Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease with pathological characteristics including synovitis,
synovial hyperplasia, infiltration of inflammatory cell and
formation of pannus (1,2). RA has a population prevalence of 0.5–1%
worldwide (3). RA has been
associated with a range of risk factors, including genetics,
smoking, environment, immune regulation and infectious agents
(3). A variety of the molecular
mechanisms on the occurrence and development of RA have been
reported (4). In addition to
inflammatory and immunological responses, increased angiogenesis
has been considered play a vital role in the early pathological
process of RA and sustains synovitis by producing proinflammatory
cytokines and chemokines (5).
Vascular endothelial growth factor (VEGF), as the most effective
proangiogenic molecule, is highly expressed in RA synovial tissue
(6). Moreover, several studies have
been suggested that signal transducer and activator of
transcription (STAT3) (7) and
nuclear factor-κB (NF-κB) (8) are
associated with the pathogenesis of RA and have an important
regulatory effect on angiogenesis (9,10).
Currently, a variety of approaches, such as thermotherapy,
anti-inflammatory therapy and gene therapy, have been suggested to
treat RA (11). However, in
consideration of the effectiveness and side effects of drugs, it is
essential to identify and develop novel therapies for RA.
The total saponins of Rhizoma Dioscorea
nipponica (TSRDN), a traditional Chinese medicine (TCM)
extracted from the roots of RDN, has been suggested to aid in
dispelling wind, eliminating dampness, alleviating pain, promoting
blood circulation and suppressing cough (12). Previous studies have indicated that
TSRDN may be able to reduce levels of total cholesterol and serum
glucose, as well as eliminate hydroxyl radicals (13,14). A
prior study showed that TSRDN may improve acute gouty arthritis by
inhibiting the expression of inflammatory factors (15). However, it remains unclear whether
TSRDN has a therapeutic effect on RA.
In the present study, a collagen-induced arthritis
(CIA) rat model was established which had similar clinical and
pathological features to human RA (16). The aim of the study was to
investigate the role and therapeutic mechanism of TSRDN in RA by
detecting the expression levels of CD31, VEGF, STAT3 and NF-κB in
CIA rats.
Materials and methods
Animal models
Approval from the Animal Ethics Committee of the
Animal Laboratory Center of Chengde Medical University (Chengde,
China) was obtained prior to using the animals for research.
Healthy Wistar rats (age, 42 days; n=168; weight, 170±20 g; half
male and female) were provided by Vital River Laboratory Animal
Technology Co. Ltd. (Beijing, China), and acclimatized to a natural
day/night cycle and given ad libitum access to food and
water at 21°C for a week before the trial.
Collagen induced-arthritis (CIA) rat model was
established using the method described by Cremer et al
(17). In brief, type II collagen
emulsion was prepared by mixing bovine type II collagen (2 g/l;
Sigma-Aldrich, St. Louis, MO, USA) in 0.05 M acetic acid with an
equal volume of complete Freund's adjuvant (Sigma-Aldrich).
Subsequently, 10% chloral hydrate (Sigma-Aldrich) was used to
anesthetize rats, then the rats were injected intradermally with
type II collagen emulsion (0.2 ml) into the back and tail roots of
rats. The second immunisation was performed with the same dose of
type II collagen emulsion after 7 days. Arthritis was evaluated by
measuring the arthritis index (AI), according to the method
described in a previous study (18).
AI was measured by the joint swelling degree and the number of
affected joints as following: 0, Normal joints; 1, swelling
slightly of toe joints; 2, swelling of toe and digit joints; 3,
swelling of foot paw below the ankle joints; and 4, entire swelling
of foot paw including ankle joints. AI was defined as the sum of
scores of the limbs joint swelling level (total potential score of
16). Rat with an AI score of ≥4 were considered to be a successful
CIA model, while rats with AI <4 were excluded from the
study.
