Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a
life-threatening immune disorder that is characterized by fever,
massive hepatosplenomegaly, pancytopenia, hypertriglyceridemia,
hypofibrinogenemia and seizures (1–3). The
disease may be inherited (primary or familial) or secondary to
infection, malignancy or rheumatological disease, and can occur at
any age (3). Infection-associated
HLH has prominent links with various viral, bacterial, fungal and
parasitic infections, including Epstein-Barr virus (EBV),
influenza, typhoid, tuberculosis, leishmaniasis and scrub typhus
(2). Scrub typhus, a febrile
rickettsial disease, is often caused by Orientia
tsutsugamushi, which is endemic in various Asian countries,
including China, India, Korea and other areas in southeast Asia
(4–6). Its clinical manifestations are
non-specific, as they typically include fever, skin rash, myalgia,
gastrointestinal disturbances and lymphadenopathy (4). Nevertheless, the majority of reported
cases of scrub typhus have shown prompt improvement with
anti-rickettsial treatment. To date, previous examples of scrub
typhus-associated HLH in children have been described only as
single case reports (5,6); therefore, the precise distribution and
spectrum of scrub typhus associated with this disorder remains
largely unknown.
The present study reports HLH and acute respiratory
distress syndrome (ARDS) occurring in 6 pediatric patients as a
complication of scrub typhus.
Patients and methods
Patients
The clinical data of 6 pediatric patients (4 boys
and 2 girls; age range, 8 months to 11 years) that were admitted to
Guangzhou Women and Children's Medical Center, Guangzhou Medical
University (Guangzhou, China) with scrub typhus complicated with
HLH and ARDS between 2010 and 2014 were retrospectively reviewed. A
history of playing outdoors in the summer or fall in rural areas
where scrub typhus was endemic, high fever (>38.5°C),
lymphadenopathy, splenomegaly and eschar lesions on covered areas
(scalp, axilla, groin or genitalia) were identified as markers of
suspected scrub typhus on admission. Laboratory diagnosis of scrub
typhus was confirmed using a Weil-Felix agglutination test or/and
the detection of elevated serum antibodies [O. tsutsugamushi
immunoglobulin M (IgM)] using immunofluorescence, as previously
described (7). Each patient was
diagnosed with HLH based on criteria proposed by the Histiocyte
Society in 2004 (8).
The present study was conducted in accordance with
the Declaration of Helsinki, and was approved by the Ethics
Committee of Guangzhou Medical University. Written informed consent
was obtained from the guardians of all participants.
Diagnostic methods
For each patient, clinical and laboratory data, and
details of the disease course, treatment and outcomes were
collected and analyzed. Blood and bone marrow samples were cultured
for bacteria, fungi and viruses. In addition, serologic and/or
nucleic acid-based diagnostic testing for EBV, cytomegalovirus
(CMV) and parvovirus B19 was conducted for all patients, as
previously described (7).
Serological testing for human immunodeficiency virus, hepatitis B
and C, mycoplasma pneumonia and TORCH toxoplasma [which includes
toxoplasmosis, other (syphilis, varicella-zoster and parvovirus
B19) were performed using the ELISA method. A bone marrow sample,
obtained by needle aspiration, was obtained from each of the
patients, and routine serological and hematological analyses were
performed using standard procedures. Hepatosplenomegaly and
lymphadenopathy were diagnosed via physical examination and
ultrasonography.
Results
Clinical features
All patients had a history of playing outdoors in
the summer or fall in rural areas where scrub typhus was endemic.
