Introduction
Type 2 diabetes mellitus (T2DM) is characterized by
a combination of insulin resistance and pancreatic β-cell
dysfunction due to metabolic exhaustion. Sustained hyperglycemia
may result in multi-system chronic complications, including micro-
and macrovascular complications, which are associated with high
morbidity and mortality. With current pharmacological agents, many
patients find it difficult to achieve good glycemic control, and
the majority of these patients will eventually require insulin
therapy (1). Insulin therapy
negatively impacts patients' daily lives and does not prevent the
occurrence of diabetic complications (2). Therefore, it is imperative that novel
strategies for optimal glycemic control or β-cell replacement are
explored.
Cellular therapies offer novel opportunities for the
treatment of diabetes. Previous clinical studies have demonstrated
the potential of stem cells for disease treatment (3–5).
Mesenchymal stem cells (MSCs) are a population of self-renewable
cells that secrete various cytokines, growth factors and
extracellular matrix molecules which have important roles in the
regulation of hematopoiesis, angiogenesis, immune and inflammatory
responses (6,7). MSCs can be easily isolated and rapidly
expanded ex vivo, exhibit no tumor formation after long-term
cultivation and express intermediate levels of major
histocompatibility complex (MHC) class I molecules but not MHC
class II on their cell surface, thus allowing allogeneic
transplantation (8,9). Moreover, MSCs are capable of homing to
injured tissues following intravenous delivery (10–12).
These properties indicate that MSCs may be used as a potential
therapeutic strategy for treating various diseases.
Previous studies have indicated that MSCs are
capable of exerting anti-diabetic effects, resulting in the partial
restoration of pancreatic islet function, increased insulin
secretion and improved insulin resistance (13–16).
Furthermore, it has been reported that single-dose MSC infusion may
ameliorate hyperglycemia (13).
Although this protocol failed to restore normoglycemia in diabetic
animals, multiple infusions of MSCs may have a role in reversing
hyperglycemia (13). Jiang et
al (14) evaluated the safety
and efficacy of allogeneic human placenta-derived mesenchymal stem
cells (PD-MSCs) in patients with a long history of T2DM. The
results demonstrated that infusion with PD-MSCs effectively
decreased plasma glucose levels, improved islet function and
induced no serious adverse effects (14). Moreover, Liu et al (15) demonstrated that treatment with
allogeneic Wharton's Jelly-derived mesenchymal stem cells (WJ-MSCs)
improved metabolic control and β-cell function in patients with
T2DM (15). However, the follow-up
time of these trials was too short to assess the long-term effect
and safety of MSCs on T2DM.
In the present pilot phase I/II study, WJ-MSCs were
used to explore the long-term safety and efficacy of WJ-MSCs
infusion in T2DM patients with a follow-up period of 36 months.
Materials and methods
Study design
The present phase I/II, 36-month, randomized
controlled study was conducted in patients diagnosed with T2DM
according to the criteria outlined by the American Diabetes
Association (17). The present study
was conducted in accordance with the Declaration of Helsinki and
was approved by the Ethical Committee of the Affiliated Hospital of
Qingdao University (Qingdao, China). Written informed consent was
obtained from all patients prior to enrollment. Throughout,
investigators remained blinded to the treatment administered. An
independent data and safety monitoring committee monitored the
safety and efficacy of the study.
Patients
Study participants were selected from patients
admitted to the Affiliated Hospital of Qingdao University for the
treatment of diabetes mellitus between September 2010 and December
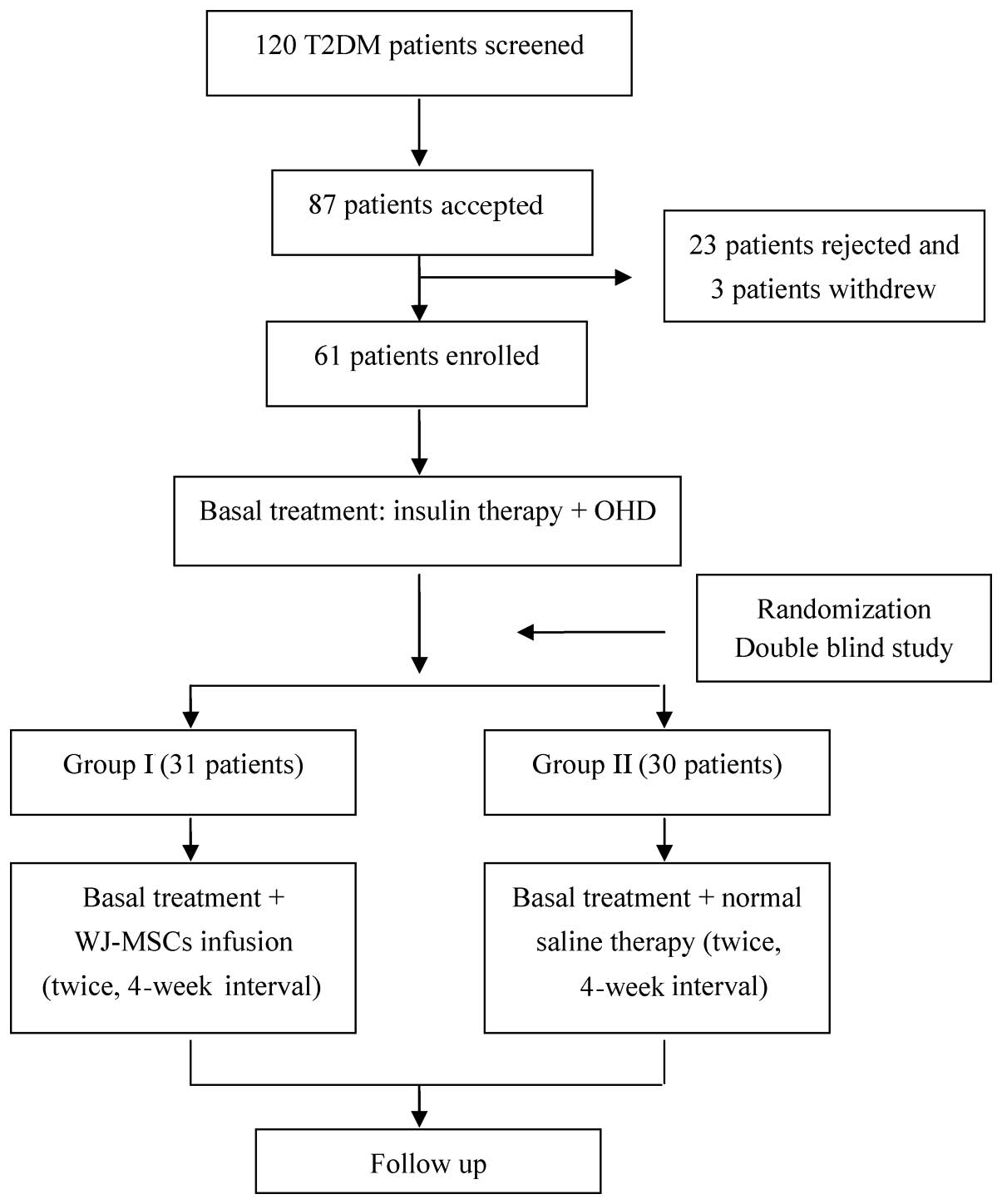
2011. A total of 87 patients met the inclusion criteria and,
following an interview, 64 patients were enrolled. Although 64
patients with T2DM were initially enrolled (Fig. 1), 2 patients in group II and one
patient in group I withdrew at the start of follow-up due to
immigration to other distant city and a lack of availability. The
remaining 61 patients completed the entire study and their data
were analyzed. Using a balanced permuted-block randomization
method, participants were divided into two groups: The WJ-MSC
treatment group (group I; n=31) and the control group (group II;
n=30). All patients were subsequently enrolled, treated and
followed-up for 36 months until April 2014 at the Stem Cell Center
of the Affiliated Hospital of Qingdao University.