Animal grouping, drug administration
and sample collection
A total of 168 Wistar rats were enrolled in our
study. Of these, 32 rats were injected with physiological saline in
an equal volume as blank control, and 136 rats underwent the
establishment of CIA model. After the first immunization 0, 10, 20,
30, 40, 50 and 60 days, 8 rats were randomly selected to undergoing
analysis of joint swelling and AI in the model rat and blank
control groups, respectively. At 14 days after the first
immunization, CIA model rats were randomly allocated into 3 groups
(n=24 per group) and lavaged with an equal volume of double
distilled water (CIA group), TSRDN (25 mg/kg/day, RDN group) or
tripterygium (12 mg/kg/day, TP group) for 21 days, respectively.
TSRDN was provided by the Department of Traditional Chinese
Medicine of Chengde Medical University and tripterygium was
obtained from Huangshi Feiyun Pharmaceutical Co., Ltd. (Hubei,
China). The rats in blank group were also lavaged with an equal
volume of solvent for 21 days. Following the treatment periods, the
rats were anesthetized with 10% chloral hydrate and sacrificed by
cervical dislocation. The knee joint synovium were harvested for
histopathological and immunohistochemical assessment, and
nucleoproteins were extracted for detection of the DNA-binding
activity of NF-κB p65.
Histopathological assessment
Specimens were fixed in 4% paraformaldehyde solution
for 24 h, then paraffin-embedded tissue samples were cut into 5-µm
sections. Sections were incubated for 4 h for deparaffinization at
65°C and dehydrated with gradient ethanol. Subsequently, the
sections were stained with hematoxylin (Sigma-Aldrich) for 5 min.
Following differentiation in 1% hydrochloric acid alcohol for 2
sec, the sections were incubated in ammonia water for 2 min, and
stained with eosin for 1 min. Ultimately, the sections were
dehydrated, cleared and mounted with neutral resin. Light
microscopy (Olympus Corporation, Tokyo, Japan) was used to observe
the sections.
Immunohistochemical analysis of CD31,
VEGF and STAT3
The sections were incubated with 3%
H2O2 to block the endogenous peroxidase. An
antigen retrieval step in 10 mM citrate buffer (pH 6.0) for 10 min
was performed. Sections were blocked using normal goat serum
(Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
at 37°C for 30 min, then incubated with rabbit anti-rat CD31 (1:50;
cat. no. sc-1506; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), VEGF (1:50; cat. no. RB-222-P0; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and STAT3 antibody (1:50; cat. no. 1122-1;
Epitomics, Inc., Burlingame, CA, USA) for 30 min, respectively, and
washed with phosphate-buffered saline (PBS). Subsequently, the
sections were incubated with goat anti-rabbit secondary antibody
(1:200; cat. no. ZB-2301; Zhongshan Golden Bridge Biotechnology
Co., Ltd.) at 37°C for 30 min. Sections were stained with
3,3′-diaminobenzidine (Zhongshan Golden Bridge Biotechnology Co.,
Ltd.) for 5 min and restained with hematoxylin for 2 min. Primary
antibody was replaced by PBS in the negative controls. Positive
CD31 expression was detected in the cytoplasm or membrane of
vascular endothelial cells, which were used for marking and
counting micro vessel density (MVD) (19). Blood vessel distribution was observed
under a microscope at a magnification of ×100. The area with the
highest MVD was selected (hot spot) for the counting of
microvascular numbers under a microscope at a magnification of ×400
in three fields, the mean was recorded as the MVD of this tissue
section. The emergence of brown-yellowish inside the cytoplasm was
considered to indicate a positive result for VEGF cells. Positive
STAT3 cells were visualized in the cytoplasm and/or nucleus with
brown-yellowish coloration. VEGF and STAT3 expression levels were
calculated as the ratio of immune positive area and the whole area.
Three fields were randomly selected and observed at a magnification
of ×400, and the mean was recorded as the VEGF and STAT3 expression
levels of this tissue section.
