Introduction
Atherosclerosis (AS) is characterized by the
accumulation of lipids in the walls of large and medium-sized
arteries, resulting in plaque formation and narrowing of the
arterial lumens (1). Although the
underlying etiology of AS remains poorly defined, it is generally
accepted that AS is not only a disorder of lipids, but also a
chronic autoimmune inflammatory disease (2). Evidence from AS-prone models suggested
that various immune cells and inflammatory cytokines were present
in atherosclerotic lesions, and that a complex imbalance existed
between pro-inflammatory and anti-inflammatory factors, indicating
that this imbalance may have an important role in AS initiation and
progression (3,4). In the immune system, CD4+
regulatory T-cells (Treg) are a master subset of
regulatory T-cells that have a critical role in limiting the
process of AS (5). It has been
reported that the transcription factor forkhead box protein 3
(Foxp3) is specifically expressed in CD4+
Treg cells, and is a key marker of CD4+
Treg cells (6). The
functions of Treg cells were deficient in patients with
immunodysregulation polyendocrinopathy enteropathy X-linked
syndrome and the scrufy (sf) mouse model due to a Foxp3 mutation
(7,8), thereby demonstrating the importance of
Foxp3 for the functions of Treg cells. Sf is an X-linked
recessive mouse mutant resulting in lethality in hemizygous males
16–25 days after birth, and is characterized by overproliferation
of CD4+CD8− T lymphocytes, extensive
multiorgan infiltration and elevation of numerous cytokines.
Furthermore, previous studies demonstrated that Foxp3 was
indispensable for the development and function of Treg
cells (7–9). Therefore, stimulating the expression of
Foxp3 and increasing the numbers of Treg cells may be
important strategies for the treatment of AS. Treg cells
mediate the immunosupression via cell-to-cell contact and secretion
of anti-inflammatory factors, including interleukin (IL)-10,
transforming growth factor-β and IL-35 (10).
IL-35, which was identified in 2007 as a member of
the IL-12 family, is a heterodimer composed of Epstein-Barr
virus-induced protein (EBI)-3 (a subunit of IL-27) and p35 (a
subunit of IL-12) (11,12). Subsequent studies determined that
IL-35 is predominantly secreted by CD4+ Treg
cells (13). Evidence from a mouse
model of rheumatoid arthritis demonstrated that IL-35 is an
anti-inflammatory cytokine that inhibits the activity of effector T
cells (Teff), improves the activity of Treg
cells, reduces the secretion of inflammatory factors and suppresses
autoimmune diseases (14). In
particular, IL-35 was observed to attenuate established rheumatoid
arthritis, which indicated that IL-35 has an important role in
maintaining the activity of Treg cells (14). In addition, it has been reported that
Ebi3 and p35 are strongly co-expressed in the majority of advanced
lesions, thus suggesting that IL-35 is associated with AS (15). Previous studies have reported that
IL-35 may have a protective effect on the progression of AS
(16,17). However, the exact role of IL-35 in AS
remains poorly understood. The present study aimed to investigate
whether exogenous intervention with IL-35 was able to attenuate the
formation of atherosclerotic lesions in advanced AS
apoE−/− mice. In addition, alterations in the
expression levels of Foxp3 in peripheral blood and atherosclerotic
lesions during the progression of AS were analyzed.
Materials and methods
Reagents
Atorvastatin calcium was purchased from AstraZeneca
(London, UK). Recombinant human IL-35 was obtained from Sino
Biological Inc. (Beijing, China). The peripheral blood mononuclear
cell (PBMC) kit was purchased from Tianjin Haoyang Biological
Products Technology, Co., Ltd. (Tianjin, China). Fluorescein
isothiocyanate (FITC)-conjugated anti-CD4 and phycoerythrin
(PE)-conjugated anti-CD25 antibodies were purchased from
eBioscience Inc. (San Diego, CA, USA). Allophycocyanin
(APC)-conjugated anti-Foxp3 antibody was obtained from Miltenyi
Biotec GmbH (Bergisch Gladbach, Germany). Anti-Foxp3 antibody was
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). SP-9000/9001/9002 SPlink Detection kits were purchased from
OriGene Technologies, Inc. (Beijing, China) Diagnostic enzyme assay
kits [total cholesterol test kit, cat. no. F002-2; triglyceride
test kit, cat. no. F001-2; high density lipoprotein-cholesterol
(HDL-C) test kit, cat. no. F003-2; and low density
lipoprotein-cholesterol (LDL-C) test kit, cat. no. F004-2] were
obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
Animals and groups
Male apoE−/− mice (age, 8
weeks old; weight, 0.02±0.003 kg) were purchased from Vital River
Laboratory in Beijing, China. The mice were maintained at room
temperature in a sterilized laboratory with food and sterilized
water ad libitum. The apoE−/− mice
were divided into two groups, as follows: The negative control
group (n=8), who received a basal diet, and the high-fat diet (HFD)
group (n=24). The normal diet and HFD, constituting 81.85% of the
basal diet, 0.15% cholesterol and 18% lard, were purchased from the
Experimental Animal Center of Anhui Medical University (Hefei,
China).
After 4 weeks, the HFD group was further divided
into three subgroups (n=8/group), as follows: i) the AS control
group, which did not received any treatment; ii) the drug control
group, in which the mice were orally administered with atorvastatin
calcium (5 mg/kg); and iii) the exogenous intervention group, in
which mice were intraperitoneally injected with IL-35 (1.2 mg/kg)
once daily for 12 weeks.