Inclusion criteria were as follows: Patients of
either sex, aged 18–60 years, with a clinical and laboratory
diagnosis of T2DM according to the criteria outlined by the
American Diabetes Association (17).
Exclusion criteria were: Any malignancies; pancreatic congenital
anomaly; positive serology for human immunodeficiency virus (HIV),
hepatitis B (HBV) or hepatitis C (HCV); underlying hematologic,
nephrologic, cardiac, psychiatric, or hepatic disease; pregnancy;
any acute or chronic infection; and any other endocrine and
metabolic disease, including hyperthyroidism, hypercortisolism,
acromegaly or chromaffin tumor.
Treatment
All patients enrolled into the present study were
assessed in the diabetic out-patient clinic for a period of 3
months prior to the initiation of therapy, and were recommended a
1,500-calorie diet and exercise routine, which composed of walking
or similar exercise for 1 h three times/week during the entire
study and follow-up period. At the initiation of therapy, all
patients had been treated with diet, exercise, oral hypoglycemic
agents [1500 mg/d dimetyl biguanide (0.5 g t.i.d.) and 4 mg/d
avandia] and insulin injections, which were considered baseline
treatment, at stable doses for at least two months.
In addition to the baseline treatment, patients in
group I were administered two WJ-MSC infusions through the veins in
the back of the hand. The infusion interval was four weeks,
according to previous studies (14,18). In
addition to the baseline treatment, patients in group II were
treated with normal saline which was administered in the same
volume of parenteral solution as WJ-MSC. All patients were admitted
to the hospital for infusion and, following infusion, all patients
remained on the same drug therapy, and diabetic diet and exercise
regimen as before.
During the 36-month follow-up, the dosages of oral
hypoglycemic agents and insulin (26–48U/day, 2–4 times/day) were
adjusted according to the patient's blood glucose. Dosages of
insulin and oral hypoglycemic agents were increased if the
patient's blood glucose had not been controlled within the normal
range [fasting plasma glucose (FPG) normal range, 70–110 mg/dl;
postprandial plasma glucose (PPG) normal range, ≤140 mg/dl].
Similarly, the dosages of insulin and oral hypoglycemic agents were
reduced if the patient's blood glucose was successfully controlled
within the normal range.
Stem cell preparation
WJ-MSCs were provided by the Human Umbilical Cord
Mesenchymal Stem Cell Bank (Shandong, China). Umbilical cords were
obtained from the healthy mothers of healthy full-term fetuses with
no familial history of DM and no history of cancer, HBV, HCV, HIV,
Epstein-Barr virus (EBV), cytomegalovirus (CMV) or syphilis
detected in serum. Umbilical cord collections were approved by the
Institutional Medical Research Ethics Committee of the local
maternity hospitals. Written informed consent was obtained from
each mother several weeks prior to delivery. WJ-MSC preparation was
performed in a laminar flow laboratory, as previously reported
(18,19). Briefly, umbilical cords were washed
twice with phosphate-buffered saline and subsequently dissected
with scissors into sections that were ~1 mm3 in volume.
Tissue sections were plated in a cell culture dish (cat no. 430597;
Corning, Inc., Palo Alto, CA, USA) in serum-free
NutriStem® MSC XF medium for MSCs (Biological
Industries, Ltd., Kibbutz Beit-Haemek, Israel). Cell cultures were
maintained in a humidified atmosphere with 5% CO2 at
37°C. Following 3 days of culture, the medium was replaced to
remove the tissue and non-adherent cells, and was subsequently
changed twice weekly thereafter. Once 80% confluence had been
achieved, the adherent cells (passage 0) were detached with 0.125%
trypsin and passaged in the cell culture dish. WJ-MSCs were
cultured and expanded in a laminar flow laboratory, which was
designed according to good manufacturing practice conditions, for
four passages to prepare the final cell products. WJ-MSCs were
sterile and qualified for aerobe, mycoplasma, HBV, HCV, HIV, EBV,
CMV, syphilis and endotoxin testing. Subsequently, cells were
stained with CD-PE and CD-FITC (from Human MSC Analysis kit; cat
no. 562245; BD Biosciences) and analyzed by flow cytometry with a
FACscalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA). It
was determined by flow cytometry that these cells highly expressed
CD90 (85.77%), CD105 (79.26%), CD73 (89.63%), and CD146 (54%), but
not CD34 (0.23%), CD45 (0.02%) and HLA-DR (0.03%). The chromosomal
karyotype of the UC-MSCs was determined as normal by metascan
karyotyping system (IMSTAR company, France).
Clinical assessment and follow-up
Medical history was obtained from each patient at
baseline, including diabetes duration, diabetes-related
complications, and clinical history of hypertension, dyslipidemia
and cardiovascular complications. Concomitant lipid-lowering,
antihypertensive and anticoagulant/antithrombotic medications were
recorded at all visits.
All patients were checked for viral infections
including HCV, HBV, HIV, and urogenital infections prior to
enrollment. In order to undergo MSC infusion, all patients were
admitted to he Affiliated Hospital of Qingdao University. On the
day of hospitalization, a primary clinical examination was
performed and the following laboratory data were collected: Height;
body weight; blood pressure; plasma glucose; glycosylated
hemoglobin; fasting serum C-peptide; full blood count; liver and
renal function tests; lipid profile tests; cardiac enzyme; cardiac
troponin; serum electrolytes; blood coagulation function;
microalbuminuria; and cancer screening test. These data were
recollected at monthly intervals for the first 3 months and then
every 3 months for the subsequent 33 months during follow-up
period. Each follow-up visit included a complete physical
examination and laboratory tests. In order to optimize diabetes
care, each participant had 24-h access to a phone line that
connected them to a physician during the follow-up period.
FPG and PPG levels were measured by an enzymatic
glucose oxidase/peroxidase colorimetric method (cat no. ECS000016;
OneTouch® Ultra, Johnson & Johnson, Shanghai,
China). C-peptide was examined via the C-peptide response test
(Roche Diagnostics GmbH, Mannheim, Germany; normal range, 1.1–4.4
ng/ml) in the fasted state and following a standardized mixed-meal
test. Glycosylated hemoglobin (HbA1c) was examined using
high-performance liquid chromatography (Bio-Rad D10; Bio-Rad
Laboratories, Inc., Hercules, CA, USA; normal range, 3.9–6.1%). The
C-peptide/glucose ratio (CPGR) was calculated to evaluate the
glycemic profile of patients at various time points according to
the following formula: C-peptide × 100/glucose.
Hypertension was diagnosed if the patient had a
history of hypertension, was receiving medication for hypertension
or had a resting recumbent blood pressure of ≥140/90 mmHg on two
separate occasions. Height and weight were measured in light indoor
clothing, without shoes, using a fixed rigid stadiometer and a Seca
scale, respectively. Body mass index (BMI; kg/m2) was
determined by dividing the weight (kg) of each patient by their
height squared (m2).