Analysis of the DNA-binding activity
of NF-κB p65
Nuclear protein was extracted using a Nuclear
Extraction Kit (Active Motif, Carlsbad, CA, USA), following the
manufacturer's instructions. A BCA Protein Assay Kit (Pierce;
Thermo Fisher Scientific, Inc., Rockford, IL, USA) was used to
detect the protein concentration. DNA-binding activity assay for
NF-κB p65 in nuclear proteins was determined using a TransAM Assay
kit (cat. no. 40096; Active Motif). Briefly, 30 µl binding buffer
or binding buffer with 2 µl specific oligonucleotides was added
into each well. Subsequently, 20 µl lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) containing nuclear
protein was incubated with connection buffer for 1 h at 25°C in the
microplates. Subsequently, DNA oligonucleotide-bound protein was
detected with anti-p65 (1:1,000) and secondary antibody (1:1,000).
Successively, the chromogenic reagent and stop buffer were added
into the each hole. Absorbance was detected at 450 nm using a
microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 11 (SPSS, Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard deviation and analyzed using
one-way analysis of variance and Q test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in body weight, joint swelling
and AI
Compared with the control group, rats in the CIA
group exhibited symptoms including listlessness, slow response,
dry, sparse hair, reduced activity and food intake. In addition,
body weight was reduced by 10.78, 14.95, 18.20, 22.04 and 19.45% by
days 20, 30, 40, 50 and 60, respectively, in the CIA group rats
compared with the control group rats at the same period (P<0.01)
(Fig. 1A). In the CIA group rats,
the thickness of right leg foot cushion was increased by 9.15-,