At the end of the experiment and prior to sacrifice
of the mice, fresh blood samples were taken intravenously from the
epicanthal folds of mice in each group using tubes containing
heparin sodium. Subsequently, the tubes were centrifuged at 500 × g
for 15 min at 22–25°C to collect PBMCs for flow cytometry. The mice
were fasted for 12 h prior to sacrifice. Following anesthetization
with 10% chloral hydrate (4.8 ml/kg), blood was collected from the
inferior vena cava for biochemical detection. The mice were then
sacrificed following chest opening from excessive loss of blood and
cardiac arrest. Aortic root sections were generated for hematoxylin
and eosin (H&E) staining and immunohistochemical analyses. All
procedures complied with and were approved by the Internal Animal
Care and Use Committee of Anhui Medical University.
Detection of serum lipids
At the end of the experiment, blood collected from
the inferior vena cava of the mice in tubes was incubated for 2 h
at room temperature, followed by centrifugation at 1,000 × g for 15
min at 22–25°C to prepare for detection of serum lipids. Total
cholesterol (TC), total triglyceride (TG), HDL and LDL levels were
detected using diagnostic enzyme assay kits.
H&E staining and
immunohistochemistry
Following anesthetization with 10% chloral hydrate,
the mice were injected into the apical muscle with normal saline
and 4% paraformaldehyde was flushed through the heart vascular
system and intercepted thoracic aorta, fixed in 4% paraformaldehyde
for 24 h, then dehydrated and embedded in paraffin longitudinally.
Aortic root sections (4 µm) were cut from the embedded hearts. To
prepare for immunohistochemical analysis, the paraffin-embedded
tissue sections were deparaffinized, immersed in phosphate-buffered
saline and blocked with 3% H2O2 solution for
30 min at room temperature to inhibit endogenous peroxidase
activity. Subsequently, the tissue sections were incubated with
normal goat serum (included in the SPlink Detection kits) at 37°C
for 30 min, followed by incubation with anti-Foxp3 antibody
overnight at 4°C. Next, the deparaffinized sections were incubated
with biotinylated goat anti-rabbit immunoglobulin G (1:200; cat.
no. SP9000-3; OriGene Technologies, Inc.), followed by
horseradish-streptavidin complex for 30 min at 37°C. Finally, the
sections were incubated with 3,3′-diaminobenzidine and stained with
hematoxylin for 2 min. Since Foxp3 is expressed in the nucleus
(18), positive staining was
indicated by brown coloration of the nucleus. Foxp3 expression was
analyzed for the vascular atherosclerotic plaques within every
section. Ten visual fields were randomly selected, and the number
of positive cells was calculated in each field to obtain the mean.
The Image-Pro Plus 5.1 Image Operation system was used to capture
images of the sections. The intimal thickness and area of a plaque
were measured using the JD-801 Pathological Image Analysis system.
The protocols for H&E staining and immunohistochemistry were
performed according to previous studies (19,20).
Flow cytometry
PBMCs were isolated from fresh peripheral blood, and
the number of cells was adjusted to a concentration of
1×106. The PBMCs were stained with FITC-conjugated
anti-CD4 and PE-conjugated anti-CD25 antibodies (1:100) for 30 min
at 22–25°C to label cell surface antigens. Subsequently, the cells
were fixed and perforated using 1 ml Fixation/Permeabilization
solution for 30 min at 4°C in the dark. The cells were repeatedly
washed with 2 ml permeabilization buffer, followed by staining with
diluted APC-conjugated anti-Foxp3 antibody at 4°C for 30 min in the
dark. Finally, the cells were washed repeatedly and resuspended at
1×106 in flow cytometry staining buffer. Flow cytometry
was performed using the Beckman Coulter Epics XL™ Flow Cytometer
(Beckman Coulter, Inc., Brea, CA, USA), and the image was analyzed
using FlowJo 7.6.1 software (http://www.flowjo.com/download-flowjo/).
Statistical analysis
Data are presented as the mean ± standard deviation.
Using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA), comparison
between groups was carried out by one-way analysis of variance. If
homogeneity of variance was found, a Student-Newman-Keuls test was
performed to analyze differences among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Exogenous IL-35 downregulates lipid
levels in apoE-/− mice
Since lipids are important in the development of AS,
the present study analyzed the levels of TC, TG, HDL and LDL in a
mouse model of AS. As is shown in Table
I and Fig. 1, the levels of TC,
TG, HDL and LDL were significantly increased in the AS group (all
P<0.01), as compared with the negative group. In addition, a
significant reduction in the levels of TC, TG, HDL and LDL
(P<0.05) was observed in the atorvastatin-treated mice, as
compared with the AS group. Treatment with IL-35 resulted in a
significant decrease in the levels of TC and TG (P<0.05), as
compared with the AS group. However, there was no significant
difference in the levels of HDL and LDL between the AS and
IL-35-treated groups (P>0.05), and between the atorvastatin- and
IL-35-treated groups (P>0.05).
 | Figure 1.Levels of serum lipids. Blood was
collected and centrifuged at 1,000 × g for 10 min to prepare for
detection of serum lipids. TC, TG, HDL and LDL contents were
analyzed by diagnostic enzyme assay kits. Representative histograms
are shown. Data are presented as the mean ± standard deviation.
