Introduction
The aim of a bone augmentation procedure in
dentistry is the repair of alveolar bone tissue (1,2). In bone
tissue repair, the use of autogenous bone grafts remains the gold
standard. Autogenous bone grafts have osteoinductive and
osteoconductive properties. Additionally, autogenous grafts contain
stem cells and growth factors and do not create an immunological
reaction. However, the requirement for a second surgical area, the
restricted amount of bone grafts, and graft resorption have
resulted in a search for alternative graft materials and treatment
methods for bone augmentation (3).
Human-derived bone grafts are more immunogenic but less osteogenic
than autogenous bone grafts, and the resorption rate of allogeneic
bone grafts is greater than that of autogenous bone grafts, with an
added risk of disease transmission (4). For these reasons, synthetic bone grafts
have been developed (2,4,5).
Alloplastic bone grafts should be tissue-compatible,
and should not be antigenic or inflammatory. Synthetic bone grafts
made of hydroxyapatite (HA) have been demonstrated to stimulate new
bone regeneration in experimental animal studies, with high
osteogenic potential compared with autologous bone grafts (2,4,5). A HA synthetic bone graft is a type of
calcium phosphate ceramic graft. HA synthetic bone grafts, compared
with autogenous bone grafts, have been shown to stimulate bone
regeneration in experimental animal studies, with excellent
stability and bone-regenerative characteristics. Due to their
composition and structure, HA bone grafts degrade slowly and are
replaced gradually by bone (4,6,7).
Biphosphonates (BPs) are used to prevent and treat
increased bone resorption in skeletal diseases. The influence of
BPs on bone healing and bone-implant connections has been
investigated. Throughout bone repair, BPs have been shown to have
anti-osteoclastic effects and, thus, a relatively pro-osteoblastic
effect (8–11).
BPs have some side effects when used systematically.
An initial influenza-like illness, renal failure and osteonecrosis
have been documented when BPs have been used systematically
(12–14). Zoledronic acid (ZA) is a strong BP in
clinical use. Single-dose intraoperative ZA application has shown
favourable effects in various models of bone repair and healing
(12). In the present study, the aim
was to evaluate the effects of locally and systemically
administered ZA with HA synthetic bone grafts on new bone
generation in a rat critical-size calvarial defect model.
Materials and methods
Animal care and ethics
The experimental design and study protocol were
approved by the Animal Ethics Committee at the University of Dicle
(Diyarbakir, Turkey). Rats were obtained from the Sabahattin Payzın
Experimental Research of Center Dicle University (Diyarbakır,
Turkey). In total, 84 female Sprague Dawley rats, aged 4–6 months
were used. Their average body weights were 280–300 g on the first
day of the experiment. The animals were kept in
temperature-controlled cages, exposed to a 12/12-h light/dark
cycle, and had ad libitum access to food and water.
Experimental protocols and surgical
procedure
First, the rats were divided randomly into four
groups, as follows: Empty control (EC) group (n=21), no bone graft
material or ZA treatment was applied; HA group (n=21), received a
HA graft without ZA therapy; HA plus local ZA (HA+LZA) group
(n=21), treated locally with ZA; and HA plus systemic ZA (HA+SZA)
group (n=21). In the HA+LZA group, each graft was soaked in ZA
solution (1 mg/ml) for 5 min and unbound ZA was not rinsed away as
described by Toker et al (4).
In the HA+SZA group, the rats received 0.1 mg/kg systemic ZA in
sterile injectable saline according to the method of Ayan et
al (12), with a HA graft.
General anaesthesia was established using ketamine.
All rats were fed with a standard diet during the experimental
period. Surgical operations were performed under sterile
conditions. Following general anaesthesia, prior to surgery, the
skull skin was shaved. A skin incision on the skull was made over
the linea media. An incision allowing reflection of a
full-thickness flap in the anterior-posterior direction was made in
the scalp in the sagittal plane. A periosteal elevator was used to
lift the flap and periosteum to access the skull bone. A
5-mm-diameter defect was made in the right side of the calvarium
with a standard trephine drill used in a low-speed handpiece under
continuous irrigation with sterile saline. During this process,
extreme care was taken not to damage the dura mater. The rats in
each group were treated as indicated above. All surgical procedures
were performed by the same surgeon (SD).