9.43-, 5.44-, 3.74- and 2.78-fold on days 20, 30, 40, 50 and 60,
respectively, compared with the control group rats (P<0.01)
(Fig. 1B). Similarly, compared with
the control group rats, the thickness of left leg foot cushion was
increased by 5.56-, 5.56-, 2.87-, 2.43- and 2.16-fold on days 20,
30, 40, 50 and 60 in the CIA group rats (P<0.01) (Fig. 1B). Furthermore, there was no swelling
of foot paw and AI value was 0 in blank group rats; however, AI
value was significantly increased at days 20, 30, 40, 50 and 60
compared with the control group rats (P<0.01) (Fig. 1C).
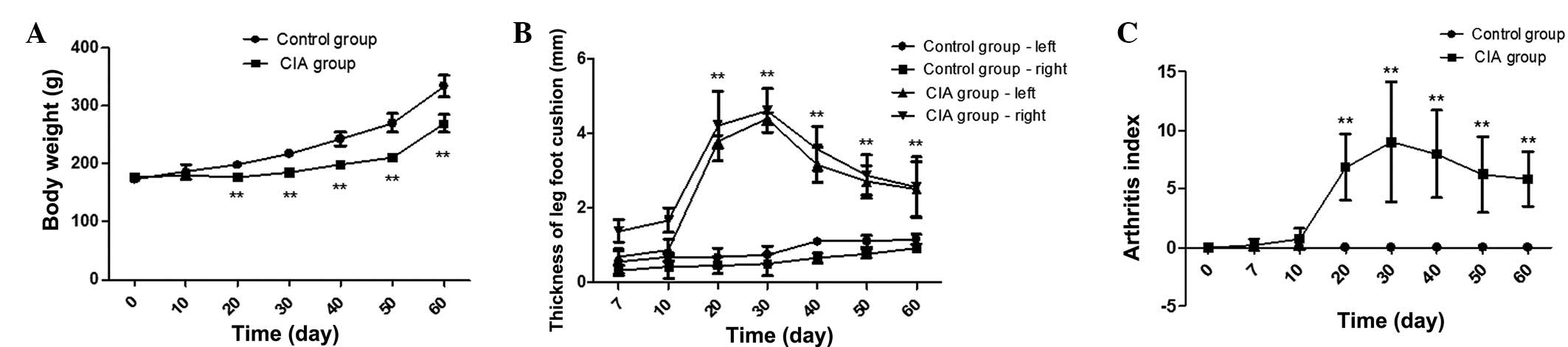 | Figure 1.Body weight, joint swelling and
arthritis index of rats in the control and CIA groups. (A) Body
weight was obviously lower at days 20, 30, 40, 50 and 60 in the CIA
group rats compared with control group rats at the same period. (B)
In the CIA group rats, the thickness of right and left leg foot
cushion was increased at days 20, 30, 40, 50 and 60 compared with
the control group rats; (C) AI value was increased at days 20, 30,
40, 50 and 60 in the CIA group compared with the control group
rats. **P<0.01 vs. control group. CIA, collagen-induced
arthritis. |
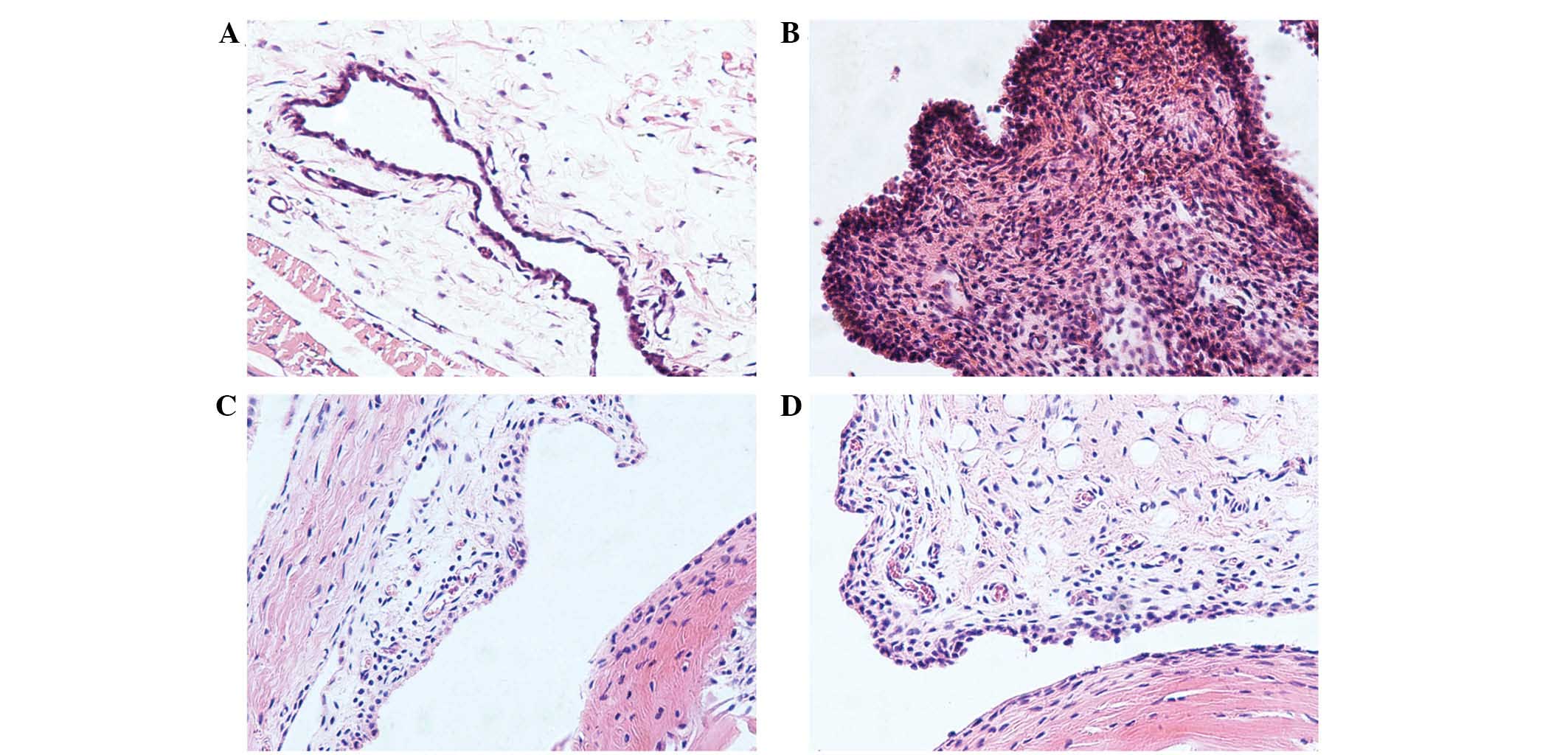
Histopathological changes of CIA rat
synovial tissue after treatment
As shown in Fig. 2A,
synovial and joint tissues in the control group rats exhibited no
lesions, and synoviocytes were arranged regularly. However, there
were obvious pathological features, including synovial hyperplasia,
formation of pannus, infiltration of inflammatory cell, cartilage
and bone erosion in the CIA group rats (Fig. 2B). Following treatment for 21 days,
these pathological features were alleviated in RDN group and TP
group compared with CIA group (Fig. 2C
and D).