Comparisons between groups were analyzed by one-way analysis of
variance and Student-Newman-Keuls test (n=8). *P<0.01, vs. the
negative group. **P<0.01, #P<0.05, vs. the AS
group. TC, total cholesterol; TG, total triglycerides; HDL,
high-density lipoprotein; LDL, low-density lipoprotein; AS,
atherosclerosis; IL-35, interleukin-35. |
 | Table I.Lipid levels in the various
groups. |
Table I.
Lipid levels in the various
groups.
| Group (n=8) | TC (mmol/l) | TG (mmol/l) | HDL (mmol/l) | LDL (mmol/l) |
|---|
| Negative | 12.42±1.47 | 1.68±0.38 |
2.57±0.36a |
2.72±0.27a |
| AS |
17.36±1.25a |
2.24±0.18a |
3.61±0.15a |
3.67±0.18a |
| Atorvastatin |
11.35±1.37b |
1.78±0.28c |
4.15±0.23c |
2.32±0.21c |
| IL-35 |
14.13±1.46c |
1.96±0.27c | 3.96±0.17 | 3.14±0.24 |
Exogenous IL-35 attenuates
atherosclerotic lesions
Changes to lesions were analyzed using H&E
staining and Image-Pro Plus software. Fig. 2A shows that the vessel wall was
smooth, and the elastic plates were clear and neat, in the negative
group. In addition, endothelial cells were arranged uniformly and
there was minimal evidence of plaque formation. Conversely, in the
AS group, eminences were diffused beneath the vascular
dissepiments, and hyperplasia of the intima was observed (Fig. 2B). Furthermore, there were a large
proportion of foam cells and cholesterol crystals, and a few
inflammatory cells; endothelial cells were disordered; the intima
appeared discontinuous; rupture and discontinuity of the internal
elastic was observed; and the arrangement of smooth muscle cells
with spindle-shaped cores was disordered. However, treatment with
atorvastatin calcium (Fig. 2C) and
IL-35 (Fig. 2D) significantly
reduced the proportions of foam cells, cholesterol crystals and
inflammatory cells.
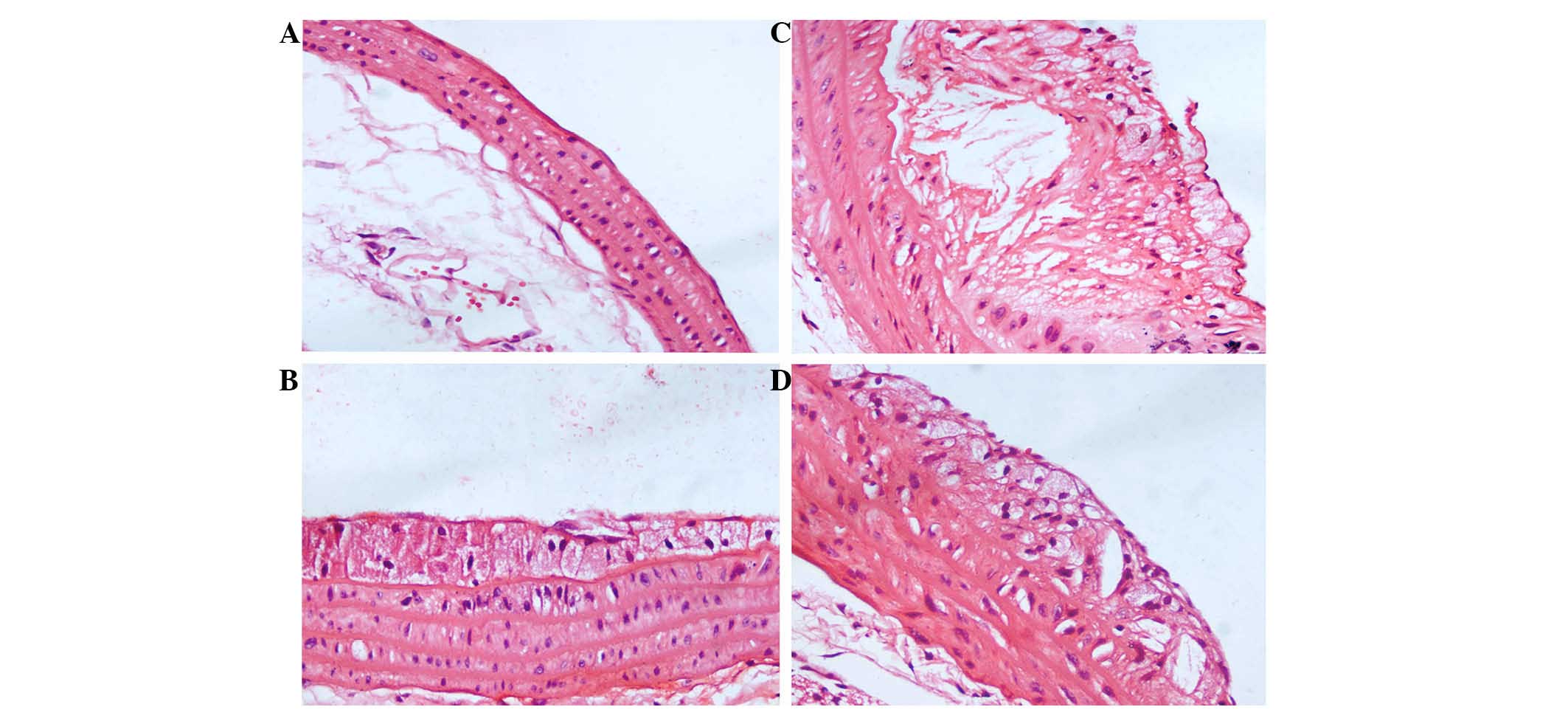 | Figure 2.Characteristics of arterial lesions.