To determine insulin sensitivity, fasting plasma
C-peptide (FPC) was used instead of fasting insulin for homeostasis
model assessment of insulin resistance (HOMA-IR) and pancreatic
islet β-cell function (HOMA-β) analysis. HOMA-IR C-peptide was
calculated using the following equation: HOMA-IR C-peptide=FPG
(mmol/l) × FPC (pmol/l)/22.5, where the denominator of 22.5 is a
normalizing factor. HOMA-β was calculated using the following
equation: HOMA-β C-peptide=20 × FPC (pmol/l)/[FPG (mmol/l] −
3.5).
Diabetic complications
Diabetic nephropathy was diagnosed when the patient
exhibited at least one of the following: i) Positive
microalbuminuria within one year, as confirmed by elevated urine
microalbumin levels in at least two of three collections; ii)
positive proteinuria, which was defined as a positive urine
dipstick test at least 1+ level; and iii) renal insufficiency, as
defined by a serum creatinine level ≥132 µmol/l. Patients without
nephropathy were defined when they had negative urine
microalbumin.
Diabetic peripheral neuropathy was diagnosed when
patients exhibited typical symptoms and/or signs of neuropathy, or
neuropathy symptoms, as defined by a Michigan Neuropathy Score ≥3
(20), and an abnormal result on the
monofilament test at the time of the follow-up visit. Information
on patient awareness of diabetic peripheral neuropathy was obtained
via an interview. Patient history of ocular surgery was surveyed
and the presence and severity of diabetic retinopathy was assessed
every 3 months by ophthalmologists. According to The International
Clinical Diabetic Macular Edema Disease Severity Scale (21), the severity of diabetic retinopathy
was categorized into five stages: i) no retinopathy; ii) mild
non-proliferative diabetic retinopathy; iii) moderate
non-proliferative diabetic retinopathy; iv) severe
non-proliferative diabetic retinopathy; and v) proliferative
diabetic retinopathy. ‘Incidence of diabetic retinopathy’ was
defined in patients with no diabetic retinopathy signs in either
eye at the baseline evaluation and mild to severe non-proliferative
diabetic retinopathy or proliferative diabetic retinopathy in
either of the eyes at follow-up visits over two consecutive years.
‘Progression of diabetic retinopathy’ was defined in patients with
mild non-proliferative diabetic retinopathy at the baseline
evaluation, and severe non-proliferative diabetic retinopathy,
proliferative diabetic retinopathy or laser photocoagulation
treatment for diabetic retinopathy at follow-up visits over two
consecutive years.
Study objectives and data
collection
The primary objective of the present study was to
evaluate the feasibility of WJ-MSC therapy and the safety of MSC
infusion during the 12-month period following treatment. Secondary
objectives were to assess the safety of MSC infusion over 36 months
in patients treated with MSC infusion and to evaluate the
therapeutic effect of MSC infusion in patients with T2DM over 36
months.
A data collection form was developed according to
the objectives of the present study. Training of researchers and
research assistants was performed during a pilot data collection
period and a case record form was standardized. Site visits by
internal and external auditors were regularly completed in order to
assure the quality of the data and the study process.
Safety assessments
Safety assessments included monitoring and recording
all adverse events. Potential safety concerns, including
hypersensitivity, infection, hemorrhage, proteinuria, myocardial
infarction, venous thromboembolic events and other arterial
thromboembolic events, were recorded. Hypoglycaemia was defined in
patients who exhibited symptoms that were suggestive of low blood
glucose and were confirmed by self-monitored blood glucose (SMBG)
measurement equivalent to <3.1 mmol/l plasma glucose. Severe
hypoglycaemia was defined as any episode requiring the assistance
of another party, regardless of whether or not a confirmatory SMBG
measurement was available.
Statistical analysis
All statistical analyses were performed using
SPSS® 15.0 software (SPSS, Inc., Chicago, IL, USA). Data
were presented as the mean ± standard deviation. Between-group
differences in the means of the baseline values of groups I and II
were analyzed using Student's t-test. Comparisons of time-dependent
changes at the time of baseline and different time points following
the treatment were performed using repeated measure analysis of
variance and post-hoc analysis with Bonferroni correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 64 patients with T2DM were initially
enrolled in the study (Fig. 1);
however, two patients in group II and one patient in group I
withdrew at the start of follow-up due to immigration to other
distant city and a lack of availability. The remaining 61 patients
completed the entire study and their data were analyzed. Overall,
the present study investigated 33 men and 28 women, with a mean age
of 52.7±6.3 years (range, 42–63 years). Baseline patient
characteristics are shown in Table
I. No significant differences in the clinical findings,
laboratory examinations or diabetic complications were detected
between the two groups prior to the initiation of the study. Cancer
screening test confirmed no cancer in all patients. The volumes of
parenteral solution of WJ-MSCs and normal saline in group I and II,
respectively, were 100 ml, and the number of WJ-MSCs was determined
according to the weight of patient. Mean cell number was
6.1±2.1×107 (1.0×106/kg; range,
5.3–8.9×107).
 | Table I.Baseline patient characteristics in
the two groups. |
Table I.
Baseline patient characteristics in
the two groups.
| Variable | Group I | Group II |
|---|
| Clinical
characteristics |
|
|
| Age
(years) | 52.43±4.88 | 53.21±8.22 |
| Sex
(n) |
|
|
|
Male | 17 | 16 |
|
Female | 14 | 14 |
|
Duration of T2DM (years) | 8.93±5.67 | 8.3±6.07 |
|
Duration of insulin therapy
(years) | 4.28±1.64 | 4.14±1.23 |
| Dose of
insulin U/d (U/kg/d) | 45.92±8.87
(0.79±0.23) | 43.09±10.3
(0.74±0.19) |
| BMI
(kg/m2) | 26.74±5.41 | 27.03±6.68 |
|
Hypertension (n) | 12 | 11 |
| Laboratory
tests |
|
|
| FPG
(mg/dl) | 148.27±27.81 | 142.31±25.88 |
| HbA1c
(%) | 7.67±1.23 | 7.54±1.31 |
| Fasting
C-peptide (ng/ml) | 1.75±0.64 | 1.83±0.59 |
|
Triglycerides (mg/dl) | 130.57±40.22 | 134.23±42.76 |
| HDL-c
(mg/dl) | 42.56±5.92 | 40.92±5.34 |
| LDL-c
(mg/dl) | 74.90±29.73 | 75.81±31.57 |
| Complications
(n) |
|
|
|
Retinopathy | 5 | 4 |
|
Neuropathy | 4 | 3 |
|
Nephropathy | 3 | 4 |
During the follow-up period, the BMI of patients in
group I marginally decreased, whereas a gradual increase in the BMI
of group II patients was detected throughout the follow up periods.
In spite of this, the differences in BMI between the two groups
were not significant. These results suggested that WJ-MSC infusion
does not affect the BMI of patients with T2DM.
