study was
diagnosed with a flow-related aneurysm (type 2 classification). The
position of the AVM relative to the aneurysm is important for
deciding the appropriate treatment strategy and surgical approach.
There has been significant debate regarding the most appropriate
therapeutic strategy for AVMs associated with an aneurysm (19–23).
Cunha et al (20) advised
that priority treatment should be given to the symptomatic lesion
or that both lesions should be treated simultaneously if the
condition permits. Batjer et al (22) suggested that an intracranial aneurysm
should be removed by microsurgery or endovascular embolization
prior to resection of the AVM, since it may avoid rupturing the
aneurysm during the surgery to remove the AVM. Conversely,
Koulouris and Rizzoli (23) insisted
that the AVM be removed first. Since numerous scholars consider
that hemodynamic mechanisms serve an important role in the
pathogenesis of the disease, the removal of AVM may lead to various
hemodynamic changes causing the aneurysm to disappear spontaneously
(6,7,9,14,20). In
the present case, the aneurysm and AVM were adjacent and the
feeding artery of the AVM was the parent artery of the aneurysm.
Therefore, the AVM and aneurysm could be removed simultaneously in
the same operation, according to Cunha et al (20). However, the aneurysm was clipped
prior to removing the AVM in order to reduce the risk of the
aneurysm rupturing during surgery.
At present, three treatment strategies have been
described for patients with coexisting AVM and aneurysm, including
microsurgery, endovascular embolization and radiotherapy. Factors,
such as the size and location of the AVM, venous drainage, hospital
equipment and the technique level of the surgeon, will determine
the requirement for treatment and the optimum therapeutic strategy.
In the present study, microsurgery was used to remove the AVM and
clip the aneurysm entirely. As a result, the prognosis of the
patient was good and the intracranial hematoma was cleared.
However, if the lesions had been deeply seated or located within an
important functional area, microsurgery treatment may not have been
the ideal choice (21).
Previous studies have reported that embolization has
a number of advantages, including minor damage to brain tissue,
fewer symptoms, effectiveness, rapid recovery and a short hospital
stay (24–26). A previous study used gamma knife
radiotherapy for treatment (27);
however, it has a long treatment cycle, its effectiveness has not
been well documented and re-bleeding may occur (28,29). The
present study describes a young pregnant woman with a large AVM,
wide draining veins and a high risk of endovascular complications.
Therefore, microsurgery was selected for treatment of the patient.
A CTA reexamination demonstrated that the operation was successful;
the AVM had been removed completely and the aneurysm was
clipped.
Previous studies have not reported particular
technical difficulties associated with the removal or embolization
of giant AVMs coexisting with an aneurysm. However, it is important
to protect the draining veins and arteries supplying blood to the
brain tissue via the AVM. Furthermore, it is important that the
intraoperative and postoperative blood pressure of the patient is
maintained relatively low in order to reduce the risk of
re-bleeding.
In conclusion, AVMs associated with intracranial
aneurysms are a complex lesion, and the treatment is dependent on
the relative location of each lesion and its classification. In the
present study, a patient with a type 2 AVM and associated aneurysm
underwent aneurysm clipping, followed by resection of the AVM in a
single operation, and showed a good clinical outcome.
References
|
1
|
Richter GT and Friedman AB: Hemangiomas
and vascular malformations: current theory and management. Int J
Pediatr. 2012:6456782012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halim AX, Singh V, Johnston SC, Higashida
RT, Dowd CF, Halbach VV, Lawton MT, Gress DR, McCulloch CE and
Young WL: UCSF BAVM Study Project. Brain Arteriovenous
Malformation: Characteristics of brain arteriovenous malformations
with coexisting aneurysms: A comparison of two referral centers.
Stroke. 33:675–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walsh FB and King AB: Ocular signs of
intracranial saccular aneurysms: Experimental work on collateral
circulation through the ophthalmic artery. Arch Ophthalmol.
27:1–33. 1942. View Article : Google Scholar
|
|
4
|
da Costa L, Wallace MC, Ter Brugge KG,
O'Kelly C, Willinsky RA and Tymianski M: The natural history and
predictive features of hemorrhage from brain arteriovenous
malformations. Stroke. 40:100–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stapf C, Mohr JP, Pile-Spellman J, Sciacca
RR, Hartmann A, Schumacher HC and Mast H: Concurrent arterial
aneurysms in brain arteriovenous malformations with haemorrhagic
presentation. J Neurol Neurosurg Psychiatry. 73:294–298. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson RC, Steinburg GK, Levy RP and
Marks MP: The management of patients with arteriovenous
malformations and associated intracranial aneurysms. Ncurosurgery.
43:202–211; discussion 211–212. 1998. View Article : Google Scholar
|
|
7
|
Shen CC and Wang YC: Surgical management
of intraeranial arteriovenous malformation associated with
aneurysms. Zhonghua Yi Xue Za Zhi (Taipei). 61:8–16. 1998.(In
Chinese). PubMed/NCBI
|
|
8
|
Wilkins RH: Natural history of
intracranial vascular malformations: A review. Neurosurgery.