We analyzed changes in the atherosclerotic lesions by hematoxylin
and eosin staining and Image-Pro Plus software (magnification,
×400). (A) In the negative group, the vessel walls were smooth, and
the elastic plates were clear and neat. Endothelial cells were
arranged uniformly, and there was almost no evidence of plaque
formation. (B) In the atherosclerosis (AS) group, there were large
numbers of foam cells and cholesterol crystals, and a few
inflammatory cells. Endothelial cells and the smooth muscle cells
with spindle-shaped cores were disordered, and rupture and
discontinuity of the internal elastic was observed. In the (C)
atorvastatin and (D) interleukin-35 groups, the proportions of foam
cells, cholesterol crystals and inflammatory cells were reduced, as
compared with the AS group. |
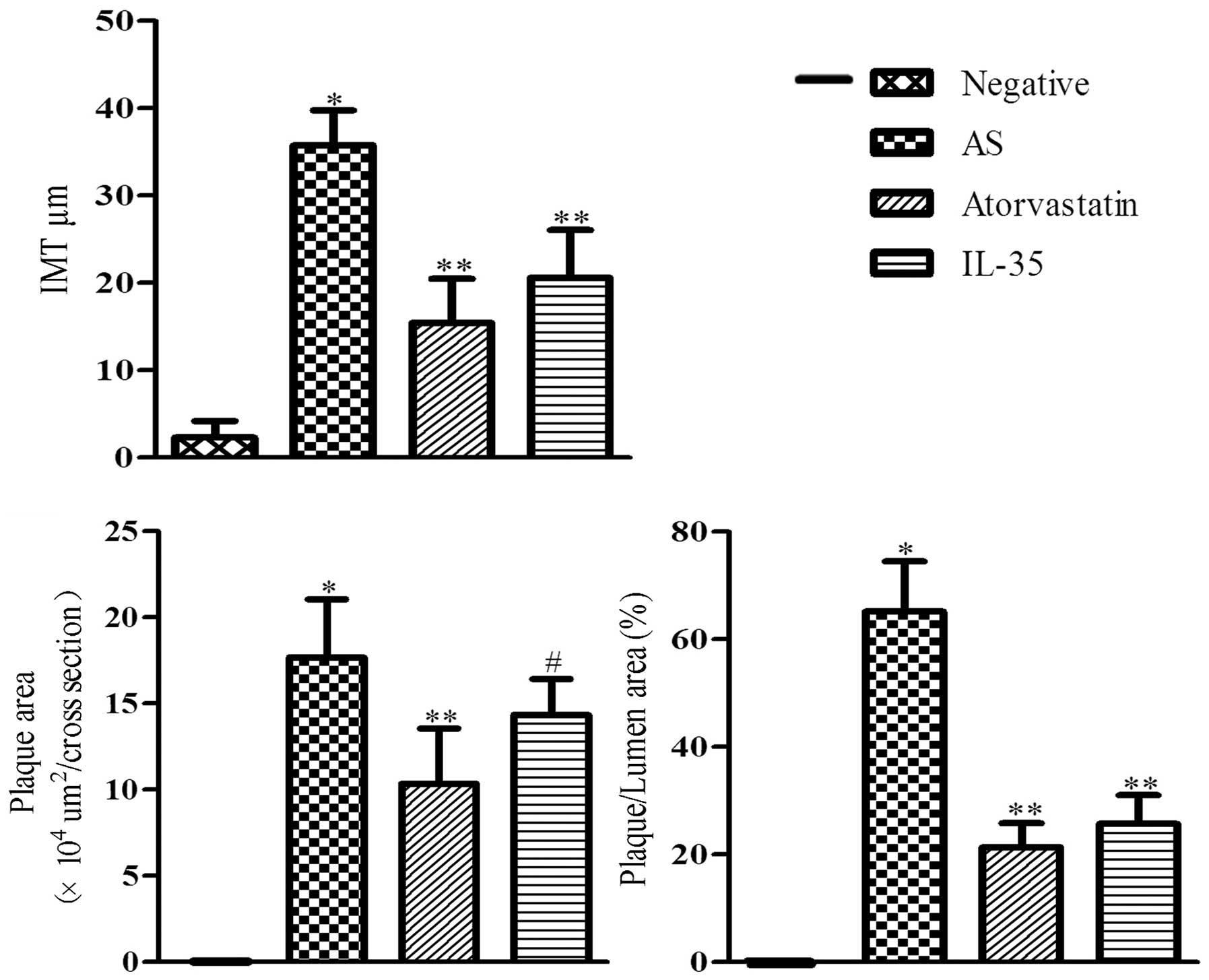
In addition, the intima, plaque area and
plaque/lumen area were measured. As is shown in Table II and Fig. 3, the HFD diet was associated with
thicker intima and larger plaque areas. As compared with the
negative group, the mean intima thickness of the AS group was
significantly increased (10.63±2.17 vs. 151.54±17.52 µm;
P<0.01). Treatment with atorvastatin or IL-35 resulted in a
significant reduction in intima thickness, which was reduced to
36.7±6.37 and 70.61±9.85 µm, respectively (P<0.01). A
significant increase in the plaque area and plaque/lumen area were
observed in the AS group, as compared with the negative group
(P<0.01). Conversely, the plaque/lumen area in the
atorvastatin-treated and IL-35-treated mice were reduced from
38.13% in the AS group to 10.24 and 24.19%, respectively. These
results suggest that IL-35 attenuates the advancement of
atherosclerotic lesions.
 | Table II.IMT and plaque areas of
apoE−/−mice. |
Table II.