The skull skin was sutured with 4/0 polyglactin
resorbable sutures. Cephalosporin antibiotic (50 mg/kg) and an
analgesic (tramadol hydrochloride, 0.1 mg/kg) were injected
intramuscularly in all animals after the surgery.
After 7, 14 and 28 days, rats were sacrificed (7
rats from each group at each time point) with an anaesthetic
overdose (ketamine at a dose 2–3-fold higher than the anaesthetic
dosage). After this, a surgical drill attached to an electrical
hand motor piece was used to harvest the calvarial bone. The
calvarial bone specimens were then separated from muscles and soft
tissues (15).
Histological and histomorphological
analysis
The original defect area and the surrounding tissues
were used for histological analysis. The specimens were fixed in
10% formaldehyde for 72 h and demineralised in 10% formic acid;
after this, they were dehydrated, embedded in paraffin wax, and
sectioned for haematoxylin and eosin staining for light microscopic
analysis. Sections 6-µm in thickness, corresponding to the bone
defect area, were evaluated by light microscopy. Osteoblast numbers
were scored in the total defect area, as follows: No osteoblast
cells, 0; low-density osteoblasts, 1; and dense osteoblasts, 2.
Osteoclast numbers were scored as follows: No osteoclasts, 0;
low-density osteoclasts, 1; and dense osteoclasts, 2. Bone
formation was scored as follows: No bone formation, 0; mild visible
bone formation, 1; moderate visible bone formation, 2; and dense
visible bone formation, 3. Images of all histological specimens