Only 1 patient had been diagnosed with scrub typhus prior to
admission. Three patients were diagnosed with pneumonia or fever of
unknown origin prior to admission to hospital, and two patients had
been suspected of having HLH. As a result of an elevated peripheral
atypical lymphocyte count (15%), 1 patient was suspected to have
EBV-associated HLH; however, this was contradicted by negative
results for EBV capsid antigen IgM antibody and EBV nuclear
antibody, and a low EBV load. The duration of fever prior to
admission ranged from 4 to 12 days. All patients exhibited
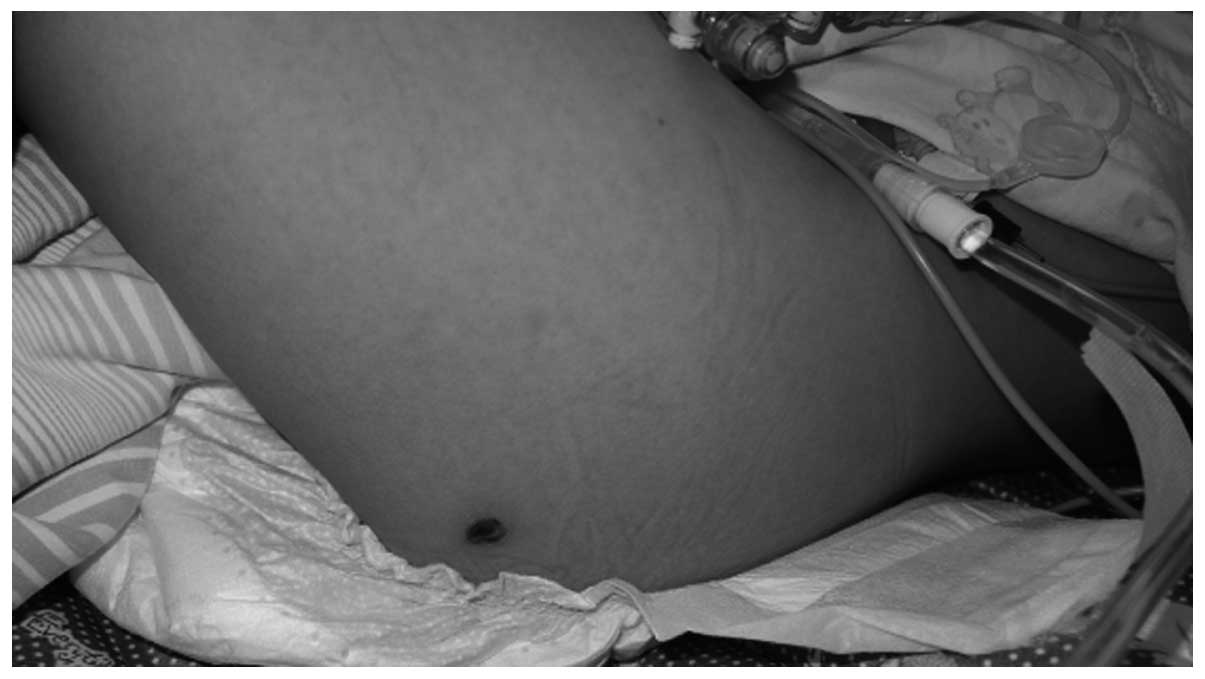
persistent or intermittent fever, eschar (Fig. 1), hepatosplenomegaly and
lymphadenopathy at the time of diagnosis. Features of the 6
patients whose data included manifestations of disease, identified
on admission or developed during hospitalization, are presented in
Table I. Gastrointestinal discomfort
manifesting with vomiting, diarrhea and abdominal pain was present
in 5 patients on admission. Seizure, hematuria and jaundice were
each present in 1 patient. In addition, 5 patients experienced
complicating ARDS and disseminated intravascular coagulation (DIC)
during hospitalization, and an intracranial hemorrhage was
identified in 1 patient.
 | Table I.Clinical and laboratory features of
scrub typhus-associated HLH in children. |
Table I.