IMT and plaque areas of
apoE−/−mice.
| Group (n=8) | IMT (µm) | Plaque area
(×105 µm2/cross section) | Plaque/lumen area
(%) |
|---|
| Negative | 10.63±2.17 | 0 | 0 |
| AS |
151.54±17.52a |
3.01±0.49a |
38.13±5.72a |
| Atorvastatin |
36.7±6.37b |
0.81±0.05b |
10.24±1.14b |
| IL-35 |
70.61±9.85b |
2.04±0.28b |
24.19±4.27b |
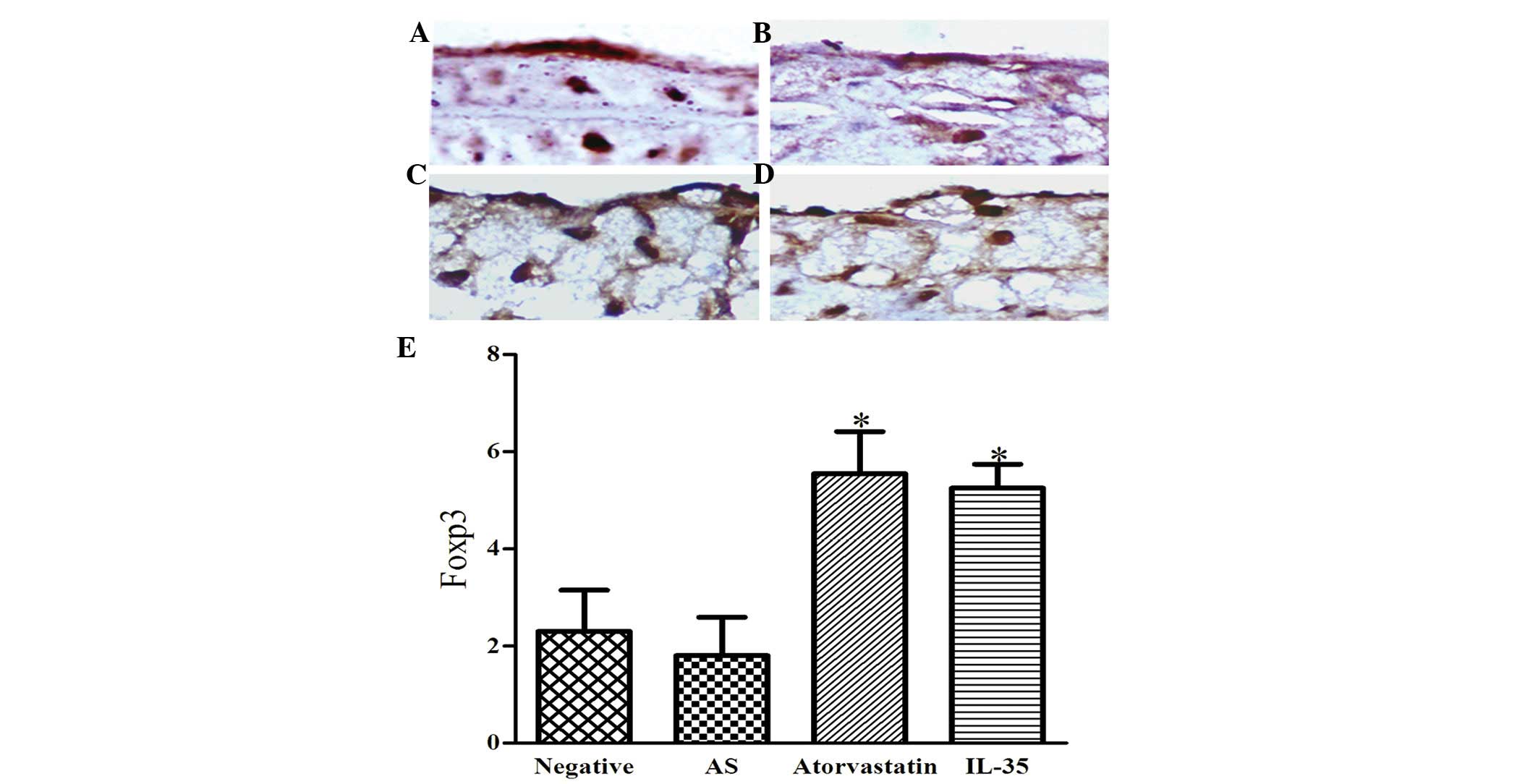
IL-35 upregulates the expression of
Foxp3 in apoE-/- mice
It has been reported that IL-35 is not only secreted
by Treg cells, but is also an inducer of Treg
cells and is important for maintaining the function of these cells
(21). Therefore, the present study
detected the effect of exogenous IL-35 on the proportions of
CD4+CD25+Foxp3+Treg/CD4+Treg
cells in apoE−/− mice using flow cytometry.
