Introduction
Mesenchymal stem cells (MSCs) originate from
mesoderm and can differentiate into three germ layers. MSCs are
widely used in cell engineering research. They are mainly derived
from bone marrow, and aer also found in fat in limited numbers
(1,2). Factors such as susceptibility to viral
infections and strong immunogenicity limits their clinical
applications (3).
Recent findings have shown that MSCs from human
umbilical cord have advantages such as large numbers, strong
proliferation and differentiation capacity and low immunogenicity
(4) compared to MSCs in the bone
marrow. MSCs originating from bone marrow differentiated into
hepatocytes in partially hepatectomized models (5). However, there are few reports on
whether human umbilical cord MSCs are capable of surviving and
differentiating into hepatocyte-like cells in partially
hepatectomized model rats.
In the present study, labeled human umbilical cord
MSCs were transplanted into partially hepatectomized model rats,
and the possibility of differentiating into hepatocytes in this
regeneration environment of liver cells was examined.
Materials and methods
Main reagents
Reagents used were: Dulbecco's modified Eagle's
medium (DMEM/F12; HyClone, Logan, UT, USA), fetal bovine serum
(FBS; Gibco, Grand Island, NY, USA), trypsin (Solarbio, Beijing,
China), PKH26 staining solution (Sigma, St. Louis, MO, USA), mouse
anti-human albumin antibody (Dako, Glostrup, Denmark), and
FITC-labeled double-antibody (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China).
After written informed consent was obtained from the
family or relatives of the patient, umbilical cord was collected
from full term cesarean section under strict sterile
conditions.
Experimental animals
Clean 6-week-old Sprague-Dawley female rats were
purchased from the Guangdong Experimental Animal Center [license
no. SCXK (Guangdong) 2008-0002]. Approval for the study and use of
the animals was obtained from the ethics committee of Xiangyang
Hospital (Hubei, China).
Isolation, cultivation and
proliferation of umbilical cord MSCs (UC-MSCs)
Four to six centimeters of healthy fetal umbilical
cord was collected under strict sterile conditions and washed with
PBS. The residual blood of the umbilical vein and the umbilical
artery were rinsed off and the outer membrane and vascular tissues
were removed. The umbilical cord was dissected into approximately 1
mm3 tissue blocks, and placed into the collagenase, the
mass fraction of which was 0.1%. After 20-h digestion at 37°C, the
solution was filtered through a 100 mesh strainer and the filtrate
with cells was collected. Subsequently, the filtrate was
centrifuged at 290 × g at 37°C for 10 min at room temperature, and
the supernatant was discarded to retain the precipitate. The
precipitate was washed twice with PBS and inoculated with a cell
density of 1×106/ml in a T-75 plastic culture flask and
cultivated in DMEM/F12 culture medium [comprising 10% (v/v) FBS,
100 µ/ml penicillin, 100 µ/ml streptomycin] under a saturated humid
environment at 37°C. After 4–5 days, 5% (v/v) of the solution was
initially altered. The non-adherent cells were discarded and the
medium was changed every 2–3 days. When the cell fusion was up to
80%, the cells were digested with 0.25% (v/v) of trypsin for 5 min.
The cells at ratio of cell passage was 1:2 and were continued to
cell expansion and cultivation.
Cell phenotype using flow cytometry
(FCM)
Fifth generation of cells with stable proliferation
were taken, and digested with 0.25% (v/v) of trypsin. PBS solution
was used to wash cells twice and each tube was adjusted to 0.1 ml
with the cell density of 1×106/ml. Subsequently, mouse
anti-human monoclonal antibodies, CD29-FITC (cat. no.: 032041-M35
with a dilution of 1:200), CD13-FITC (cat. no.: 032041-M19 with a
dilution of 1:500), CD44-FITC (cat. no.: 032041-M48 with a dilution
of 1:500), CD31-FITC (cat. no.: 032041-M37 with a dilution of
1:500), CD106-FITC (cat. no.: 032041-M78 with a dilution of 1:200),
CD45-FITC (cat. no.: 032041-M52 with a dilution of 1:500) were
added and incubated at 4°C. The cells were washed with PBS once,
and detected using Cytomics™ FC500 FCM (Beckman Coulter, Brea, CA,
USA), followed by analysis with Cytometer 1.0 software (Frederick,
MD, USA).