Clinical and laboratory features of
scrub typhus-associated HLH in children.
| Patients | Gender/age | Diagnosis prior to
admission | Duration of fever
prior to admission, days |
Fever/eschar/lymphadenopathy/hepatosplenomegaly | Other
presentations | Blood profile Hg
<90 g/l/PLT <100×109/l/WBC
<4×109/l | Biochemistry ALT
>120 U/l/LDH >1,000 U/l/FTG >3 mmol/l/ALB <20 g/l | Coagulation PT >15
sec/APTT >45 sec/FIB <1.5 g/l | Ferritin >1,500
µg/ml/hemophago-cytosis |
|---|
| Case 1 | M/8 m | Pneumonia | 9 | +/+/+/+ | Respiratory and
abdom-, inal symptoms, seizure, intracranial hemorrhage | +/+/+ | +/+/+/+ | +/+/+ | +/+ |
| Case 2 | F/1 y 3 m | Pneumonia | 4 | +/+/+/+ | Respiratory and
abdom-, inal symptoms, jaundice | +/+/− | +/+/+/+ | +/+/+ | +/+ |
| Case 3 | M/7 y | Pneumonia | 12 | +/+/+/+ | Respiratory and
abdominal symptoms | +/+/+ | +/−/+/+ | +/+/+ | +/+ |
| Case 4 | F/7 y | Scrub typhus | 9 | +/+/+/+ | Respiratory and
abdominal symptoms | +/+/+ | −/+/+/+ | +/+/+ | +/+ |
| Case 5 | M/11 y | EBV-HLH | 7 | +/+/+/+ | Respiratory
symptoms | +/+/− | +/+/+/+ | +/+/+ | +/+ |
| Case 6 | M/7 y | HLH | 7 | +/+/+/+ | Respiratory and
abdom-inal symptoms, hematuria | +/+/+ | +/−/+/+ | +/+/+ | +/+ |
Laboratory data
Serological results for human immunodeficiency
virus, parvovirus B19, hepatitis B and C, CMV, EBV, mycoplasma
pneumonia and TORCH toxoplasma [which includes toxoplasmosis, other
(syphilis, varicella-zoster and parvovirus B19), rubella, CMV and