Detection of CD31, VEGF and STAT3 in
CIA rat synovial tissue after treatment
Immunohistochemical analysis using anti-CD31
antibody showed that the number of CD31-labeled microvessels was
larger, and the level of MVD was higher in the CIA group rat
synovial tissue than the control group rats (P<0.01) (Table I; Fig.
3). Compared with CIA group, the level of MVD was reduced in
RDN and TP group rat synovial tissues (P<0.01) (Table I; Fig.
3), while there was no significant difference between the RDN
and TP groups (P>0.05) (Table I;
Fig. 3).
 | Figure 3.Immunohistochemical analysis of CD31,
VEGF and STAT3 expression in the synovial tissues of the various
groups. Compared with control group, the expression of CD31, VEGF
and STAT3 were all increased in CIA group rat synovial tissue;
however, total saponins of RDN or tripterygium appeared to inhibit
the expression of these proteins (magnification, ×400). CIA,
collagen-induced arthritis; RDN, Rhizoma Dioscoreae
nipponicae; TP, tripterygium; VEGF, vascular endothelial growth
factor; STAT3, signal transducer and activator of transcription
3. |
 | Table I.MVD level and expression of VEGF and
STAT3 in various groups. |
Table I.
MVD level and expression of VEGF and
STAT3 in various groups.
| Group | Dose (mg/kg/day) | MVD (n=8) | VEGF (n=8) | STAT3 (n=8) |
|---|
| Control | – |
3.00±0.71 |
0.0014±0.0002 |
0.448±0.354 |
| CIA | – |
9.20±1.30a |
0.1167±0.0207a |
11.669±2.374a |
| RDN | 25 |
4.80±0.45b |
0.0452±0.0078b |
3.281±1.374b |
| TP | 12 |
5.00±0.82b |
0.0420±0.0057b |
2.836±1.625b |
The expression of VEGF and STAT3 protein was
significantly increased in CIA group rat synovial tissue compared
with the control group rats (P<0.01) (Table I; Fig.
3). However, the VEGF and STAT3 expression levels were lower in
the RDN and TP group rat synovial tissues compared with the CIA
group rats (P<0.01) (Table I;
Fig. 3), and no significant
difference was detected between the RDN and TP groups (P>0.05)
(Table I; Fig. 3).
DNA-binding activity of NF-κB p65 in
CIA rat synovial tissue after treatment
Compared with the control group, a significant
increase (P<0.01) (Fig. 4) in the
DNA-binding activity of NF-κB p65 was observed in CIA group
synovial tissue. After treatment with RDN and TP, the DNA-binding
activity of NF-κB p65 was reduced (P<0.01) (Fig. 4), while there was no significant
difference between the RDN and TP groups (P>0.05) (Fig. 4).