Fluorescence-labeled UC-MSCs in
vitro
Cells were digested into single cell suspension.
Cells (1×107) were centrifuged at 500 rpm for 5 min to
form a loose cell mass. The supernatant was discarded and the rest
was added into 1 ml dilution C to resuspend the cells.
Subsequently, the PKH26 dye solution that was diluted by dilution C
was added to make a final concentration of 2 mmol/l. The cells were
mixed with the dye solution, and incubated at 25°C for 5 min. The
same volume of serum was then added to terminate the reaction. The
same volume of serum-containing culture solution was added to
dilute the solution, and centrifuged for 10 min at 100 × g,
followed by washing 3 times. Then, 2×106/ml of cell
suspension was formed with culture medium and cell staining was
observed under a fluorescence microscope (Thermofisher, Beijing,
China).
Development of the partially
hepatectomized rat model
Six-week-old Sprague-Dawley rats were anesthetized
by injecting 2% (35 mg/kg) pentobarbital in the abdominal cavity.
The rats were placed on a sterile operating table in a supine
position, followed by disinfection of the abdomen skin with alcohol
prior to dissecting the abdominal cavity. Subsequently, the thorax
of the rat was gently squeezed to visualize the liver clearly and
the liver lobe was double ligated at the hepatic pedicle of the
diaphragmatic lobe of the liver. The hepatic vein was cut and the
portal vein was separated to inject slowly the 0.5 ml cell
suspension (approximately 1×106 cells), already marked
with the staining solution, PKH26, by using a 1 ml syringe. The
bleeding was quickly stopped by pressing, and spraying a small
amount of penicillin solution into the abdominal cavity, after
which it was sutured. Once the rat recovered from the anaesthesia,
regular feeding was continued.
Observation of the sliced liver and
staining using albumin immunofluorescence
After cell transplantation, parts of the liver were
dissected during the subsequent three weeks. The sections (5 µm)
were frozen, and observed by fluorescence microscopy to confirm
whether there were any red-labeled cells in the liver.
Subsequently, the anti-albumin antibody (cat. no.: K08531 with a
dilution of 1:100) was added for incubation at 4°C overnight. The
following day, tissues were warmed briefly at 37°C for 30 min,
followed by washing twice with PBS. FITC-labeled secondary
antibodies were added at 37°C for 1 h, and washed 3 times with PBS.
The sections were mounted with glycerol and observed immediately
under a fluorescence microscope.
Results
Isolation, cultivation and morphologic
observation of human umbilical cord MSCs
The single cells obtained by collagenase digestion
began to adhere within 24 h in primary culture. After 5 days, the
majority of cells presented the phenomenon of adherence, and most
of the cells were of diamond shape (Fig.
1A). Each 2 days, the cells were passaged once, and thereafter
the cells proliferated rapidly and the number of passages went up
to 20 generations. After the passaging, the cells were of high
purity, uniform shape, and grew in a spiral shape (Fig. 1B).