herpes infections] were negative in all patients. Two patients
(cases 3 and 6) were diagnosed with infection with
Staphylococcus epidermidis and Hemophilus influenzae,
respectively. Viral serologic results revealed the presence of
respiratory syncytial virus in 1 patient (case 4). The laboratory
findings at the time of diagnosis are presented in Table I. Thrombocytopenia was detected in
all patients with cytopenia involving two or three cell types,
simultaneously. Coagulopathy with prolonged prothrombin time (PT)
and/or activated partial thromboplastin time were noted in all
patients. Markedly elevated serum ferritin (>1,500 µg/ml) values
were observed in all patients. Elevated lactate dehydrogenase
(>1,000 U/l) was detected in 4 (66.7%) patients and elevated
alanine aminotransferase was exhibited by 5 (83/3%) patients.
According to the diagnostic criteria for HLH proposed by the
Histiocyte Society (8),
hypertriglyceridemia is diagnosed when the serum triglyceride is ≥3
mmol/l (normal range, 0.23–1.70 mmol/l) and hypofibrinogenemia is
diagnosed when serum fibrinogen is ≤1.5 g/l (normal range,
2.00–4.00 g/l). During the early stage of the disease,
hypertriglyceridemia and hypofibrinogenemia were observed in 5
(83.3%) and 4 (66.7%) patients, respectively. During the late stage
of the disease, hypertriglyceridemia and hypofibrinogenemia were
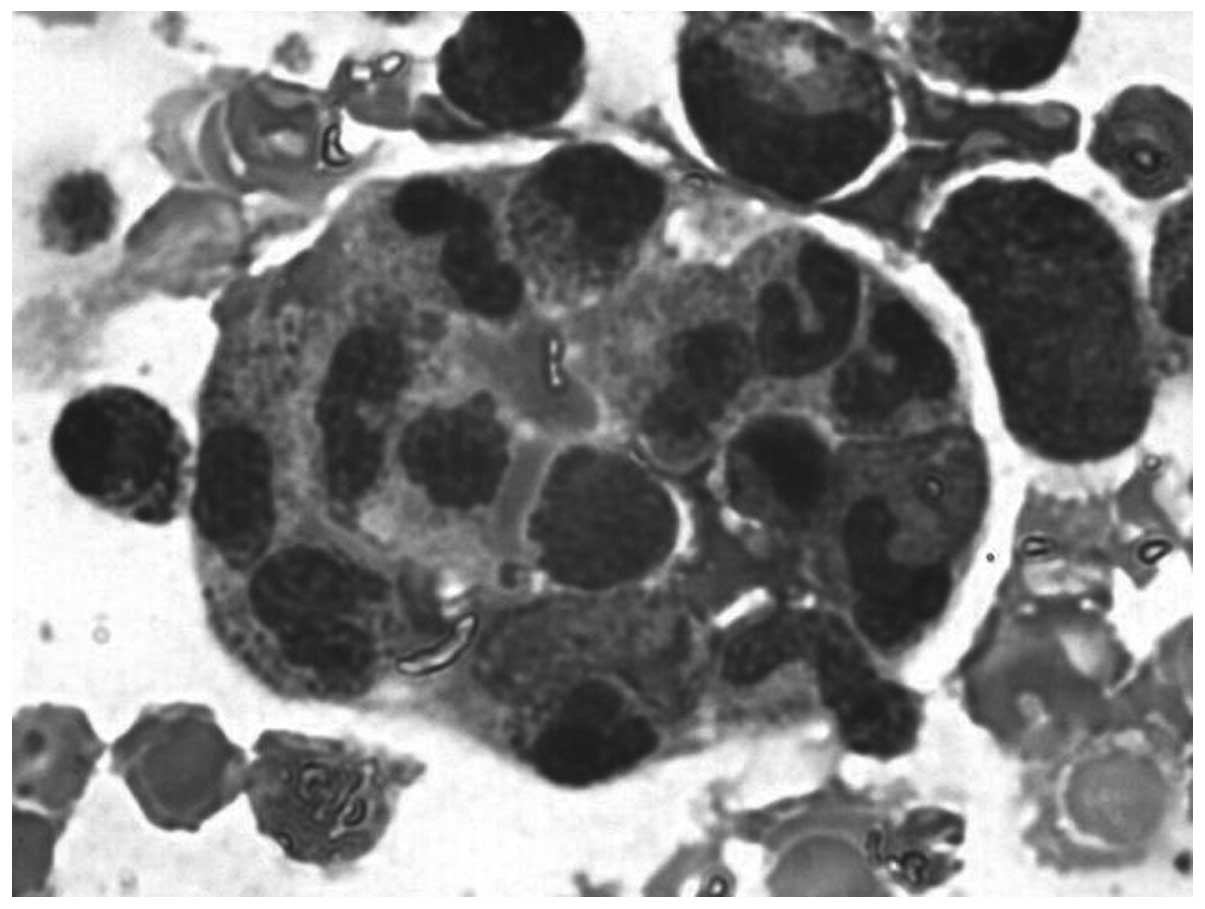
observed in all 6 patients. Hemophagocytosis (Fig. 2) was identified in all patient bone