were captured with a digital camera attached to a light microscope
(Olympus Bx51; Olympus Corporation, Tokyo, Japan) with original
magnification and saved on a computer (4,5). Imaging
software (Olympus DP71; Olympus Corporation) was used for
histomorphometric analysis.
Statistical analysis
For statistical analysis, SPSS software was used
(version 22; IBM SPSS, Armonk, NY, USA). Following the healing
period, mean values and standard deviations were calculated. The
differences between groups were tested with one-way analysis of
variance for parameters that showed a normal distribution. For
identification of the specific groups with significant differences,
Tukey's honest significant difference test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Healing and bone formation
In the EC group, healing was characterised by thin
fibrous connective tissue filling the defects, due to no bone graft
material or treatment being applied. In addition, no regenerative
bone formation was detected. At 28 days, the amount of new bone
formation in all study groups had increased in comparison with that
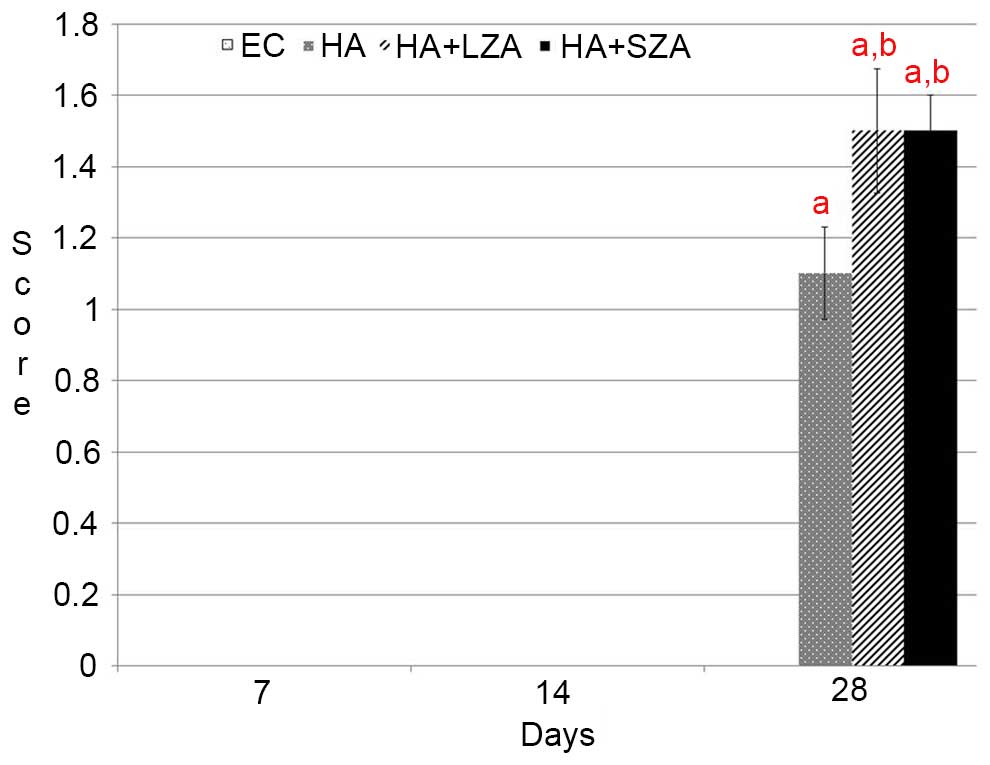
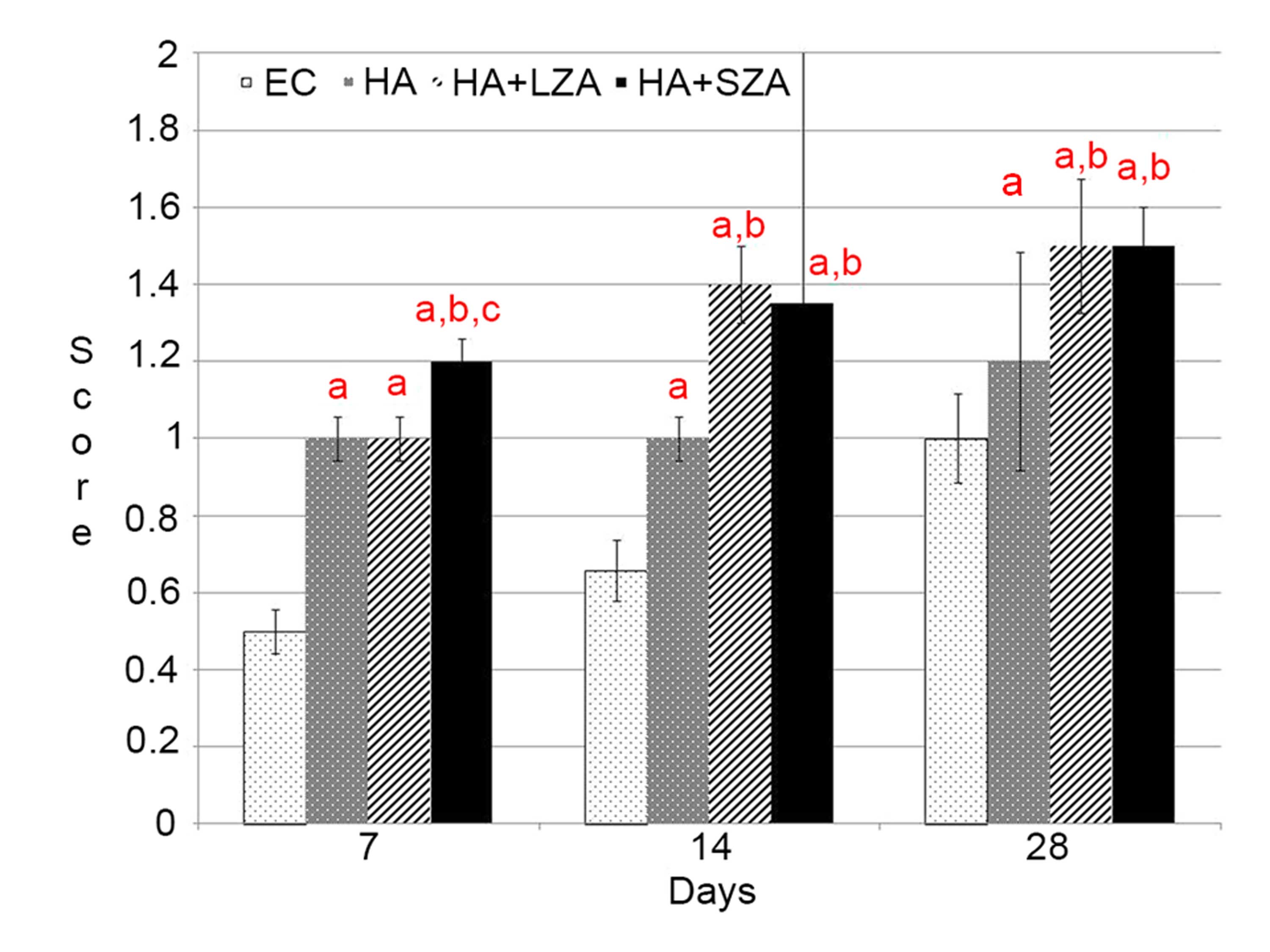
in the EC group (P<0.05). Semi-quantitative analyses
demonstrated that there was new bone formation in groups HA,
HA+LZA, and HA+SZA at 28 days. The two routes of ZA administration
resulted in significantly higher new bone formation than in group
HA (P<0.05). However, no significant difference was observed
between the two routes of ZA administration at 28 days (P>0.05).