WJ-MSC infusion ameliorates
hyperglycemia in patients with T2DM
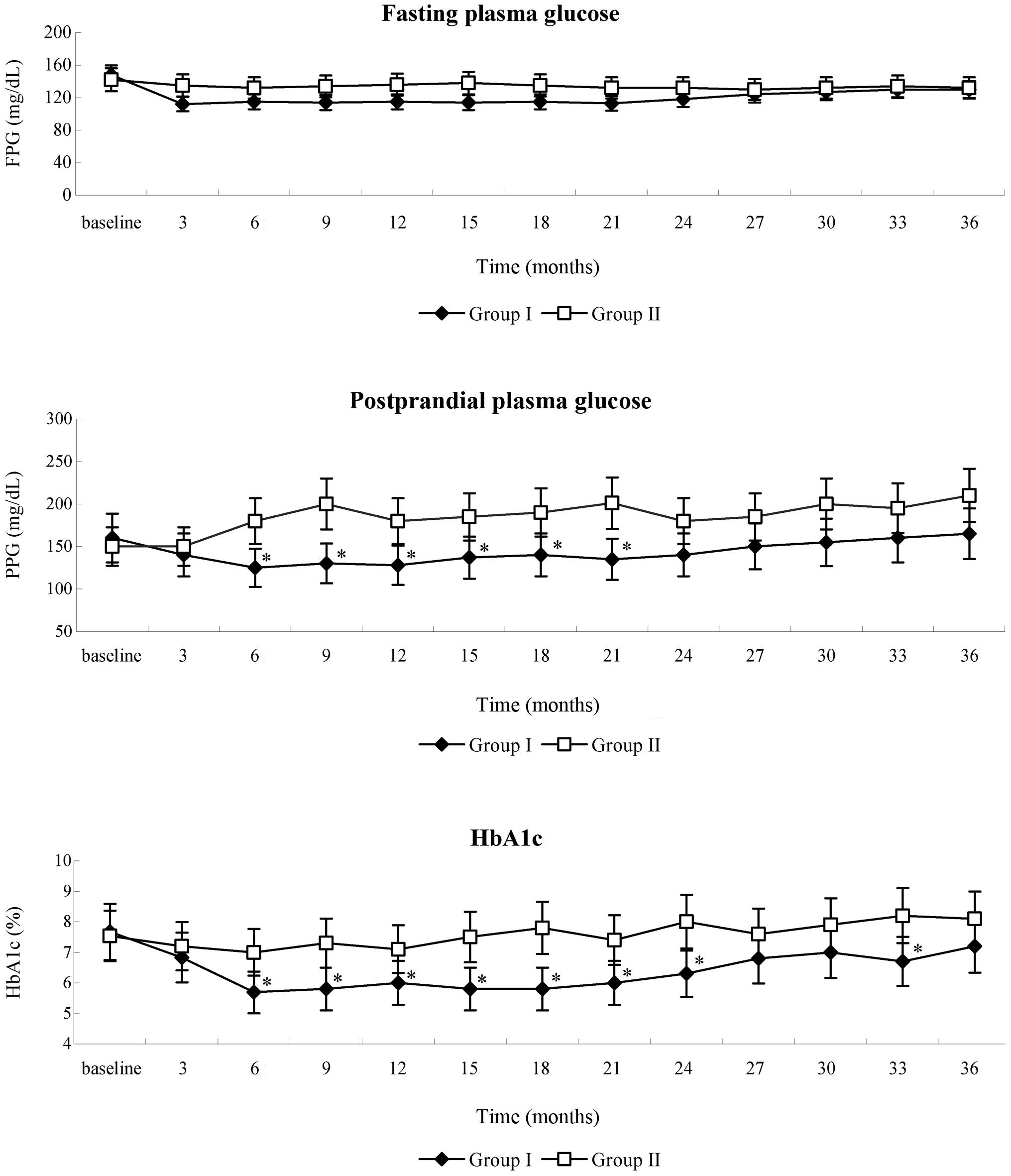
Following WJ-MSC infusion, a gradual reduction in
the FPG of patients in group I was detected during the follow-up.
FPG levels were at their lowest by the third month post-therapy
(baseline, 148.3±27.8 mg/dl; 3 months, 112±18.7 mg/dl) and remained
stable for the following 18 months, after which, FPG moderately
increased during the remaining follow-up time. FPG levels of
patients in group II remained consistent for the initial 15 months
then began to increase, necessitating the addition of insulin and
oral hypoglycemic agents in order to maintain FPG levels within the
normal range. No significant differences in FPG levels were
detected between the two groups (Fig.
2). PPG levels in the patients in group I were lowest at the
sixth month post-therapy and remained stable for 18 months.
Compared with group II, levels of PPG in group I significantly
decreased from 6–21 months post-therapy (P<0.05). Although PPG
levels moderately increased after 24 months post-therapy, improved
control was retained during follow-up, as compared with the higher
and larger fluctuations of PPG detected in group II patients during
the whole follow-up period (Fig.
2).
Following WJ-MSC infusion therapy, a gradual
decrease in HbA1c was detected in the patients in group I and the
lowest level was at the sixth month of follow-up (baseline,
7.67±1.23%; 6 months, 5.69±0.79%), after which HbA1c remained
stable for 18 months, then exhibited slight fluctuations over the
remaining follow-up period. In group II post-therapy, HbA1c levels
remained marginally reduced for 15 months then began to fluctuate
due to the addition of oral hypoglycemic agents and insulin. HbA1c
was significantly decreased in group I, as compared with group II,
between 6 and 24-months post-therapy and at 33-months post-therapy
(P<0.05; Fig. 2). These results
suggest that WJ-MSC infusion is able to decrease hyperglycemia in
T2DM patients.
WJ-MSC infusion improves β-cell
function and insulin sensitivity in patients with T2DM
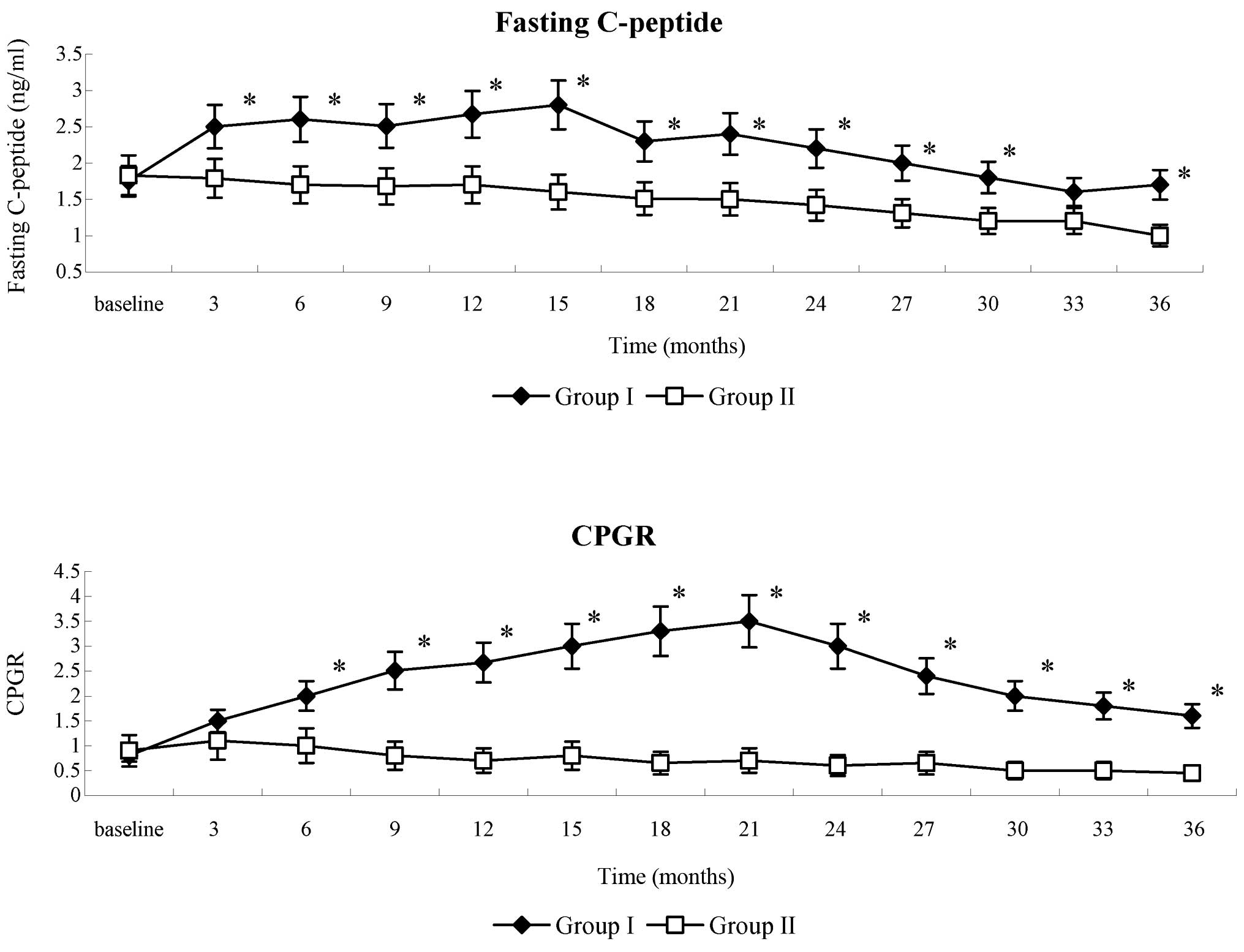
Following WJ-MSC infusion, the levels of fasting
serum C-peptide in patients in group I decreased at month 1, then
progressively increased at month 3 and remained constant for 15
months, with a slight decrease at month 18. At the end of
follow-up, the mean levels of fasting C-peptide in group I remained
higher than the baseline. In group II patients, fasting C-peptide
levels gradually decreased. Fasting C-peptide levels were
significantly increased in group I, as compared with group II,
throughout the entire follow-up period (P<0.001), with the
exception of month 33 post-therapy (Fig.