Discussion
RA is an autoimmune, systemic disease that may lead
to the destruction of joints and subsequent disability (20). In the present study, we successfully
established a CIA rat model to investigate the therapeutic
potential of TSRDN. The results showed that TSRDN alleviated
synovial hyperplasia, infiltration of inflammatory cells and the
formation of pannus in CIA rats, as well as inhibiting the level of
MVD, the expression of VEGF and STAT3, and the DNA-binding activity
of NF-κB p65.
Angiogenesis involves the formation of new blood
vessels from preexisting ones, and serves a crucial function in
tissue development and repair (21).
The formation of new microvessels within the synovium is a major
feature of RA, which induced the development of pannus and resulted
in the cartilage erosion and destruction, indicating that
anti-angiogenesis may be an effective therapeutic strategy for RA
(22). VEGF and its receptor play a
vital role in angiogenesis by regulating endothelial cell
proliferation, migration and lumen formation (23,24).
Previous studies have demonstrated that compared to healthy
individuals, serum and synovial tissue VEGF concentrations were
significantly elevated in patients with RA (25,26).
Furthermore, the expression levels of VEGF and its specific
receptors, Flk-1 and Flt-1 were associated with arthritis severity
and the degree of neovascularization (27). Similarly, the present results
suggested that the level of MVD and the expression of VEGF protein
were significantly increased in CIA rat synovial tissue, which was
decreased after TSRDN or tripterygium treatment. In addition, TSRDN
appeared to alleviate synovial hyperplasia and pannus formation, as
indicated by observing the histological structure of synovial
membrane tissues. Tripterygium is a commonly used TCM for the
treatment of RA (28). The presents
results indicated that VEGF may be involved in the occurrence and
development of CIA by regulating angiogenesis, and TSRDN may be a
potential therapeutic drug for CIA.
To further investigate the mechanism of TSRDN in
angiogenesis and RA, we detected the expression levels of STAT3 and
NF-κB. The expression levels of various cytokines are known to be
significantly increased in RA synovial tissues, and the majority of
these are regulated via the JAK-STAT signaling pathway (7). Furthermore, the pathological process of
RA may be improved by blocking JAK-STAT pathway (6,29).
Shouda et al (30)
demonstrated that removing the functional domain of STAT3
attenuated CIA, and the hyperactivation of STAT3 may result in the
spontaneous development of autoimmune arthritis (31). Furthermore, Krause et al
(32) proposed a mechanism by which
STAT3 may promote the pathogenesis of RA by suppressing the
apoptosis of synovial fibroblasts. STAT3 plays a crucial regulatory
role in angiogenesis by upregulating VEGF expression (9,33,34).
Notably, we demonstrated that the expression of STAT3 protein was
significantly increased in CIA rat synovial tissue, and decreased
following TSRDN treatment, indicating that TSRDN may inhibit the
pathogenesis of CIA by downregulating STAT3 expression.
In addition, NF-κB, a protein with the function of
promoting gene transcription, has been shown to actively
participate in pathological processes such as joint inflammation
(35). NF-κB has been shown to be
widely expressed in RA synovial cells (36), which is consistent with the present
results. Previous studies have shown that NF-κB may be involved in
regulating angiogenesis (37), and
that inhibiting the activity of NF-κB in pancreatic cancer may
prevent the formation of new blood vessels (38). The present study showed that the
DNA-binding activity of NF-κB p65 could be inhibited by TSRDN.
Therefore, we speculated that NF-κB was involved in the inhibiting
effect of TSRDN on CIA, by regulating angiogenesis.
In conclusion, the present results suggested that
CIA rat synovial tissue had a large number of new blood vessels,
which was consistent with increased expression of VEGF protein;
however, TSRDN could inhibit this phenomenon. We speculated that
TSRDN might prevent angiogenesis by inhibiting the expression of
STAT3 and the DNA-binding activity of NF-κB p65, thereby played a
positive role in improving CIA. TSRDN may be a potential
therapeutic drug for CIA, and VEGF may be involved in the
development of CIA by regulating angiogenesis. Furthermore, TSRDN
could inhibit the pathogenesis of CIA by downregulating STAT3
expression. NF-κB was involved in the inhibiting effect of TSRDN on
CIA.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30873420).