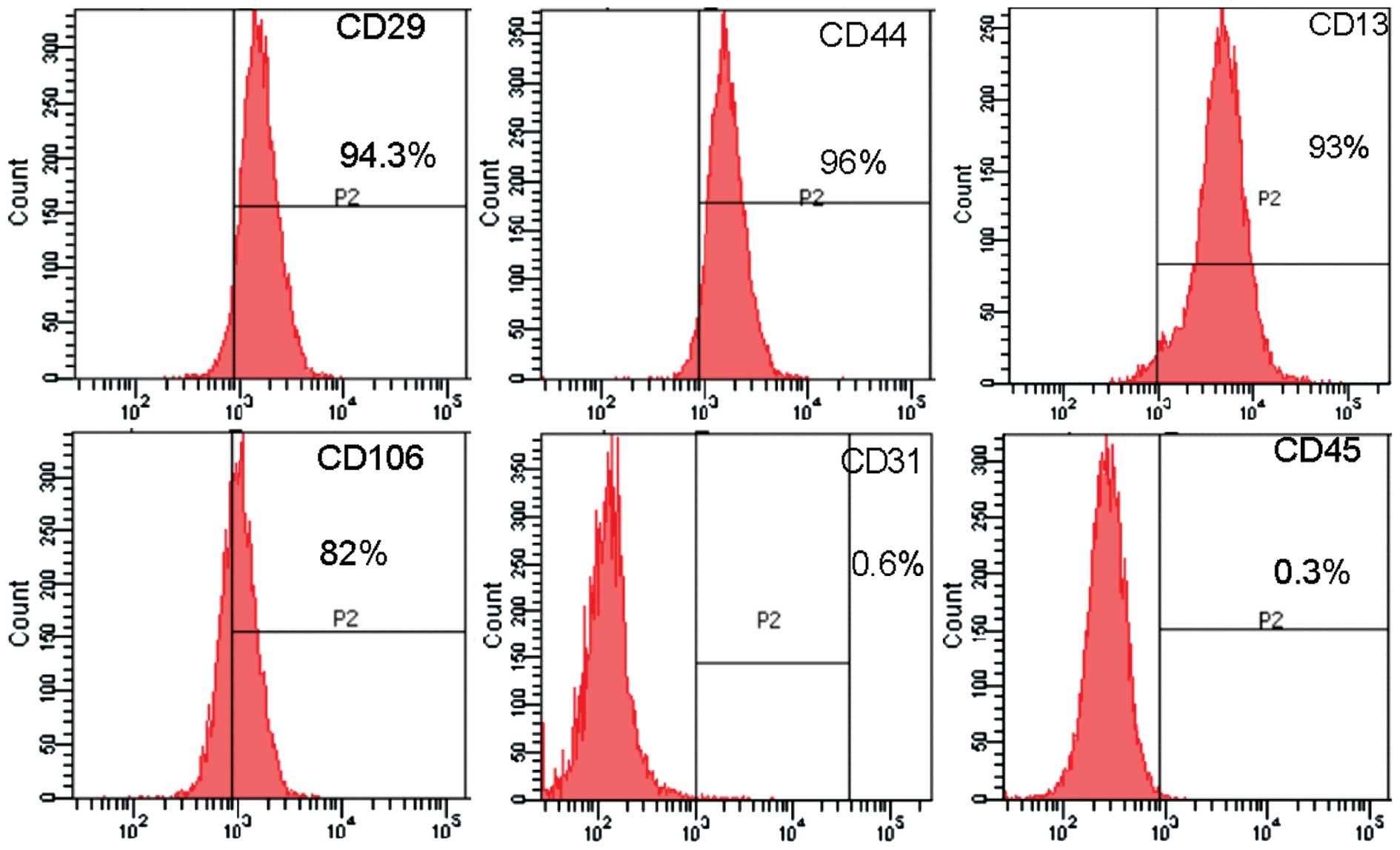
Cell phenotype analysis of human
umbilical cord MSCs
Using FCM, the cells showed stromal markers and
adhesion molecules CD29, CD44, CD13, and indicated a low expression
of CD106. By contrast, the cells did not show any sign of
endothelial cell marker CD31 and hematopoietic stem cell flag CD45
(Fig. 2), indicating that these
cells had features of stem cells, which was in line with the
requirements of this experiment.
Observation of in vitro PKH26 staining
of the human umbilical cord MSCs
After staining, the marker was distinguished for 22
h (Fig. 3A), at 45 h (Fig. 3B) and observed in the suspended state
(Fig. 3C),. The red fluorescence
marked in the human umbilical cord MSCs was observed under
fluorescence microscopy. No significant difference was observed in
cell growth, morphology and function after passaging between the
labeled and unlabeled cells.