3). The CPGR gradually increased in group I during the initial
21 months of the follow-up period, followed by a gradual decline to
the end of the follow-up. A gradual decrease in CPGR was detected
in group II patients. CPGR values were significantly increased in
group I, as compared with group II, throughout the entire follow-up
period (P<0.001).
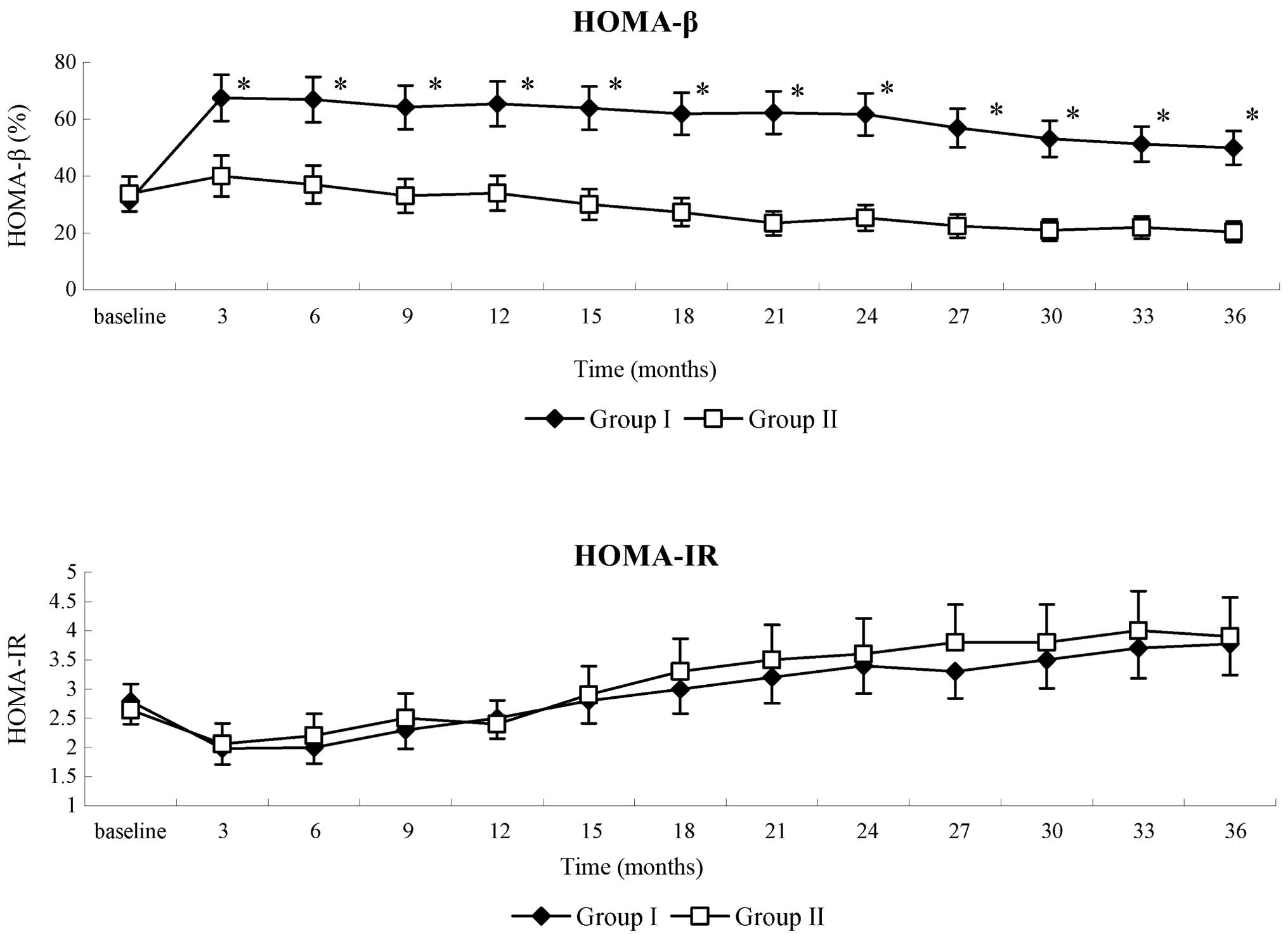
The levels of fasting glucose and C-peptide of
patients in the two groups were all within HOMA limits. HOMA-β in
group I patients significantly increased during the follow-up
period, as compared with the baseline (P<0.05); whereas in group
II patients, HOMA-β gradually decreased. There were significant
differences in HOMA-β between the two groups (P<0.05; Fig. 4). HOMA-IR was also evaluated in the
present study. Although a decrease in HOMA-IR was detected in group
I patients between 18 and 33 months post-therapy, as compared with
the group II patients, the difference in HOMA-IR between two groups
was not statistically significant. HOMA-IR in group II patients
gradually increase throughout the follow-up period (Fig. 4). These results suggested that WJ-MSC
infusion could enhance the function of islet β-cells in T2DM
patients.