On days 7 and 14, no new bone formation was detected in any group
(P>0.05). Overall, the mean new bone area in the EC group was
significantly lower than that in groups HA, HA+LZA, and HA+SZA
(P<0.05). Additionally the results demonstrated no significant
difference in new bone area between groups HA+LZA and HA+SZA
(P<0.05; Figs. 1 and 2).
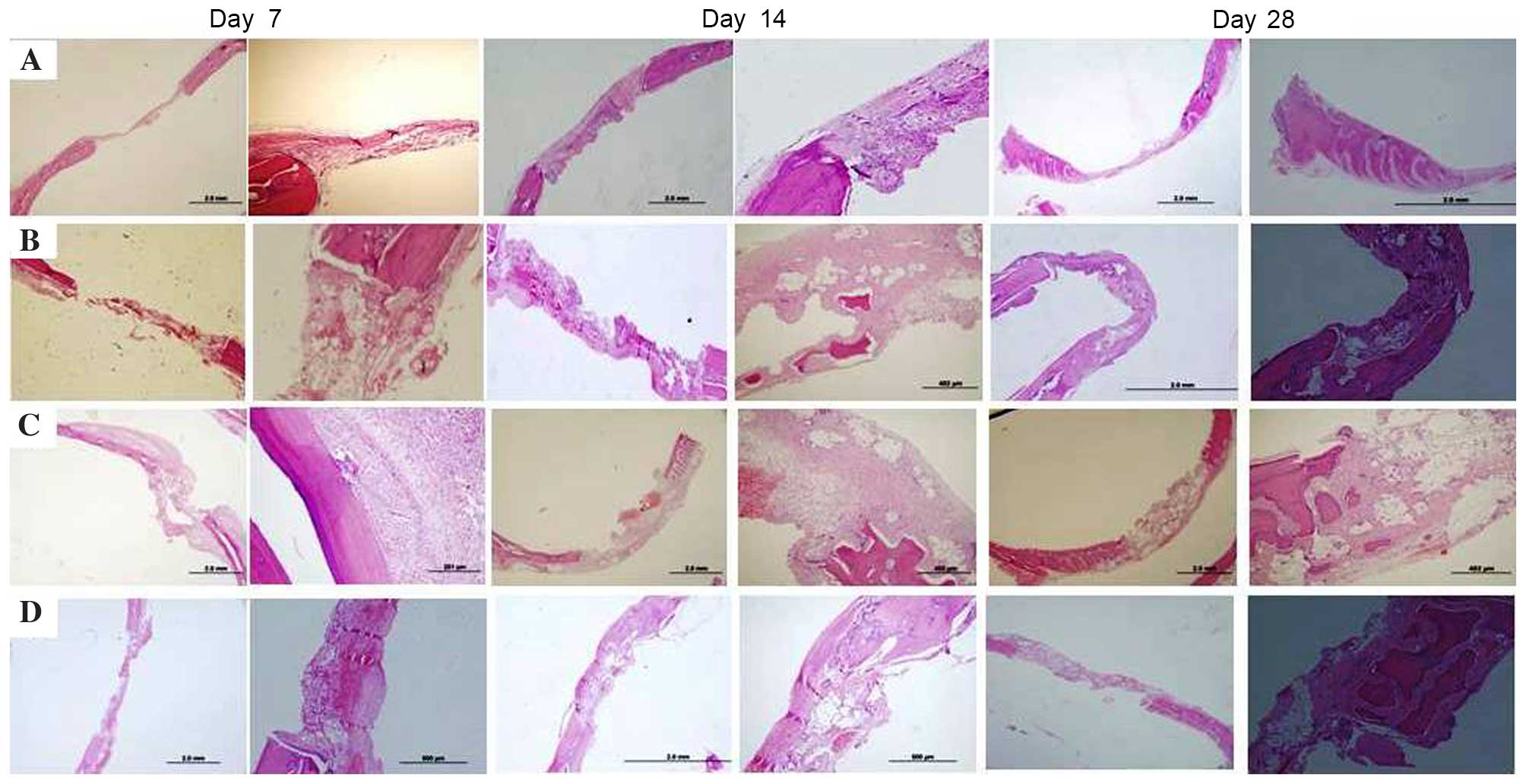 | Figure 1.Histopathological findings of the (A)
EC group, (B) HA group, (C) HA+LZA group and (D) HA+SZA group on
days 7, 14 and 28. Haematoxylin and eosin staining; magnification
×2 and ×4 for left and right images at each time point,
respectively. In the EC group, healing was characterised by thin
fibrous connective tissue filling the defects, and no regenerative
bone formation was detected at any time point. By day 28, new bone
formation was visible in all three treatment groups. ZA
administration, either systemically or locally, resulted in
increased bone formation and greater numbers of osteoclasts and
osteoblasts in comparison with those in the HA group. EC, empty
control; HA, hydroxyapatit LZA, local zoledronic acid; SZA,
systemic zoledronic acid. |
Osteoclast numbers
At day 28, osteoclast numbers in groups HA+LZA and
HA+SZA were significantly higher than those in the EC and HA groups
(P<0.05). Osteoclast numbers in groups HA+LZA and HA+SZA were
not significantly different from each other (P>0.05); however,
both were significantly higher compared with the osteoclast number
in group HA (P<0.05). At day 14, osteoclast numbers were