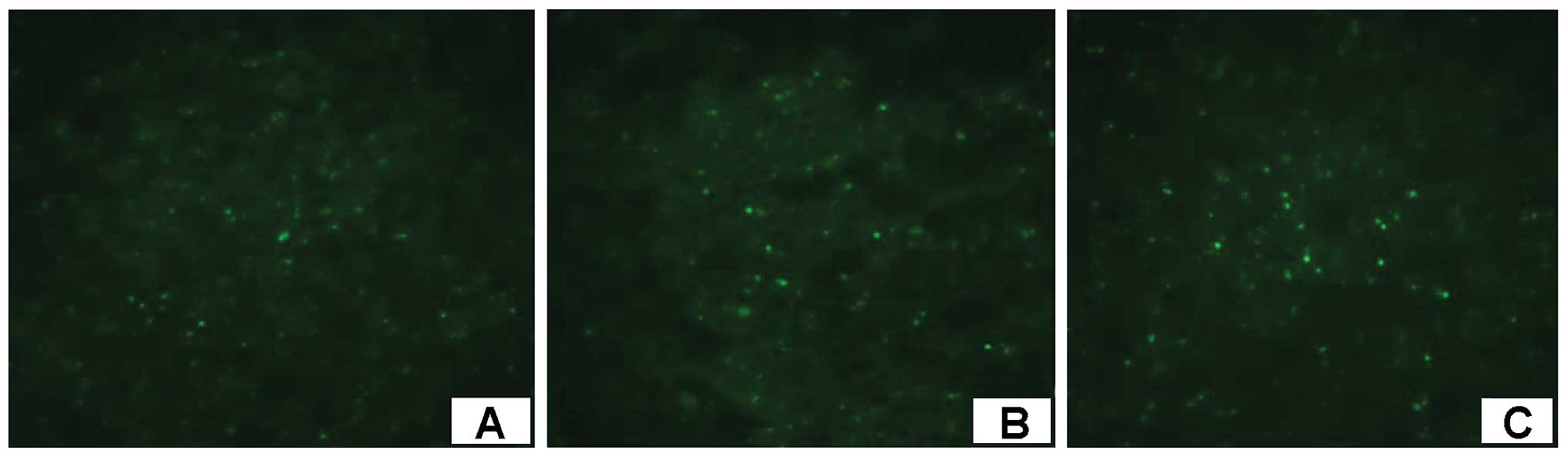
Positioning of MSCs in the liver and
determining the expression of cell albumin by utilizing
immunofluorescence
Rats were sacrificed in the first, second, and third
week, after which the abdominal cavity was opened to observe the
liver. There were clear and broad adhesions between the liver and
surrounding tissues, the surface of the liver was uneven, and at
the ligature where part of the liver was cut, the tissue was firm
or hard. The rat liver was dissected to make frozen sections and
the position of the labeled cells in liver was observed under the
fluorescence microscope. The tagged red fluorescence cells were
scattered in the liver and some were embedded in the liver panel
(Fig. 4A-C). Due to cell
differentiation, the red fluorescence gradually faded and after
immunofluorescence staining, labeled cells with albumin staining
were detected as positive, and excited green fluorescence (Fig. 5A-C), indicating that the human
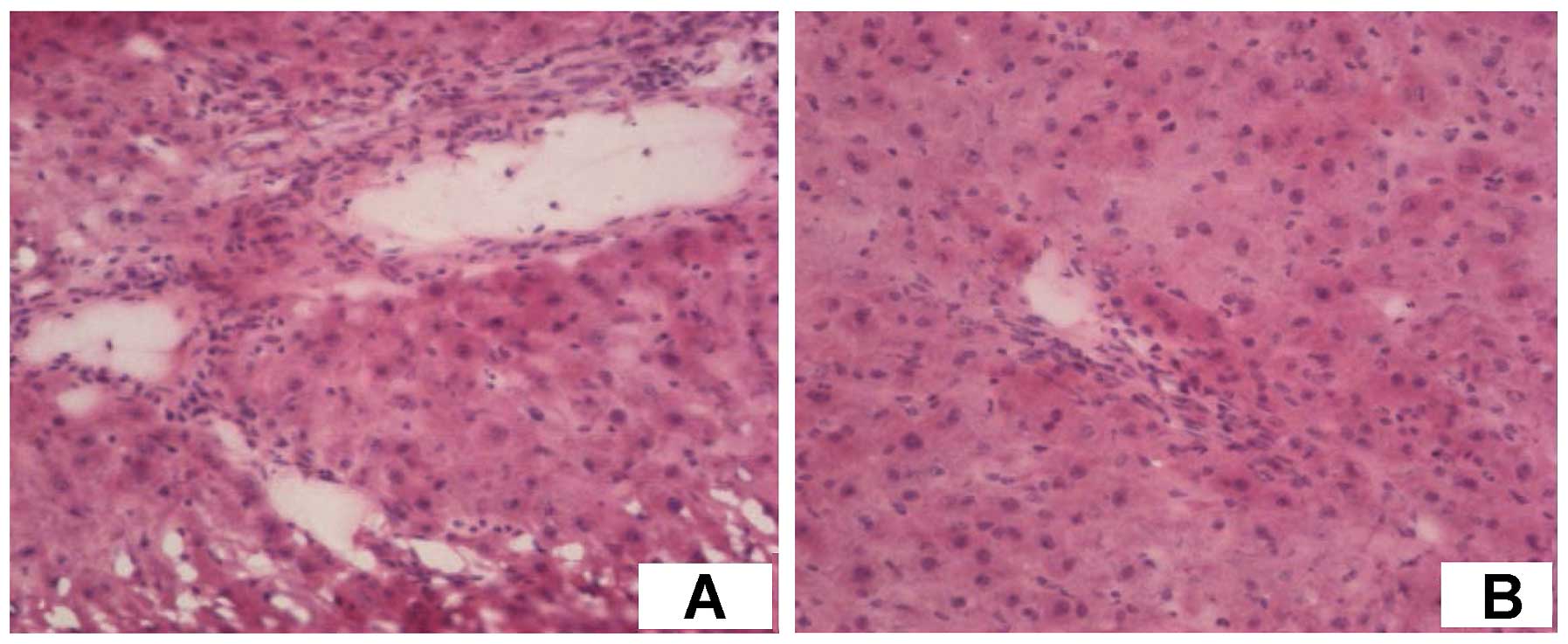
umbilical cord produces white protein. In addition, after H&E
staining there were a large number of cells aggregating around the
hepatic sinusoid, and hyperplasia was relatively active (Fig. 6A and B).
Discussion
As a temporary organ, the umbilical cord is a
relatively simple structure, mainly rich in Wharton's jelly of
collagen as well as vascular and mesenchymal elements (6). Many experiments have shown that with
suitable induction in vitro, human umbilical cord MSCs may
differentiate into mesodermal cells, such as osteoblasts, muscle
cells, or ectodermal and endodermal liver cells, such as neural
glial cells (7,8). Umbilical cord is a rich source of stem
cells that are easier to culture and proliferate, giving UC-MSCs
great clinical value.
In the present study, using collagenase digestion,
we successfully isolated and cultured human umbilical cord MSCs. To
provide an in-depth understanding of how the transplanted cells
in vivo repaired damaged tissues, many cell labeling methods
have been used (9). Achievement of
an appropriate, effective, and practical cell labeling technique,
remains a challenge. PKH26 is a lipophilic fluorescent dye that
irreversibly binds to the cell membrane (10,11). It
is excited in red fluorescence and in the exposure of 527-nm
wavelength exerted little influence on cell viability and
proliferation ability. It is therefore a relatively good tracing
marker in vivo. The cell fluorescence labeled by PKH26 may
be kept inside the body at least for one month (12,13).