References
|
1
|
Harris ED Jr: Rheumatoid arthritis.
Pathophysiology and implications for therapy. N Engl J Med.
322:1277–1289. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feldmann M, Brennan FM and Maini RN: Role
of cytokines in rheumatoid arthritis. Annu Rev Immunol. 14:397–440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gabriel SE: The epidemiology of rheumatoid
arthritis. Rheum Dis Clin North Am. 27:269–281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Eng J Med. 365:2205–2219. 2011.
View Article : Google Scholar
|
|
5
|
Marrelli A, Cipriani P, Liakouli V,
Carubbi F, Perricone C, Perricone R and Giacomelli R: Angiogenesis
in rheumatoid arthritis: A disease specific process or a common
response to chronic inflammation? Autoimmun Rev. 10:595–598. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fava RA, Olsen NJ, Spencer-Green G, Yeo
KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF and Brown LF:
Vascular permeability factor/endothelial growth factor (VPF/VEGF):
Accumulation and expression in human synovial fluids and rheumatoid
synovial tissue. J Exp Med. 180:341–346. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walker JG and Smith MD: The Jak-STAT
pathway in rheumatoid arthritis. J Rheumatol. 32:1650–1653.
2005.PubMed/NCBI
|
|
8
|
Tak PP, Gerlag DM, Aupperle KR, van de
Geest DA, Overbeek M, Bennett BL, Boyle DL, Manning AM and
Firestein GS: Inhibitor of nuclear factor kappaB kinase beta is a
key regulator of synovial inflammation. Arthritis Rheum.
44:1897–1907. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z and Han ZC: STAT3: A critical
transcription activator in angiogenesis. Med Res Rev. 28:185–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smolen JS, Aletaha D, Koeller M, Weisman
MH and Emery P: New therapies for treatment of rheumatoid
arthritis. Lancet. 370:1861–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan W, Ji L, Hang S and Shun Y: New ionic
liquid-based preparative method for diosgenin from Rhizoma
dioscoreae nipponicae. Pharmacogn Mag. 9:250–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang TS, Liang SJ, Lii CK and Liu SY:
Protective effect of water yam (Dioscorea alata L.) extract on the
copper driven fenton reaction and X-ray induced DNA damage in
vitro. Phytother Res. 18:325–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon JR, Lee JS, Lee CH, Kim JY, Kim SD
and Nam DH: Effect of ethanol extract of dried Chinese yam
(Dioscorea batatas) flour containing dioscin on gastrointestinal
function in rat model. Arch Pharm Res. 29:348–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao L, Dong W, Lu F and Liu S: An improved
acute gouty arthritis rat model and therapeutic effect of rhizoma
dioscoreae nipponicae on acute gouty arthritis based on the
protein-chip methods. Am J Chin Med. 40:121–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wooley PH, Luthra HS, Stuart JM and David
CS: Type II collagen-induced arthritis in mice. I. Major
histocompatibility complex (I region) linkage and antibody
correlates. J Exp Med. 154:688–700. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cremer M: Type II collagen-induced
arthritis in ratsHandbook of Animal Models for the Rheumatic
Diseases. Greenwald RA and Diamond HS: 1. CRC Press; Boca Raton,
FL: pp. 17–27. 1988
|
|
18
|
Welles WL and Battisto JR: Suppression of
adjuvant arthritis by antibodies specific for collagen type II.