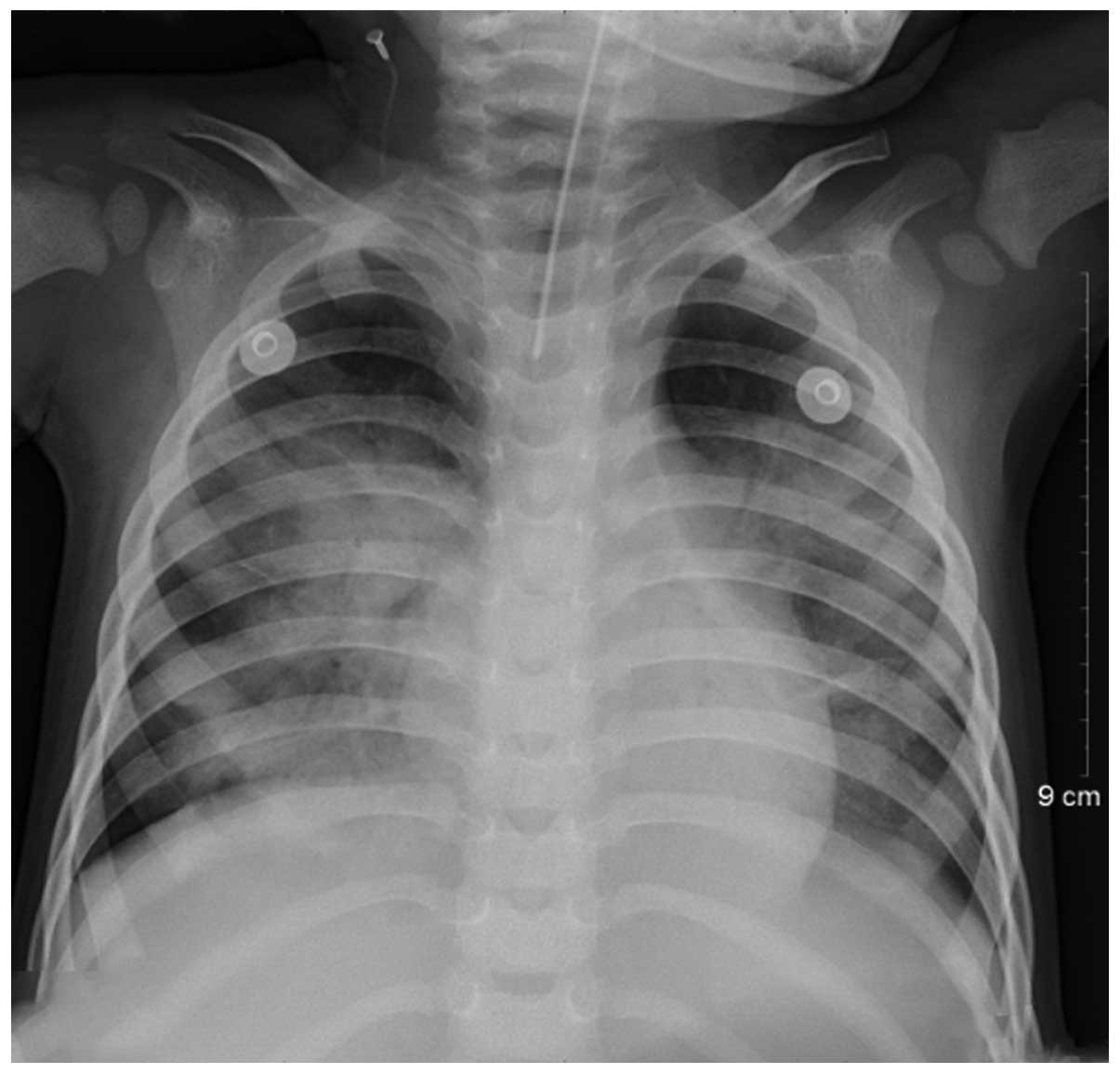
marrow biopsies. Lung infiltrates and consolidation were the most
common features observed by imaging (Fig. 3).
Treatments and outcomes
As shown in Table
II, all patients received anti-rickettsial antibiotics
following the diagnosis of scrub typhus. Anti-rickettsial
antibiotic treatment included azithromycin (10 mg/kg; once daily;
Pfizer, Inc., New York, NY, USA) for three consecutive days every
week for 2–3 weeks, and doxycycline hyclate (2.2 mg/kg twice daily,
which was altered to once daily following normothermia; Hainan
Kangli Pharmaceutical Co., Ltd., Hainan, China) for 7–10 days if
the patient was >7 years old. All patients were administered
intravenous immunoglobulin (IVIG) and mechanical ventilation due to
complications related to ARDS, and 5 patients were administered
systemic steroid therapy (intravenous 2 mg/kg methylprednisolone
1–2 times/day for 3–7 consecutive days; Pfizer, Inc.). Plasma
exchange and continuous renal replacement therapy (CRRT) was
performed on 1 patient. One patient succumbed to DIC and multiple
organ failure (MOF) on day 7 of hospitalization. Five patients
showed remission. The duration of hospital stay ranged between 7
and 24 days. To date, of the 5 survivors, 1 patient has been lost
to follow-up, and the remaining patients are in remission with
excellent general health.
 | Table II.Treatments and outcomes of scrub
typhus associated HLH in children. |
Table II.