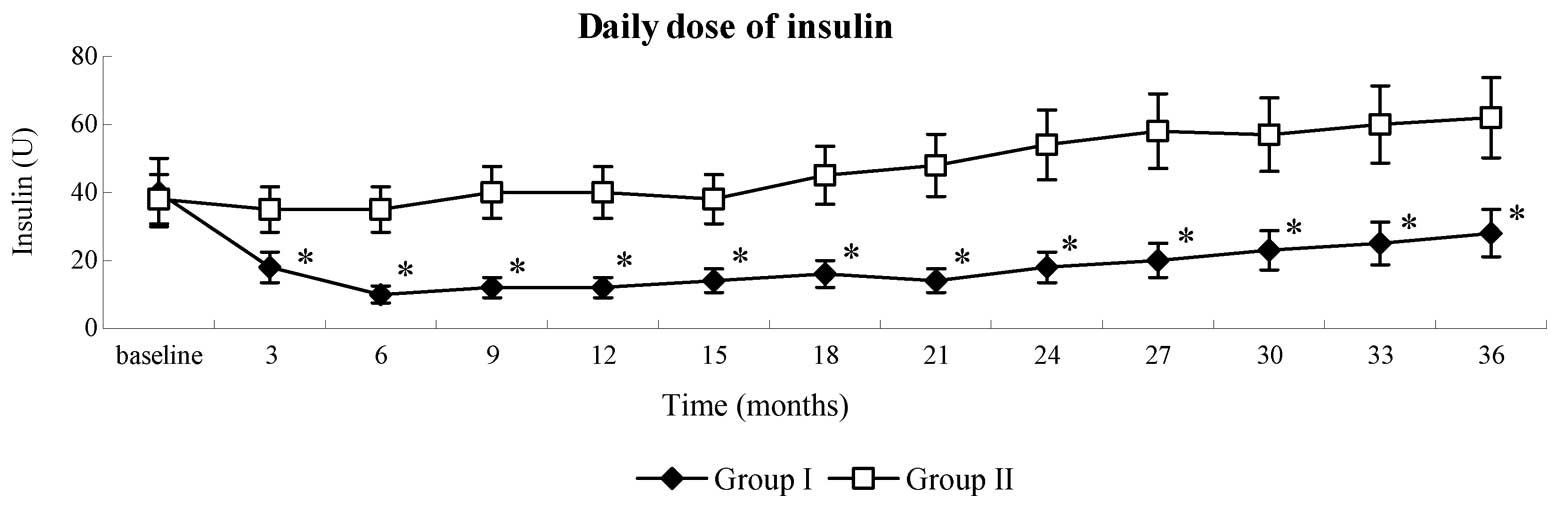
WJ-MSC infusion decreases the
requirement for insulin and oral hypoglycemic agents in patients
with T2DM
Following WJ-MSC infusion, patients in group I
receiving insulin therapy exhibited a gradual reduction in the
dosage of insulin required. Insulin withdrawal was demonstrated in
32.3% (10/31) of patients in group I, ranging from 3–11 months
(7.9±3.6 months) post-WJ-MSC infusion. These patients remained
insulin-free for 12.5±6.8 months. In total, 58.1% (18/31) of
patients in group I exhibited a ≥50% reduction in insulin
requirement, in five of the remaining 13 patients, daily insulin
dosage was reduced by 15–50%; whereas the insulin dosage
requirements of 8 patients were maintained or reduced by <15%.
In group II patients, the dose of insulin required per day
gradually increased after one year. In 47% (14/30) of patients,
insulin dosage increased by >50% from the baseline. In the 16
remaining patients, insulin dosage increased by 15–45%. The
difference between the two groups was significant (P<0.001)
throughout the follow-up period, and the serial changes in the mean
doses of insulin required are presented in Fig. 5.
By the end of the follow-up period, 19% (6/31) of
patients in group I who received oral anti-diabetic agents were
completely drug-free 3 months post-treatment (data not shown).
These patients did not relapse and exhibited good blood glucose
control with only diet and exercise intervention required. A total
of 16% (5/31) of patients terminated oral hypoglycemic drug (OHD)
treatment at the same time as insulin treatment. The mean duration
of drug discontinuance was 13.7±5.3 months. Nine of the remaining
20 patients reduced OHD by varying degrees; whereas the other 11
patients remained on the baseline dose of OHD. In group II, 77%
(23/30) of patients increased the dosage of OHD by varying degrees
(data not shown). This indicated that WJ-MSC infusion may decrease
the dosage of insulin and oral hypoglycemic agents in T2DM
patients..
WJ-MSC infusion reduces the incidence
of diabetic complications
By the end of the follow-up period, in group I,
there was no increase in the incidence of diabetic complications,
including diabetic retinopathy (5/31; 16.1%), neuropathy (4/31;
12.9%) and nephropathy (3/31; 9.7%). In group II, the incidence of
diabetic complications increased as hypothesized. Four patients
were newly diagnosed with diabetic retinopathy (total, 8/30;
26.7%), three patients were newly diagnosed with diabetic
neuropathy (total, 6/30; 20%) and three patients were newly
diagnosed with diabetic nephropathy (total, 7/30; 23.3%). There was
a statistically significant difference between the incidence of
diabetic complications in the two groups (P=0.007; data not shown).
This indicated that WJ-MSC infusion may reduce the incidence of
diabetic complications.
Adverse events
No serious adverse reactions, including fever,
chills, liver damage, hypersensitivity, infection, hemorrhage,
proteinuria, myocardial infarction, venous thromboembolic events or
other arterial thromboembolic events, were detected following
WJ-MSC infusion in any of the patients who completed the study
protocol, and no chronic side effects or lingering effects were
detected during the follow-up. None of the patients enrolled in the
present study developed severe hypoglycemia; whereas, 41 episodes
of minor hypoglycemia were detected in 41 patients (group I, n=23;
group II, n=18).
Discussion
Previous studies and clinical trials have
demonstrated that MSCs are capable of reducing glucose levels in
animals or subjects with type 1 and type 2 diabetes (14,15,18,19). Our
preliminary animal studies also suggested that the intervenous
infusion of WJ-MSC promoted the increase of β-cells in the
pancreatic islet of diabetic mice and rats, thus inducing an
increased level of insulin and decreased blood glucose (19,22). The
present study was conducted in order to explore the long-term
effect and safety of WJ-MSC in patients with T2DM. The present
results demonstrated that WJ-MSCs were was able to: i) Improve the
function of islet β-cells, as indicated by the increase in fasting
C-peptide and HOMA-β; ii) ameliorate hyperglycemia, as indicated by
the decrease of FPG, PPG, HbA1c and the dosage of oral hypoglycemic
agents and insulin therapy; and iii) reduce the incidence of
diabetic complications, although the sample size was not large
enough to assess the incidence of diabetic complications.
Accumulating evidence has indicated that paracrine
signaling initiated by MSCs, which involves the secretion of
various angiogenic growth factors and cytokines [such as vascular
endothelial cell growth factor (VEGF) and basic fibroblast growth
factor), anti-inflammatory and anti-apoptotic molecules (such as
interleukin-6 and −10, and tumor necrosis factor-α), may be
responsible for the therapeutic effect of MSCs (23–26). A
clinical trial conducted by Jiang et al (14) suggested that infusion of PD-MSCs
represented a simple, safe and effective therapeutic approach for
T2DM patients with a six-month follow-up time. Furthermore, Liu
et al (15) have previously
demonstrated that treatment with WJ-MSC may improve metabolic
control and β-cell function in patients with T2DM. These findings
are consistent with the results of the present study; however,
their respective follow-up periods were not adequate to demonstrate
the long-term effect of MSCs on T2DM. In the present study, the
follow-up period was 36 months, and the results demonstrated that
ideal glycemia control due to WJ-MSC infusion was achieved at the
third month post-therapy and was sustained for 18 months, as
confirmed by the fasting C-peptide and HOMA-β results. These
results indicated that two infusions of WJ-MSC may effectively
maintain good glycemic control for ~21 months. After this point,
due to the attenuation of the WJ-MSC effect and a gradual decrease
in β-cell function, blood glucose levels began to rise. Repetitive
WJ-MSC infusions may help to maintain good blood glucose control in
the long-term; however, future studies with larger sample sizes are
required in order to investigate this.