As is shown in Fig. 4A-C, there was
no significant difference in the proportions of
CD4+CD25+Foxp3+Treg/CD4+Treg
cells between the negative and AS groups, although the ratio of
Foxp3+ Treg/CD4+ Treg
cells appeared reduced in the AS group. However, treatment of the
AS mice with atorvastatin or IL-35 resulted in a significant
increase in the proportions of
CD4+CD25+Foxp3+Treg/CD4+Treg
cells (P<0.01; Fig. 4A, D and E).
There was no significant difference between the mice in the
IL-35-treated and atorvastatin-treated groups (P>0.05). These
results suggest that IL-35 treatment may upregulate the expression
of Foxp3 in the peripheral blood in apoE−/−
mice.
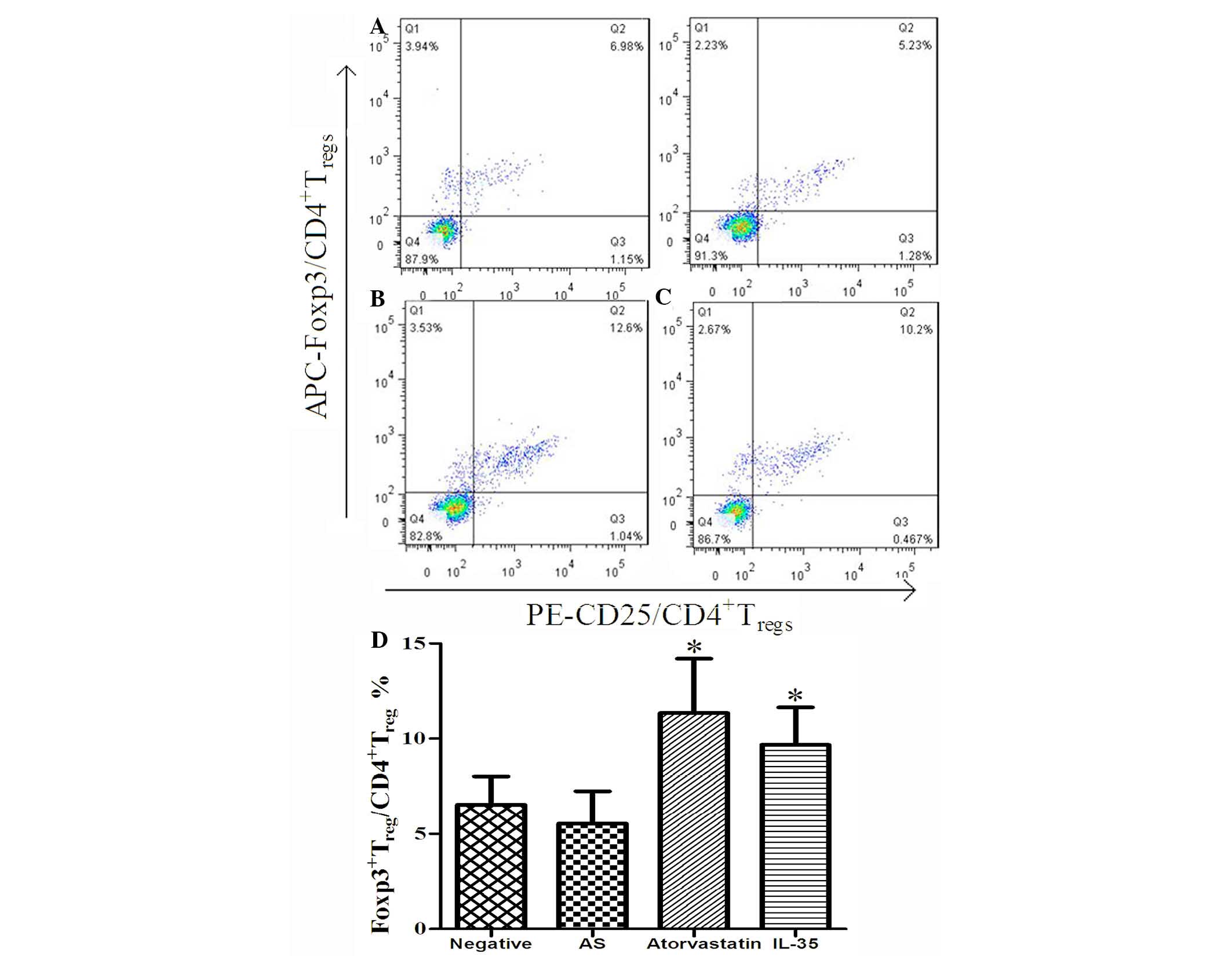 | Figure 4.Plasma levels of Foxp3. Peripheral
blood mononuclear cells were collected from each group, and the
proportions of CD4+ CD25+ Foxp3+
Treg/CD4+ T-cells were analyzed by flow
cytometry, and quantified using FlowJo 7.6.1 software. (A)
Histogram. Data are presented as the mean ± standard deviation.
*P<0.01, vs. the AS group. (B) Negative group, (C) AS group, (D)
atorvastatin group and (E) IL-35 group. Foxp3, forkhead box protein
3; AS, atherosclerosis; IL-35, interleukin-35; APC,
allophycocyanin; PE, phycoerythrin. |
To further verify this conclusion, the expression of
Foxp3 in atherosclerotic lesions was detected by
immunohistochemistry (Fig. 5). The
positive expression of Foxp3 in the nucleus was indicated by the
formation of brown spheres. Notably, the levels of Foxp3 were
markedly reduced in the AS group, as compared with the other
groups. Therefore, the levels of Foxp3 were significantly higher in
the atorvastatin and IL-35 groups, as compared with the AS group
(P<0.01). These results were consistent with the results of the
flow cytometry, and suggest that intervention with IL-35 increases
the expression of Foxp3 in the peripheral blood and atherosclerotic
lesions of apoE−/− mice.