Treatments and outcomes of scrub
typhus associated HLH in children.
| Patient | IVIG/steroids | Anti-rickettsial
antibiotics | Mechanical
ventilation | Other supportive
therapy | Complications | Hospital stay
(days) | Outcome |
|---|
| Case 1 | +/+ | Azithromycin | + (9 days) | Blood products | ARDS, DIC, MOF | 7 | DNS |
| Case 2 | +/+ | Azithromycin | + (10 days) | Blood products | ARDS, DIC | 22 | Remission |
| Case 3 | +/+ | Doxycycline | + (11 days) | Blood products | ARDS | 21 | Remission |
| Case 4 | +/+ | Doxycycline | + (14 days) | Blood products | ARDS, DIC | 24 | Remission |
| Case 5 | +/− | Doxycycline |
− | Blood products | DIC | 14 | Remission |
| Case 6 | +/+ | Doxycycline | + (9 days) | Blood products, PE
and CRRT | ARDS, DIC | 16 | Remission |
Discussion
Scrub typhus, a febrile disease caused by
rickettsial infection from O. tsutsugamushi, is common in
Asia-Pacific countries, including China, India, Korea and other
areas in southeast Asia (4,9). Scrub typhus is manifested clinically by
a high fever, rash, splenomegaly and lymphadenopathy, which can
disappear in a few days (4).
However, severe manifestations caused by complications of MOF,
ARDS, shock and DIC may also occur (6,10,11). The
basic pathology of scrub typhus is small vessel vasculitis,
particularly of the lung, heart, brain and kidney (11). The initial clinical symptoms of scrub
typhus are non-specific, and patients often present to the
physician with the appearance of a common fever of unknown origin.
There is a high chance of misdiagnosis of the disease at the early
stage; in the current study, 5 of 6 patients were not diagnosed
with scrub typhus on admission. A combination of the following
clinical manifestations can aid in the diagnosis of the disease:
Fever of unknown origin, hepatosplenomegaly and regional
lymphadenopathy with eschar lesions on covered areas of skin.
Patients with severe complications have a reported
mortality rate as high as 30% without proper treatment (10). Recent reports have suggested that the
cytokine storm associated with the immune response to O.
tsutsugamushi infection may be involved in the pathogenesis of
complicated scrub typhus (12–14).
Once the cytokine cascade has been triggered, the condition can
become a potentially life-threatening illness. In the present
study, the majority of the patients experienced ARDS and DIC, and
satisfied the diagnostic criteria of HLH. Therefore, the possible
diagnosis of secondary HLH should also been considered alongside
scrub typhus. Scrub typhus associated-HLH in children is rarely
reported. The formal guidelines for evaluating patients with
suspected O. tsutsugamushi infection-associated HLH have not
been established. Clinicians are recommended to consider scrub
typhus as a possible etiology while handling cases of HLH in the
epidemic areas.
Although hemophagocytosis is absent in the initial
bone marrow aspirate in the majority of cases and thrombocytopenia
is not a common phenomenon in patients with scrub typhus (3,15), which
was observed upon diagnosis for all of the patients in the present
study, it was noted that all serum ferritin expression levels were
markedly increased to >1,500 µg/ml, and lactate dehydrogenase
expression levels were markedly elevated (>1,000 U/l). Serum
ferritin is a particularly sensitive indicator that reflects the
disease activity, and can be used as an indicator for curative
effect and the prognosis of HLH (16). Markedly elevated serum ferritin
expression levels may occur due to inflammatory cytokines promoting
ferritin synthesis (16). The
clinical and laboratory findings in the current study suggest that
the activation of monocyte/macrophage cell-induced inflammatory
cytokines develops during scrub typhus infection and reflects the
severity of the disease in the patients. It is possible that a
number of the patients may have had primary, not secondary, HLH,
because genetic testing was not performed. One patient had an
elevated peripheral atypical lymphocyte count (15%), demonstrating
that scrub typhus can result in infectious mononucleosis.