significantly higher in groups HA, HA+LZA and HA+SZA than in group
EC (P<0.05). No significant difference was observed among groups
HA, HA+LZA and HA+SZA with respect to osteoclast numbers
(P>0.05; Figs. 1 and 3).
Osteoblast numbers
At day 28, the numbers of osteoblasts in groups HA,
HA+LZA, and HA+SZA were significantly higher than in the EC group
(P<0.05). There was no significant difference in osteoblast
number between groups HA+LZA and HA+SZA at day 28 (P>0.05),
although osteoblast cell numbers in groups HA+LZA and HA+SZA were
significantly higher than in the HA group (P<0.05). At day 14,
osteoblast numbers in groups HA+LZA and HA+SZA were significantly
higher than in the EC and HA groups (P<0.05). Osteoblast numbers
were also significantly higher in the HA group than in the EC group
(P<0.05). Newly regenerated bone formation was not detected in
the EC group (P>0.05). No significant difference was detected in
new bone formation between groups HA+LZA and HA+SZA (P>0.05;
Figs. 1 and 4).
Discussion
Rat calvarial defects are considered a preferred
experimental model for bone regeneration in experimental studies,
as poor vascular supply and membranous structures inhibit natural
healing (4,5). In the present study, a 5-mm
critical-size defect model in rat calvaria was used. The reason for
using a defect of this size is that in bone defects greater than
this, healing with scarring occurs as opposed to bone regeneration,
resulting in defect cavity formation. This was confirmed in the
present study; no new bone formation was detected in the control
defects (4,5).