During the follow up period, a decrease in fasting
C-peptide was detected in the first month post-WJ-MSC infusion.
Based on the paracrine effect of WJ-MSC, this effect may be due to
the factors stimulated by WJ-MSC, including pancreatic duodenal
homeobox-1 and transforming growth factor-β1, which may directly or
indirectly have a role in the active metabolic effect (27), and decrease hyperglycemia, blood
glucose fluctuation and the need for endogenous insulin, thus
inducing the decrease in fasting C-peptide.
The therapeutic effect induced by WJ-MSC infusion in
the present study permitted the termination of treatment with oral
hypoglycemic agents and insulin in some patients; however, some of
these patients required these agents or insulin to decrease the
hyperglycemia once again. The therapeutic effect induced by WJ-MSC
infusion may be due to the part restoration of islet function or
the increase of both islet α- and β-cells, which have previously
been demonstrated in a animal model of diabetes (19). There have also been contradictory
reports concerning the association between injection times and the
therapeutic effect of WJ-MSC infusion on diabetes (13,28,29).
Ezquer et al (28)
demonstrated that a single-injection of MSCs into diabetic mice
induced an improved therapeutic effect, as compared with multiple
injections. Conversely, other studies demonstrated that multiple
intravenous infusions were able to reverse hyperglycemia in
experimental diabetic animals, whereas a single infusion of MSCs
could not (13,29). It is believed that the therapeutic
effect of WJ-MSC infusion was associated with the injection times,
cell types and number (28,29). In the present study, WJ-MSC infusion
was implemented via two injections, according to our previous
animal studies and clinical trials (18,22,30), and
perhaps multiple injections would have been more beneficial.
The role of insulin resistance in the development of
type 2 diabetes has been investigated extensively, and it has been
demonstrated that glucose transporter-4 (GLUT4), insulin receptor
substrate 1 (IRS-1) and Akt are crucial for glucose uptake and
insulin resistance (31–33). In a previous study, Si et al
(16) demonstrated that MSCs
infusion improved insulin sensitivity by upregulating GLUT4
expression and elevating phosphorylated IRS-1 and Akt levels in
tissues targeted by insulin, and concluded that infusion with MSCs
was able to ameliorate insulin resistance. The results of the
present study demonstrated that HOMA-IR of patients in group I
decreased following WJ-MSC infusion, although this decrease was not
significant when compared with patients in group II. This
interesting phenomenon demonstrated that the improvement of insulin
sensitivity may not be the dominant therapeutic effect induced by
WJ-MSC infusion. Future studies with larger samples, multiple
infusions of WJ-MSC and longer follow-up periods are required in
order to investigate this.
Diabetic complications, which predominantly occur
during the latter phase of diabetes due to poor glycemic control,
remain severe and life-threatening. In the present study, there was
no increase in the incidence of diabetic complications in the
patients of group I, whereas in group II, the incidence of diabetic
retinopathy, neuropathy and nephropathy increased. This indicated
that infusion with WJ-MSCs may reduce the incidence of diabetic
complications; this result was consistent with a previous study by
Jiang et al (14). Although
the underlying mechanisms of this therapeutic effect of WJ-MSC
remain unclear, the cytokines secreted by WJ-MSC, including
insulin-like growth factor, VEGF and hepatocyte growth factor, may
directly or indirectly improve islet function and the associated
complications (34–36).
No serious adverse reactions, including fever,
chills, liver damage or immune rejection response, were observed
following WJ-MSC infusion. Moreover, no positive results were
detected for renal and cardiac function, blood coagulation function
and tumor screening tests during the 36-month follow-up period.
These results suggested that infusion with WJ-MSC may represent a
safe therapeutic approach for the treatment of patients with
T2DM.
In conclusion, the findings of the present study
suggested that WJ-MSC infusion may effectively ameliorate
hyperglycemia, improve islet β-cell function and reduce the
incidence of diabetic complications over a sustained period of
time. Despite the fact that WJ-MSC infusion does not appear to
attenuate insulin resistance, WJ-MSC infusion may have therapeutic
potential as a novel agent for the treatment of T2DM. Further
follow-up and large-scale placebo-controlled clinical studies are
required to fully elucidate the role of WJ-MSC in the treatment of
T2DM. Their application in therapeutic regimens may be useful in
treating diabetes and its complications.
Acknowledgements
The present study received technical support from
the Human Umbilical Cord Mesenchymal Stem Cell Bank.
References
|
1
|
Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan
H, Naeshiro N, Honda Y, Miyaki D, Kawaoka T, Tsuge M, et al:
Efficacy and safety of sitagliptin for the treatment of
nonalcoholic fatty liver disease with type 2 diabetes mellitus.
Hepatogastroenterology. 61:323–328. 2014.PubMed/NCBI
|
|
2
|
Hinnen DA: Therapeutic Options for the
Management of Postprandial Glucose in Patients With Type 2 Diabetes
on Basal Insulin. Clin Diabetes. 33:175–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poole J, Mavromatis K, Binongo JN, Khan A,
Li Q, Khayata M, Rocco E, Topel M, Zhang X, Brown C, et al: Effect
of progenitor cell mobilization with granulocyte-macrophage
colony-stimulating factor in patients with peripheral artery
disease: A randomized clinical trial. JAMA. 310:2631–2639. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Zhou
H, Yin Z, Chen Y, Zhang Y, Wang S, et al: Targeting insulin
resistance in type 2 diabetes via immune modulation of cord
blood-derived multipotent stem cells (CB-SCs) in stem cell educator
therapy: Phase I/II clinical trial. BMC Med. 11:1602013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haller MJ, Wasserfall CH, Hulme MA,
Cintron M, Brusko TM, McGrail KM, Wingard JR, Theriaque DW, Shuster
JJ, Ferguson RJ, et al: Autologous umbilical cord blood infusion
followed by oral docosahexaenoic acid and vitamin D supplementation
for C-peptide preservation in children with type 1 diabetes. Biol
Blood Marrow Transplant. 19:1126–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling W, Zhang J, Yuan Z, Ren G, Zhang L,
Chen X, Rabson AB, Roberts AI, Wang Y and Shi Y: Mesenchymal stem
cells use IDO to regulate immunity in tumor microenvironment.
Cancer Res. 74:1576–1587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gharibi T, Ahmadi M, Seyfizadeh N,
Jadidi-Niaragh F and Yousefi M: Immunomodulatory characteristics of
mesenchymal stem cells and their role in the treatment of multiple
sclerosis. Cell Immunol. 293:113–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pischiutta F, D'Amico G, Dander E, Biondi
A, Biagi E, Citerio G, De Simoni MG and Zanier ER:
Immunosuppression does not affect human bone marrow mesenchymal
stromal cell efficacy after transplantation in traumatized mice
brain. Neuropharmacology. Nov 15–2013.(Epub ahead of print).