With division of cells, the fluorescent dye was almost equally
distributed into two daughter cells and the fluorescence intensity
of the daughter cells also decreased. As the cells continued to
differentiate, the red fluorescence gradually faded (14–17).
Several studies have shown that the MSCs of bone marrow or fat of a
model rat successfully differentiated into liver cells in other
partially hepatectomized model rats. The method in MSCs was induced
to differentiate them into hepatic cells in vivo avoiding
the difficulties and limitations in vitro and making MSCs
directly involved in the liver injury repair (18–20). As
this experiment was heterogeneous allograft and the
microenvironment was different in vivo there was a high
chance of immune rejection. FCM detected that surface markers of
human umbilical cord MSCs were the same as the fetal lung
tissue-derived MSCs (21–23), but did not express HLA-DR, which was
the main factor to cause the immune response, suggesting that the
relative immunogenicity of human umbilical cord MSCs was relatively
weak and was appropriate to be transplanted between different
individuals (24–27). At the same time, after portal vein
transplantation, the cells directly reached the liver, which
provided a better microenvironment for cell growth. Therefore, it
is feasible to observe the positioning and differentiation of cells
in the liver in an improved manner. Partial liver resection is the
optimal model of liver regeneration. Liver resection caused an
increase in hepatocyte growth signals, such as metabolic
nutritional factors and neurohormones, providing a good
microenvironment for the regeneration of liver cells (28–31). The
growth signals in the blood also induced the stem cells to express
hepatocyte markers (32). In this
experiment, a heterogeneous stem cell transplantation model was
established on the basis of the experimental model of partial
hepatectomy. This was similar to the clinical experimental model,
as the donor cells were screened and prepared in advance and were
ready for immediate use. Transplanted cells were successfully
implanted and survived for a long time in rats, indicating that
this method is safe, reliable, and there were no significant
hyperacute or acute rejection of the transplantation. Liver after
partial hepatectomy regenerated significantly within 2 weeks, and
finished regeneration within three months. In this process, the
residual liver cells regenerated and died simultaneously (11), thus in this study, the liver of the
model rat was cut at the first, second and third week and frozen.
Under a fluorescent microscope, it was evident that stem cells were
scattered in the liver with intact cell structures. Part of the
liver cells were embedded in the hepatic plate with liver cell
morphology, and expression of albumin was detected with anti-human
albumin antibody. Along with cell differentiation, the red
fluorescence faded away, while the green fluorescence, which
represented the albumin expression was enhanced, indicating that
after transplantation the human umbilical cord MSCs were able to
differentiate into hepatocytes in vivo, and participate in
the regeneration of liver cells. We used anti-human albumin
antibody, despite taking the differentiation potential of human
umbilical cord MSCs into account, to exclude the interference of
albumin generated by the liver cells of rats and prevent the
generation of false positives.
In conclusion, human umbilical cord MSCs were
implanted into the model rats via portal vein transplantation. This
confirmed that the human umbilical cord MSCs differentiated into
hepatocytes in the allograft and liver regeneration environment and
there was no significant adverse reactions without the use of
immunosuppressants. By combining the experience of clinical
practice, the umbilical cord MSCs can become a promising cell
source for bioartificial liver system and liver cell
transplantation and bring hope to patients with advanced liver
cancer. However, this is only an experimental animal study, thus,
it is difficult to assess correctly the long-term treatment effect,
and there remains a gap between the experimental and clinical
application, which needs further study.
Acknowledgements
The study was funded by the Science and Technology
funded projects of Guangdong Province (grant no.
2010B031600248).
References
|
1
|
Mezey E and Chandross KJ: Bone marrow: a
possible altern ative source of cells in the adult nervous system.
Eur J Pharmacol. 4:297–302. 2000. View Article : Google Scholar
|
|
2
|
Fukuda K and Preck D: Reprogramming of
bone marrow mesenchymal stem cells into cardiomyocytes. C R Biol.