Discussion
At present, the exact mechanism underlying AS is
poorly understood. IL-35 is a heterodimer composed of EBI-3 and p35
subunits that is predominantly secreted by CD4+
Foxp3+ Treg cells (11–13).
Previous studies reported that IL-27α/p28, IL-27β/Ebi3, IL-12α/p35
and IL-12β/p40 were detectable in the majority of established
lesions, but only p35 and Ebi3 subunit levels were increased in the
lesions following treatment (22,23),
thus suggesting that IL-35 was associated with AS. Furthermore,
increased expression levels of IL-35 were associated with
attenuation of AS in a previous study (17). Therefore, the present study aimed to
verify whether exogenous IL-35 was able to attenuate the formation
of atherosclerotic lesions in apoE−/− mice.
It was demonstrated that advanced lesions were attenuated, and
aortic intimal thickness and plaque/lumen area were significantly
reduced, following treatment of AS mice with IL-34, thus suggesting
that exogenous IL-35 was able to relieve advanced AS.
Immunomodulation is a key factor in the pathogenesis
of AS (24). The imbalance between
anti-inflammatory and pro-inflammatory factors leads to lipid
deposition in the walls of large and medium-sized arteries, causing
AS of varying severities (3). The
present study used AS mice treated with atorvastatin calcium as the
normal drug group, since atorvastatin calcium has been widely used
as a traditional lipid-suppressing drug (25). The experimental results demonstrated
that atorvastatin calcium and IL-35 treatment were able to
significantly attenuate the formation of atherosclerotic lesions.
However, atorvastatin calcium and IL-35 were observed to be
different in terms of the rate at which they slowed lipid
deposition: Although there was a significant difference between the
atorvastatin and AS groups, no significant difference was observed
between the IL-35 and AS groups. These results suggested that the
mechanisms of IL-35 were different from those of atorvastatin
calcium. In addition, the expression levels of Foxp3 were
significantly increased in apoE−/− mice
treated with IL-35, thus Foxp3 may be a novel target for detecting
the benefits of IL-35 and its mode of action.
IL-35 is predominantly secreted by CD4+
Foxp3+ Treg cells (26). Evidence from rheumatoid arthritis
mice suggested that IL-35 was able to inhibit the activity of
Teff cells, promote the activity of
Tregcells, reduce the expression of inflammatory factors
and suppress autoimmune diseases, thereby attenuating the
established rheumatoid arthritis (14). These findings indicated that IL-35
has an important role in maintaining the activity of
Treg cells (14). The
present study demonstrated that, as compared with the AS group, the
expression levels of Foxp3 were significantly increased in the
plasma of the IL-35 and atorvastatin groups. Furthermore, the
expression levels of Foxp3 were significantly increased in the
atherosclerotic lesions of the IL-35- and atorvastatin-treated
groups, as compared with the AS group. These results suggested
that, with the drug alleviating the advanced atherosclerosis
plaque, the expression of Foxp3 was improved. Notably, there were
no significant differences in the expression levels of Foxp3 in
both the plasma and atherosclerotic lesions between the
atorvastatin and IL-35 groups. A possible explanation for this is
that, since IL-35 is an anti-inflammatory factor, it may not only
be secreted by CD4+ Treg cells, but also
promote the conversion of the conventional T-cells into
CD4+ Treg cells, which secrete more IL-35 to
mediate the immunosuppression (27).
Conversely, IL-35 has been demonstrated to promote the conversion
of conventional T-cells into a novel Foxp3−
Treg cell (iTr35), which is characterized by Foxp3
independence and is dependent on the secretion of IL-35 to exert
its function (28). This function
for IL-35 was also demonstrated in an experiment involving human
rhinoviruses by Seyerl et al (29). Further research is required to
overcome these challenges. In addition, although both atorvastatin
and IL-35 attenuated the atherosclerotic lesions, previous studies
have suggested that atorvastatin may cause adverse reactions
associated with muscle toxicity (30), and even tumorigenesis (31). Therefore, IL-35 may be a more
desirable option for the treatment of AS. In our future studies, we
will continue to analyze the association between IL-35 and other
inflammatory factors in the process of alleviating advanced AS, so
as to further explore its underlying mechanism.
In conclusion, the present study demonstrated that
IL-35 may be a novel therapeutic target for preventing and treating
AS. Since the specific mechanisms underlying the role of IL-35 in
AS are unclear, further studies are required to investigate the
mechanism of action of IL-35.
Acknowledgements
The present study was supported by the grants from
the National Natural Science Foundation of China (nos. 81270372,
81070232 and 81300223), the Anhui Academic and Technology Leader
Candidate Scientific Research Fund, and the Doctor Scientific
Research Start Fund of the First Affiliated Hospital of Anhui
Medical University.
References
|
1
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samson S, Mundkur L and Kakkar VV: Immune
response to lipoproteins in atherosclerosis. Cholesterol.
2012:5718462012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK: Inflammation, atherosclerosis
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Libby P: Inflammation in atherosclerosis.