PubMed/NCBI
|
|
9
|
Zhang Y, Cai W, Huang Q, Gu Y, Shi Y,
Huang J, Zhao F, Liu Q, Wei X, Jin M, et al: Mesenchymal stem cells
alleviate bacteria-induced liver injury in mice by inducing
regulatory dendritic cells. Hepatology. 59:671–682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heldman AW, DiFede DL, Fishman JE,
Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR,
Suncion VY, McNiece IK, et al: Transendocardial mesenchymal stem
cells and mononuclear bone marrow cells for ischemic
cardiomyopathy: The TAC-HFT randomized trial. JAMA. 311:62–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conforti A, Biagini S, Del Bufalo F,
Sirleto P, Angioni A, Starc N, Li Pira G, Moretta F, Proia A,
Contoli B, et al: Biological, functional and genetic
characterization of bone marrow-derived mesenchymal stromal cells
from pediatric patients affected by acute lymphoblastic leukemia.
PloS One. 8:e769892013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Cheng H, Hua R, Yang J, Dai G,
Zhang Z, Wang R, Qin C and An Y: Effects of bone marrow mesenchymal
stromal cells on gross motor function measure scores of children
with cerebral palsy: A preliminary clinical study. Cytotherapy.
15:1549–1562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou
Q, Tong C, Ti D, Dong L, Cheng Y, et al: Multiple intravenous
infusions of bone marrow mesenchymal stem cells reverse
hyperglycemia in experimental type 2 diabetes rats. Biochem Biophys
Res Commun. 436:418–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang
X, Shao Y, Yang S and Han ZC: Transplantation of placenta-derived
mesenchymal stem cells in type 2 diabetes: A pilot study. Front
Med. 5:94–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Zheng P, Wang X, Dai G, Cheng H,
Zhang Z, Hua R, Niu X, Shi J and An Y: A preliminary evaluation of
efficacy and safety of Wharton's jelly mesenchymal stem cell
transplantation in patients with type 2 diabetes mellitus. Stem
Cell Res Ther. 5:572014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y,
Shen J, Cheng Y, Fu X and Han W: Infusion of mesenchymal stem cells
ameliorates hyperglycemia in type 2 diabetic rats: Identification
of a novel role in improving insulin sensitivity. Diabetes.
61:1616–1625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lambert M: ADA releases revisions to
recommendations for standards of medical care in diabetes. Am Fam
Physician. 85:514–515. 2012.PubMed/NCBI
|
|
18
|
Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H,
Chen Y, Zhao W, Jia Z, Yan S and Wang Y: Long term effects of the
implantation of Wharton's jelly-derived mesenchymal stem cells from
the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr
J. 60:347–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Wang F, Sun R, Wang Z, Yu X, Wang L,
Gao H, Zhao W, Yan S and Wang Y: Effect of combined therapy of
human Wharton's jelly-derived mesenchymal stem cells from umbilical
cord with sitagliptin in type 2 diabetic rats. Endocrine.
45:279–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zilliox LA, Ruby SK, Singh S, Zhan M and
Russell JW: Clinical neuropathy scales in neuropathy associated
with impaired glucose tolerance. J Diabetes Complications.
29:372–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu L, Fernandez-Loaiza P, Sauma J,
Hernandez-Bogantes E and Masis M: Classification of diabetic
retinopathy and diabetic macular edema. World J Diabetes.
4:290–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu J, Wang Y, Wang F, Wang L, Yu X, Sun R,
Wang Z, Wang L, Gao H, Fu Z, et al: Effect and mechanisms of human
Wharton's jelly-derived mesenchymal stem cells on type 1 diabetes
in NOD model. Endocrine. 48:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, Li
H, Wang YW, Ren J and Wu ZB: Treatment of foot disease in patients
with type 2 diabetes mellitus using human umbilical cord blood
mesenchymal stem cells: Response and correction of immunological
anomalies. Curr Pharm Des. 19:4893–4899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Josse J, Velard F, Mechiche Alami S, Brun
V, Guillaume C, Kerdjoudj H, Lamkhioued B and Gangloff SC:
Increased internalization of Staphylococcus aureus and cytokine
expression in human Wharton's jelly mesenchymal stem cells. Biomed
Mater Eng. 24(Suppl 1): S27–S35. 2014.
|
|
25
|
Wang Y, Xue M, Xuan YL, Hu HS, Cheng WJ,
Suo F, Li XR, Yan SH and Wang LX: Mesenchymal stem cell therapy
improves diabetic cardiac autonomic neuropathy and decreases the
inducibility of ventricular arrhythmias. Heart Lung Circ.
22:1018–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman MJ, Regn D, Bashratyan R and Dai
YD: Exosomes released by islet-derived mesenchymal stem cells
trigger autoimmune responses in NOD mice. Diabetes. 63:1008–1020.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bassi ÊJ, Moraes-Vieira PM, Moreira-Sá CS,
Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva
A and Câmara NO: Immune regulatory properties of allogeneic
adipose-derived mesenchymal stem cells in the treatment of
experimental autoimmune diabetes. Diabetes. 61:2534–2545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ezquer F, Ezquer M, Simon V and Conget P:
The antidiabetic effect of MSCs is not impaired by insulin
prophylaxis and is not improved by a second dose of cells. PloS
One. 6:e165662011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho JH, Tseng TC, Ma WH, Ong WK, Chen YF,
Chen MH, Lin MW, Hong CY and Lee OK: Multiple intravenous
transplantations of mesenchymal stem cells effectively restore
long-term blood glucose homeostasis by hepatic engraftment and
β-cell differentiation in streptozocin-induced diabetic mice. Cell
Transplant. 21:997–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu J, Li C, Wang L, Zhang X, Zhang M, Gao
H, Yu X, Wang F, Zhao W, Yan S and Wang Y: Long term effects of the
implantation of autologous bone marrow mononuclear cells for type 2
diabetes mellitus. Endocr J. 59:1031–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan N, Sun H, Wang Y, Zhang L, Xia Z, Peng
L, Hou Y, Shen W, Liu R and Peng Y: Midkine, a potential link
between obesity and insulin resistance. PloS One. 9:e882992014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee BC and Lee J: Cellular and molecular
players in adipose tissue inflammation in the development of
obesity-induced insulin resistance. Biochim Biophys Acta.
1842:446–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Y, Pereira RO, O'Neill BT, Riehle C,
Ilkun O, Wende AR, Rawlings TA, Zhang YC, Zhang Q, Klip A, et al:
Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake
independent of mTORC1 and GLUT4 translocation. Mol Endocrinol.
27:172–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huat TJ, Khan AA, Pati S, Mustafa Z,
Abdullah JM and Jaafar H: IGF-1 enhances cell proliferation and
survival during early differentiation of mesenchymal stem cells to
neural progenitor-like cells. BMC Neurosci. 15:912014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Milanesi A, Lee JW, Li Z, Da Sacco S,
Villani V, Cervantes V, Perin L and Yu JS: β-cell regeneration
mediated by human bone marrow mesenchymal stem cells. PloS One.
7:e421772012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin P, Zhang X, Wu Y, Li L, Yin Q, Zheng
L, Zhang H and Sun C: Streptozotocin-induced diabetic rat-derived
bone marrow mesenchymal stem cells have impaired abilities in
proliferation, paracrine, antiapoptosis and myogenic
differentiation. Transplant Proc. 42:2745–2752. 2010. View Article : Google Scholar : PubMed/NCBI
|