325:1027–1038. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu SC, Xu YY, Li Y, Xu B, Sun Q, Li F and
Zhang XG: Construction of tissue engineered skin with human
amniotic mesenchymal stem cells and human amniotic epithelial
cells. Eur Rev Med Pharmacol Sci. 19:4627–4635. 2015.PubMed/NCBI
|
|
5
|
He J, Cai Y, Luo LM and Liu HB: Hypoxic
adipose mesenchymal stem cells derived conditioned medium protects
myocardial infarct in rat. Eur Rev Med Pharmacol Sci. 19:4397–4406.
2015.PubMed/NCBI
|
|
6
|
Forraz N and McGuckin CP: The umbilical
cord: a rich and ethical stem cell source to advance regenerative
medicine. Cell Prolif. 44(Suppl 1): 60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu LL, Song YP, Wei XD, Fang BJ, Zhang YL
and Li YF: Comparative characterization of mesenchymal stem cells
from human umbilical cord tissue and bone marrow. J Exp Hematol.
16:140–146. 2008.(In Chinese).
|
|
8
|
Zhan YT, Wang Y, Wei L, Liu B, Chen HS,
Cong X and Fei R: Differentiation of rat bone marrow stem cells in
liver after partial hepatectomy. World J Gastroenterol.
12:5051–5054. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blute JW, Douglas T, Witwer B, Zhang SC,
Strable E, Lewis BK, et al: Magnetodendrimers allow endosomal
magnetic labeling the in vivo tracking of stem cells. Nat
Biotechnol. 19:1141–1147. 2011. View Article : Google Scholar
|
|
10
|
Ji KH, Xiong J, Fan LX, Meng HK and Liu
HQ: Rat marrow derived multipotent adult progenitor cells
differentiate into skin epidermal cells in vivo. J Dermatol.
36:403–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallace PK and Muirhead KA: Cells tracking
2007: a proliferation of probes and applications. Immunol Invest.
36:527–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haas J, Bauer P, Rolf A and Wree A:
Immunocytochemical characterization PKH26 labelled an
intracerebrally transplanted neonatal cells. Acta Histochem.
102:273–280. 2011. View Article : Google Scholar
|
|
13
|
Fox D, Kouris GJ, Blumofe KA, Heilizer TJ,
Husak V and Greisler HP: Optimizing fluorescent labeling of
endothelial cells for tracking during long term studies of
autologous transplantation. J Surg Res. 86:9–16. 2015. View Article : Google Scholar
|
|
14
|
Weiss ML and Troyer DL: Stem cells in the
umbilical cord. Stem Cell Rev. 2:155–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Tian XB, Hu RY, Xu FB and Zhao JM:
Mechanism of BMP and TG2 in mesenchymal stem cell osteogenesis. Eur
Rev Med Pharmacol Sci. 19:4214–4219. 2015.PubMed/NCBI
|
|
16
|
Xue Z, Niu LY, An G, Guo YS, Lv SC and Ren
XP: Expression of recombinant BMP-7 gene increased ossification
activity in the rabbit bone mesenchymal stem cells. Eur Rev Med
Pharmacol Sci. 19:3056–3062. 2015.PubMed/NCBI
|
|
17
|
Hendrikx PJ, Martens CM, Hagenbeek A, Keij
JF and Visser JW: Homing of fluorescently labeled murine
hematopoietic stem cells. Exp Hematol. 24:129–140. 1996.PubMed/NCBI
|
|
18
|
Oyagi S, Hirose M, Kojima M, Okuyama M,
Kawase M, Nakamura T, Ohgushi H and Yagi K: Therapeutic effect of
transplanting HGF-treated bone marrow mesenchymal cells into
CCl4-injured rats. J Hepatol. 44:742–748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Tang CL, Chen WJ, Zhang Q and
Wang SL: Dynamic compression combined with exogenous SOX-9 promotes
chondrogenesis of adipose-derived mesenchymal stem cells in PLGA
scaffold. Eur Rev Med Pharmacol Sci. 19:2671–2678. 2015.PubMed/NCBI
|
|
20
|
Han YF, Sun TJ, Han YQ, Xu G, Liu J and
Tao R: Clinical perspectives on mesenchymal stem cells promoting
wound healing in diabetes mellitus patients by inducing autophagy.