Arterioscler Thromb Vasc Biol. 32:2045–2051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chistiakov DA, Sobenin IA and Orekhov AN:
Regulatory T cells in atherosclerosis and strategies to induce the
endogenous atheroprotective immune response. Immunol Lett.
151:10–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nik Tavakoli N, Hambly BD, Sullivan DR and
Bao S: Forkhead box protein 3: Essential immune regulatory role.
Int J Biochem Cell Biol. 40:2369–2373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunkow ME, Jeffery EW, Hjerrild KA,
Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF
and Ramsdell F: Disruption of a new forkhead/winged-helix protein,
scurfin, results in the fatal lymphoproliferative disorder of the
scurfy mouse. Nat Genet. 27:68–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bacchetta R, Passerini L, Gambineri E, Dai
M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C,
Matthes-Martin S, Lawitschka A, et al: Defective regulatory and
effector T cell functions in patients with FOXP3 mutations. J Clin
Invest. 116:1713–1722. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horis S, Momura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1507–1061. 2003.
|
|
10
|
Collison LW, Pillai MR, Chaturvedi V and
Vignali DA: Regulatory T cell suppression is potentiated by target
T cells in a cell contact, IL-35-and IL-10-dependent manner. J
Immunol. 182:6121–6128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collison LW and Vignali DA:
Interleukin-35: Odd one out or part of the family? Immunol Rev.
226:248–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wirtz S, Billmeier U, Mchedlidze T,
Blumberg RS and Neurath MF: Interleukin-35 mediates mucosal immune
responses that protect against T-cell-dependent colitis.
Gastroenterology. 141:1875–1886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS and Vignali DA:
The inhibitory cytokine IL-35 contributes to regulatory T-cell
function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niedbala W, Wei XQ, Cai B, Hueber AJ,
Leung BP, McInnes IB and Liew FY: IL-35 is a novel cytokine with
therapeutic effects against collagen-induced arthritis through the
expansion of regulatory T cells and suppression of Th17 cells. Eur
J Immunol. 37:3021–3029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kempe S, Heinz P, Kokai E, Devergne O,
Marx N and Wirth T: Epstein-barr virus-induced gene-3 is expressed
in human atheroma plaques. Am J Pathol. 175:440–447. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Lin YZ, Shi Y and Ji QW: IL-35: A
potential target for the treatment of atherosclerosis. Pharmazie.
68:793–795. 2013.PubMed/NCBI
|
|
17
|
Lin Y, Huang Y, Lu Z, Luo C, Shi Y, Zeng
Q, Cao Y, Liu L, Wang X and Ji Q: Decreased plasma IL-35 levels are
related to the left ventricular ejection fraction in coronary
artery diseases. PLoS One. 7:e524902012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudensky AY: Regulatory T cells and Foxp3.
Immunol Rev. 241:260–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Dai M and Jia W: Paeonol attenuates
high-fatdiet-induced atherosclerosis in rabbits by
anti-inflammatory activity. Planta Med. 75:7–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park K, Lee DG, Kim SW and Paick JS:
Dimethylarginine dimethylaminohydrolase in rat penile tissue:
Reduced enzyme activity is responsible for erectile dysfunction in
a rat model of atherosclerosis. Int J Impot Res. 21:228–234. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang B, Dai S, Dong Z, Sun Y, Song X, Guo
C, Zhu F, Wang Q and Zhang L: The modulation of endoplasmic
reticulum stress by chemical chaperone upregulates immune negative
cytokine IL-35 in apolipoprotein E-deficient mice. PLoS One.
9:e877872014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kempe S, Heinz P, Kokai E, Devergne O,
Marx N and Wirth T: Epstein-barr virus-induced gene-3 is expressed
in human atheroma plaques. Am J Pathol. 175:440–447. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fredman G and Spite M: Recent advances in
the role of immunity in atherosclerosis. Circ Res. 113:e111–e114.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Profumo E, Buttari B, Saso L and Rigano R:
Pleiotropic effects of statins in atherosclerotic disease: Focus on
the antioxidant activity of atorvastatin. Curr Top Med Chem.
14:2542–2551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao Q, Pan Y, Wang Y, Wang H, Xiong S, Li
Q, Wang J, Tao L, Wang Z, Wu F, et al: Regulatory T cells-derived
IL-35 promotes the growth of adult acute myeloid leukemia blasts.
Int J Cancer. 137:2384–2393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Mai J, Virtue A, Yin Y, Gong R, Sha
X, Gutchigian S, Frisch A, Hodge I, Jiang X, et al: IL-35 is a
novel responsive anti-inflammatory cytokine-a new system of
categorizing anti-inflammatory cytokines. PloS One. 7:e336282012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seyerl M, Kirchberger S, Majdic O, Seipelt
J, Jindra C, Schrauf C and Stöckl J: Human rhinoviruses induce
IL-35-producing Treg via induction of B7-H1 (CD274) and
sialoadhesin (CD169) on DC. Eur J Immunol. 40:321–329. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson PD, Clarkson PM and Rosenson RS:
National Lipid Association Statin Safety Task Force Muscle Safety
Expert Panel: An assessment of statin safety by muscle experts. Am
J Cardiol. 97:69C–76C. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vural K and Tuğlu MI: Neurotoxic effect of
statins on mouse neuroblastoma NB2a cell line. Eur Rev Med
Pharmacol. 15:985–991. 2011.
|