16:421–430. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown RD Jr, Wiebers DO and Forbes GS:
Unruptured intracranial aneurysms and arteriovenous malformations:
Frequency of intracranial hemorrhage and relationship of lesions. J
Neurosurgery. 73:859–863. 1990. View Article : Google Scholar
|
|
10
|
Jennett B, Teasdale G, Braakman R,
Minderhoud J and Knill-Jones R: Predicting outcome in individual
patients after severe head injury. Lancet. 1:1031–1034. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin NA and Vinters HV: Arteriovenous
malformations. Neurovascular Surgery. Carter LP, Spetzler RF and
Hamilton MG: McGraw-Hill. (New York, NY). 875–903. 1995.
|
|
12
|
Spetzler RF and Martin NA: A proposed
grading system for arteriovenous malformations. J Neurosurg.
65:476–483. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Changaris DG, McGraw CP, Richardson JD,
Garretson HD, Arpin EJ and Shields CB: Correlation of cerebral
perfusion pressure and Glasgow Coma Scale to outcome. J Trauma.
27:1007–1013. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steiner T, Juvela S, Unterberg A, Jung C,
Forsting M and Rinkel G: European Stroke Organization: European
stroke organization guidelines for the management of intracranial
aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 35:93–112.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Komotar RJ, Schmidt JM, Starke RM,
Claassen J, Wartenberg KE, Lee K, Badjatia N, Connolly ES Jr and
Mayer SA: Resuscitation and critical care of poor-grade
subarachnoid hemorrhage. Neurosurgery. 64:397–410; discussion
410–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gabriel RA, Kim H, Sidney S, McCulloch CE,
Singh V, Johnston SC, Ko NU, Achrol AS, Zaroff JG and Young WL:
Ten-year detection rate of brain arteriovenous malformations in a
large, multiethnic, defined population. Stroke. 41:21–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marks MP, Lane B, Steinberg GK and Chang
PJ: Haemorrhage in intracerebral arteriovenous malformations:
Angiographic determinants. Radiology. 176:807–813. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perata HJ, Tomsick TA and Tew JM Jr:
Feeding artery pedicle aneurysms: Association with parenchymal
hemorrhage and arteriovenous malformation in the brain. J
Neurosurg. 80:631–634. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoh BL, Putman CM, Budzik RF and Ogilvy
CS: Surgical and endovascular flow disconnection of intracranial
pial single-channel arteriovenous fistulae. Neurosurgery.
49:1351–1363; discussion 1363–1364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cunhae Sa MJ, Stein BM, Solomon RA and
McCormick PC: The treatment of associated intracranial aneurysm and
arteriovenous malformations. J Neurosurg. 77:853–859. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Passacantilli E, Pichierri A, Guidetti G,
Santoro A and Delfini R: Surgical treatment of pial cerebellar
arteriovenous fistulas with aneurysm of the main feeding artery.
Surg Neurol. 65:90–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Batjer H, Suss RA and Samson D:
Intracranial arteriovenous malformations associated with aneurysms.
Neurosurgery. 18:29–35. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koulouris S and Rizzoli HV: Coexisting
intracranial aneurysm and arteriovenous malformations: Case report.
Neurosurgery. 8:219–222. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ibrahim T and Brophy BP: Subarachnoid
haemorrhage in a patient with a left temporal arteriovenous
malformation and an associated anterior communicating artery
aneurysm. J Clin Neurosci. 18:1414–1416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kouznetsov E, Weill A, Ghostine JS,
Gentric JC, Raymond J and Roy D: Association between posterior
fossa arteriovenous malformations and prenidal aneurysm rupture:
Potential impact on management. Neurosurg Focus. 37:E42014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meisel HJ, Mansmann U, Alvarez H, Rodesch
G, Brock M and Lasjaunias P: Cerebral arteriovenous malformations
and associated aneurysms: Analysis of 305 cases from a series of
662 patients. Neurosurgery. 46:793–800; discussion 800–802. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao E, Young WL, Pile-Spellman J, Joshi S,
Duong H, Stieg PE and Ma Q: Cerebral arteriovenous malformation
feeding artery aneurysms: A theoretical model of intravascular
pressure changes after treatment. Neurosurgery. 41:1345–1356;
discussion 1356–1358. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grady C, Tanweer O, Zagzag D, Jafar JJ,
Huang PP and Kondziolka D: Delayed hemorrhage from the tissue of an
occluded arteriovenous malformation after stereotactic
radiosurgery: Report of 3 cases. J Neurosurg. 10:1–6. 2016.
View Article : Google Scholar
|
|
29
|
Lv X and Li Y: The clinical
characteristics and treatment of cerebral AVM in pregnancy.
Neuroradiol J. 28:385–388. 2015. View Article : Google Scholar : PubMed/NCBI
|