Eur Rev Med Pharmacol Sci. 19:2666–2670. 2015.PubMed/NCBI
|
|
21
|
Javazon EH, Beggs KJ and Flake AW:
Mesenchymal stem cells: paradoxes of passaging. Exp Hematol.
32:414–425. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Lu Y, He DM and Zhang Y:
Isolation, purification and identification of mesenchymal stem
cells derived from human umbilical cord. J Jinan University.
17:367–372. 2009.(In Chinese).
|
|
23
|
Jia Z: Basic therapy and clinic of
hepatopathy cells. Beijing: People's Medical Publishing House; pp.
139–140. 2005
|
|
24
|
Ma J, Duan FL, Yan FG, Li WX, Wang X, Chen
XY, Gao TH, Zhu WL and Wang ZQ: Serum from partial hepatectomy rat
and hepatocyte growth factor stimulate bone marrow cell expressing
albumin and alpha fetoprotein. Zhonghua Gan Zang Bing Za Zhi.
12:410–413. 2004.(In Chinese). PubMed/NCBI
|
|
25
|
Li JW and Wu X: Mesenchymal stem cells
ameliorate LPS-induced acute lung injury through KGF promoting
alveolar fluid clearance of alveolar type II cells. Eur Rev Med
Pharmacol Sci. 19:2368–2378. 2015.PubMed/NCBI
|
|
26
|
Zhao YF, Luo YM, Xiong W, Ding W, Li YR,
Zhao W, Zeng HZ, Gao HC and Wu XL: Mesenchymal stem cell-based FGF2
gene therapy for acute lung injury induced by lipopolysaccharide in
mice. Eur Rev Med Pharmacol Sci. 19:857–865. 2015.PubMed/NCBI
|
|
27
|
Garcea G and Maddern GJ: Liver failure
after major hepatic resection. J Hepatobiliary Pancreat Surg.
16:145–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun TJ, Tao R, Han YQ, Xu G, Liu J and Han
YF: Wnt3a promotes human umbilical cord mesenchymal stem cells to
differentiate into epidermal-like cells. Eur Rev Med Pharmacol Sci.
19:86–91. 2015.PubMed/NCBI
|
|
29
|
Roseti L, Serra M, Canella F, Munno C,
Tosi A, Zuntini M, Pandolfi M, Sangiorgi L, Biso P, Pittalis MC, et
al: In vitro gene and chromosome characterization of expanded bone
marrow mesenchymal stem cells for musculo-skeletal applications.
Eur Rev Med Pharmacol Sci. 18:3702–3711. 2014.PubMed/NCBI
|
|
30
|
Xu Y, Sun DC, Wei ZT, Hong BF and Yang Y:
Experimental study on transplantation of autologous minced muscle
with human umbilical cord mesenchymal stem cells for urethral
reconstruction. Eur Rev Med Pharmacol Sci. 18:3412–3419.
2014.PubMed/NCBI
|
|
31
|
Yao XL, Li L, He XL, Cui L, Kuang W and
Tang M: Activation of β-catenin stimulated by mechanical strain and
estrogen requires estrogen receptor in mesenchymal stem cells
(MSCs). Eur Rev Med Pharmacol Sci. 18:3149–3155. 2014.PubMed/NCBI
|
|
32
|
Zhu XW, Zuo JL, Liu YH, Zang R, Li YK,
Wang X and Li JM: Osteogenesis of umbilical mesenchymal stem cells
is enhanced in absence of DNA methyltransferase 3B (DNMT3B) through
upregulating Runx2 expression. Eur Rev Med Pharmacol Sci.
18:3004–3009. 2014.PubMed/NCBI
|