BP pretreatment can be useful to prevent bone graft
resorption. Additionally, bone cell culture studies have indicated
that BPs can increase bone formation indicators at very low
concentrations (5,12). Due to their direct action on
osteoclasts, it is evident that BPs may affect the bone formation
process. Osteoclast cell function may be changed by the production
of an osteoclastic inhibitory factor secreted by osteoblasts
following BP administration. During the bone remodelling process,
osteoblastic cells control the activity of osteoclastic cells. BPs
increase the proliferation and maturation of osteoblastic cells and
reduce apoptosis (4,5,12). This
supports the hypothesis that BPs may have an anabolic effect on
bone tissue cells and thus increase bone tissue formation. As such,
the target cells of BPs may include members of the osteoblastic
cell family (12,16). It has been shown that BPs can
increase the proliferation of osteoblasts and the synthesis of
collagen and osteocalcin by bone cells at the cellular level
(4,5). In the present study, histological
analysis indicated that the newly formed bone area was larger in
all study groups at the end of the study (at 28 days) compared with
that in the EC group. Systemic and topical application of ZA
resulted in significantly more bone formation than was observed in
group HA, with no significant difference between the two
application routes of ZA administration at day 28. New bone
formation was not observed in the EC group. In terms of new bone
formation, no significant difference was observed between groups
HA+LZA and HA+SZA. This result confirms the results of earlier
studies regarding bone augmentation with local and systemic ZA
application and the association between bone tissue cells and BPs
(4,5).
In the present study, it was hypothesized that ZA
would activate osteoblastic cells and increase osteogenesis. Mixing
the grafts with BP solution prior to application on the bone
defects seemed to be a reasonable approach. Treating the bone with
local BP may facilitate bone tissue healing without systemic
effects. In earlier studies, it was reported that local application
of BP solution on an allograft increased osteogenesis (4–6). ZA is a
strong BP that is used clinically. A single dose of ZA administered
intraoperatively has shown favourable effects in various models of
bone repair and healing (12).
Systemic BP application has been used widely in the treatment of
various systemic skeletal metabolic bone diseases, such as Paget's
disease, hypercalcaemia of malignancy and post-menopausal
osteoporosis (12,17,18). It
is clear that BPs in bone tissues inhibit bone turnover and, thus,
bone tissue loss (12,19). The present study confirmed thi ZA
treatment of the bone graft, locally and systemically, increased
osteogenesis of the graft material and enabled bone formation,
compared with that in the control and graft-only groups. In this
study, at day 28, favourable effects of local and systemic BP were
observed in groups HA+LZA and HA+SZA in terms of newly regenerated
bone formation, which is consistent with previous reports (4,5,10–12,20,21).
However, in terms of new bone formation and osteoblast and
osteoclast numbers, no significant difference was observed between
groups HA+LZA and HA+SZA. As the amount of new bone formation in
the HA+LZA and HA+SZA groups was similar, a statistically
significant difference was not detected between the two groups for
osteoblast and osteoclast numbers. The bone formation results can
be explained by the osteoblast and osteoclast numbers observed in
the two groups.
The type of application and dose of BP are key
factors in the understanding of bone tissue and BP interaction.
Previous studies have indicated that BPs cause a biphasic effect,
stimulating cellular reproduction and the formation of bone cell
tissues at low concentrations and restricting these processes at
higher concentrations (4,22–25). In
a study using an experimental periodontitis model, the preventative
effects of BPs were investigated in alveolar bone tissue
destruction at two doses. It was demonstrated that treatment with
BPs in the experimental group, given either as a prophylactic or
therapeutic medication, significantly inhibited inflammatory tissue
destruction and alveolar bone resorption in comparison with the
saline-treated control group (21).
Myoung et al (26)
investigated the effects of a BP at a dose of 0.01 mg/kg/day on the
expression of bone tissue regeneration-related genes following
autogenous bone graft application in an experimental rat model.