Pediatric therapeutic strategies for scrub
typhus-associated HLH are not well established in the literature.
As aforementioned, profound hypercytokinemia serves a key role in
the pathophysiology of severe HLH. Controlling hypercytokinemia is
the first step in saving a patient's life (16,17). The
patients in the present study were not treated according to the
2004-HLH protocol (consisting of etoposide, cyclosporine and
dexamethasone); they were treated with anti-rickettsial antibiotics
and immunomodulatory therapy (IVIG and/or corticosteroids)
alongside supported treatment (blood products and mechanical
ventilation). In addition, plasma exchange and CRRT were performed
in 1 patient. Steroids are frequently used in combination with IVIG
to treat cases of virus-associated HLH. Hyperinflammation can be
treated with corticosteroids, which are cytotoxic for lymphocytes
and inhibit the expression of cytokines and the differentiation of
dendritic cells (3,15). Furthermore, IVIG is effective in
hyperferritinemia (18). Previous
reports suggest that infection-associated HLH has a severe clinical
course and a poor prognosis, emphasizing the importance of early
diagnosis and prompt therapy to shorten the disease course, and
reduce mortality and mortality (16,19,20). The
prognosis of scrub typhus-associated HLH is unknown. In the current
study, one patient (16.7%) succumbed to DIC and MOF, which
demonstrated that there is a relatively low rate of mortality
resulting from scrub typhus-associated HLH. The results
demonstrated that anti-rickettsial treatment and the use of
immunoregulatory therapy may be effective in the treatment of the
inflammatory cytokine storm experienced in children with scrub
typhus-associated HLH. The laboratory results of patient 6 improved
markedly following plasma exchange and CRRT. These treatments can
remove large quantities of cytokines from the plasma and may be a
beneficial method of treatment. However, there were too few
patients included the current study to draw any conclusions, and
further studies are required to confirm a therapeutic strategy for
scrub typhus-associated HLH.
ARDS and DIC are serious and very high-frequency
complications of HLH in the context of scrub typhus compared with
other causes. This can be explained by vasculitis caused by the
pathogen itself, and is exacerbated by the induced inflammatory
cytokine storm, since most cases were improved following treatment
with anti-rickettsial and immunosuppressive therapy. In the current
study, only 1 patient, an 8-month old boy, succumbed to refractory
bleeding and MOF despite receiving aggressive treatment, which
included the administration of anti-rickettsial antibiotics and
immunomodulatory therapies (IVIG and corticosteroid) with
additional supporting treatments (blood products and mechanical
ventilation). This patient had a 9-day delayed diagnosis and
experienced complications with MOF, DIC and septic shock on
admission. Delayed diagnosis (and delayed initial anti-rickettsial
treatment), multiple severe complications and young age may be the
primary causes of mortality. It may be suggested that an early
diagnosis and appropriate treatment may improve the clinical
outcomes and reduce the complications associated with HLH in
children.
In conclusion, the present study demonstrates that
HLH should be considered as a diagnosis in severe pediatric cases
of scrub typhus. The majority of the patients in the current study
had a positive outcome. Early recognition of this syndrome, prompt
treatment and supportive treatment in a pediatric intensive care
unit are key factors for survival. Further studies are required to
confirm a therapeutic strategy for scrub typhus-associated HLH;
anti-rickettsial antibiotic treatment combined with an
immunomodulatory treatment (steroids and IVIG) may be beneficial in
such cases, instead of treatment according to the 2004-HLH
protocol. In addition, plasma exchange and CRRT may be beneficial
treatments for severe scrub typhus-associated HLH; however, more
data are required to clarify the optimal treatment protocol and the
expected outcomes.