Immunol Commun. 10:673–685. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anic GM, Thompson RC, Nabors LB, Olson JJ,
Browning JE, Madden MH, Murtagh FR, Forsyth PA and Egan KM: An
exploratory analysis of common genetic variants in the vitamin D
pathway including genome-wide associated variants in relation to
glioma risk and outcome. Cancer Causes Control. 23:1443–1449. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamanishi Y, Boyle DL, Pinkoski MJ,
Mahboubi A, Lin T, Han Z, Zvaifler NJ, Green DR and Firestein GS:
Regulation of joint destruction and inflammation by p53 in
collagen-induced arthritis. Am J Pathol. 160:123–130. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semerano L, Clavel G, Assier E, Denys A
and Boissier MC: Blood vessels, a potential therapeutic target in
rheumatoid arthritis? Joint Bone Spine. 78:118–123. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrara N: Role of vascular endothelial
growth factor in the regulation of angiogenesis. Kidney Int.
56:794–814. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carrato A, Gallego-Plazas J and
Guillen-Ponce C: Anti-VEGF therapy: A new approach to colorectal
cancer therapy. Expert Rev Anticancer Ther. 6:1385–1396. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SS, Joo YS, Kim WU, Min DJ, Min JK,
Park SH, Cho CS and Kim HY: Vascular endothelial growth factor
levels in the serum and synovial fluid of patients with rheumatoid
arthritis. Clin Exp Rheumatol. 19:321–324. 2001.PubMed/NCBI
|
|
26
|
Ballara S, Taylor PC, Reusch P, Marmé D,
Feldmann M, Maini RN and Paleolog EM: Raised serum vascular
endothelial growth factor levels are associated with destructive
change in inflammatory arthritis. Arthritis Rheum. 44:2055–2064.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu J, Kasama T, Kobayashi K, Yoda Y,
Shiozawa F, Hanyuda M, Negishi M, Ide H and Adachi M: Vascular
endothelial growth factor expression and regulation of murine
collagen-induced arthritis. J Immunol. 164:5922–5927. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao X, Cush JJ, Garret M and Lipsky PE: A
phase I study of ethyl acetate extract of the chinese antirheumatic
herb Tripterygium wilfordii hook F in rheumatoid arthritis. J
Rheumatol. 28:2160–2167. 2001.PubMed/NCBI
|
|
29
|
Walker JG, Ahern MJ, Coleman M, Weedon H,
Papangelis V, Beroukas D, Roberts-Thomson PJ and Smith MD: Changes
in synovial tissue Jak-STAT expression in rheumatoid arthritis in
response to successful DMARD treatment. Ann Rheum Dis.
65:1558–1564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shouda T, Yoshida T, Hanada T, Wakioka T,
Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, et
al: Induction of the cytokine signal regulator SOCS3/CIS3 as a
therapeutic strategy for treating inflammatory arthritis. J Clin
Invest. 108:1781–1788. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Atsumi T, Ishihara K, Kamimura D, Ikushima
H, Ohtani T, Hirota S, Kobayashi H, Park SJ, Saeki Y, Kitamura Y
and Hirano T: A point mutation of Tyr-759 in interleukin 6 family
cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp
Med. 196:979–990. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krause A, Scaletta N, Ji JD and Ivashkiv
LB: Rheumatoid arthritis synoviocyte survival is dependent on
Stat3. J Immunol. 169:6610–6616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB,
Lee CN and Hsieh CY: Interleukin-6 promotes cervical tumor growth
by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene.
22:1517–1527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Makarov SS: NF-kappaB in rheumatoid
arthritis: A pivotal regulator of inflammation, hyperplasia, and
tissue destruction. Arthritis Res. 3:200–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Martin R, Hoeth M, Hofer-Warbinek R and
Schmid JA: The transcription factor NF-kappa B and the regulation
of vascular cell function. Arterioscler Thromb Vasc Biol.
20:E83–E88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiong HQ, Abbruzzese JL, Lin E, Wang L,
Zheng L and Xie K: NF-kappaB activity blockade impairs the
angiogenic potential of human pancreatic cancer cells. Int J
Cancer. 108:181–188. 2004. View Article : Google Scholar : PubMed/NCBI
|