References
|
1
|
Verbsky JW and Grossman WJ: Hemophagocytic
lymphohistiocytosis: Diagnosis, pathophysiology, treatment and
future perspectives. Ann Med. 38:20–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domachowske JB: Infectious triggers of
hemophagocytic syndrome in children. Pediatr Infect Dis J.
25:1067–1068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janka GE: Familial and acquired
hemophagocytic lymphohistiocytosis. Annu Rev Med. 63:233–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henter JI, Horne A, Aricó M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski
J and Janka G: HLH-2004: Diagnostic and therapeutic guidelines for
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
48:124–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han DK, Baek HJ, Shin MG, Kim JW, Kook H
and Hwang TJ: Scrub typhus-associated severe hemophagocytic
lymphohistiocytosis with encephalomyelitis leading to permanent
sequelae: a case report and review of the literature. J Pediatr
Hematol Oncol. 34:531–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon HJ, Yoo IH, Lee JW, Chung NG, Cho B,
Kim HK and Kang JH: Life-threatening scrub typhus with
hemophagocytosis and acute respiratory distress syndrome in an
infant. J Trop Pediatr. 59:67–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koraluru M, Bairy I, Varma M and
Vidyasagar S: Diagnostic validation of selected serological tests
for detecting scrub typhus. Microbiol Immunol. 59:371–374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI
and Lee SH: Scrub typhus: Clinical, pathologic and imaging
findings. Radiographics. 27:161–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamarasu K, Malathi M, Rajagopal V,
Subramani K, Jagadeeshramasamy D and Mathai E: Serological evidence
for wide distribution of spotted fevers & typhus fever in Tamil
Nadu. Indian J Med Res. 126:128–130. 2007.PubMed/NCBI
|
|
10
|
Kim DM, Kim SW, Choi SH and Yun NR:
Clinical and laboratory findings associated with severe scrub
typhus. BMC Infect Dis. 10:1082010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CS, Hwang JH, Lee HB and Kwon KS: Risk
factors leading to fatal outcome in scrub typhus patients. Am J
Trop Med Hyg. 81:484–488. 2009.PubMed/NCBI
|
|
12
|
Cascio A, Pernice LM, Barberi G, Delfino
D, Biondo C, Beninati C, Mancuso G, Rodriguez-Morales AJ and Iaria
C: Secondary hemophagocytic lymphohistiocytosis in zoonoses. A
systematic review. Eur Rev Med Pharmacol Sci. 16:1324–1337.
2012.
|
|
13
|
Valsalan R, Kosaraju K, Sohanlal T and
Kumar PS: Hemophagocytosis in scrub typhus. J Postgrad Med.
56:301–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YC, Chao TY and Chin JC: Scrub
typhus-associated hemophagocytic syndrome. Infection. 28:178–179.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nahum E, Ben-Ari J, Stain J and Schonfeld
T: Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of
multiple organ failure. Pediatr Crit Care Med. 1:51–54. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imashuku S: Clinical features and
treatment strategies of Epstein-Barr virus-associated
hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol.
44:259–272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freeman HR and Ramanan AV: Review of
haemophagocytic lymphohistiocytosis. Arch Dis Child. 96:688–693.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Emmenegger U, Frey U, Reimers A, Fux C,
Semela D, Cottagnoud P, Spaeth PJ and Neftel KA: Hyperferritinemia
as indicator for intravenous immunoglobulin treatment in reactive
macrophage activation syndromes. Am J Hematol. 68:4–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin YK, Xie ZD, Yang S, Lu G and Shen KL:
Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis: A
retrospective study of 78 pediatric cases in mainland of China.
Chin Med J (Engl). 123:1426–1430. 2010.PubMed/NCBI
|
|
20
|
Yasunaga H, Horiguchi H, Kuwabara K,
Hashimoto H and Matsuda S: Delay in tetracycline treatment
increases the risk of complications in Tsutsugamushi disease: Data
from the Japanese diagnosis procedure combination database. Intern
Med. 50:37–42. 2011. View Article : Google Scholar : PubMed/NCBI
|