They demonstrated that the BP inhibited osteoclastic function and
triggered osteoblasts to secrete an inhibitor of osteoclast-related
resorption. In another animal model study, BPs were administered
systemically at a dose of 0.25 mg/day for 8 weeks, and it was shown
that alendronate stimulated bone regeneration in autogenous bone
grafts (20). In the present study,
to compare the systemic effects of ZA with those of local ZA
pre-treatment of the bone graft, systematic ZA was used at a dose
of 0.1 mg/kg according to Ayan et al (12) and local ZA at a concentration of 1
mg/ml according to Toker et al (4). The results suggest that favourable
effects occurred in the HA+LZA and HA+SZA groups regarding new bone
formation, compared with the graft-only group, which is consistent
with the findings of Ayan et al (12) and Toker et al (4).
BPs primarily reach revascularised sections of bone
tissue when used systemically, but not the unvascularised graft
(4). However, long-term BP use has
been associated with osteonecrosis of the jaw (13,14).
Local BP treatment of bone tissues provides protection against bone
resorption, without any broader skeletal effects (4). Additionally, in local BP pretreatment,
the majority of the BP adsorbs to the bone surface of cancellous
bone while a small volume stays free in solution between the
trabeculae (4,10). Furthermore, topical treatment of an
allograft with a BP has been shown to inhibit bone graft resorption
(4). Another experimental study
using a synthetic bone graft suggested that a single dose of local
BP pretreatment combined with the bone graft improved bone tissue
regeneration in the rat mandible (27). A study investigating the influence of
systemic BPs on synthetic bone graft osteogenesis in a
posterolateral spinal fusion porcine model showed that BPs at a
dose of 10 mg/day did not inhibit bone formation within the
synthetic bone graft and did not demonstrate differences in
trabecular bone volume between treatment and control groups
(28). In the present study,
favourable effects were observed with topical BP pretreatment at 1
mg/ml concentration in the HA+LZA group, as previously reported
(11,27).
In the present study, ZA was administered
systemically as a single dose of 0.1 mg/kg (12,29–32).
According to previous reports, the plasma concentration of BPs
declines progressively over 28 days (12,32). A
repeat dose of ZA could be administered 28 days after the initial
single dose, if required. The administration of an intra-operative
single dose of 0.1 mg/kg ZA was considered to be sufficient for the
bone healing period in the present study, according to Ayan et
al (12). Thus, for the
comparison of local and systemic single BP administration in the
present study, a 28-day experimental period was selected because of
the use of a single local application of ZA with the HA graft.
In conclusion, the present study demonstrated that
systemic and local BP treatment can increase bone formation in HA
grafts in a rat critical-size defect model, compared with that in
rats treated with graft alone. Considering the risks associated
with systemic BP therapy, we suggest that further studies focusing
on local and systemic applications of ZA at different doses and/or
concentrations and different graft materials may be effective in
identifying methods for the enhancement of healing using bone graft
materials.
Acknowledgements
The authors thank Dr Selcuk Ilhan (Department of
Pharmacology, Faculty of Medicine, Firat University) for helpful
advice with the statistical analysis.
References
|
1
|
Buser D, Brägger U, Lang NP and Nyman S:
Regeneration and enlargement of jaw bone using guided tissue
regeneration. Clin Oral Implants Res. 1:22–32. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ezirganli S, Polat S, Baris E, Tatar I and
Celik HH: Comparative investigation of the effects of different
materials used with a titanium barrier on new bone formation. Clin
Oral Implants Res. 24:312–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alam S, Ueki K, Nakagawa K, Marukawa K,
Hashiba Y, Yamamoto E, Sakulsak S and Iseki N: Statin-induced bone
morphogenetic protein (BMP) 2 expression during bone regeneration:
An immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 107:22–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toker H, Ozdemir H, Ozer H and Eren K: A
comparative evaluation of the systemic and local alendronate
treatment in synthetic bone graft: A histologic and
histomorphometric study in a rat calvarial defect model. Oral Surg
Oral Med Oral Pathol Oral Radiol. 114(5 Suppl): S146–S152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toker H, Ozdemir H, Ozer H and Eren K:
Alendronate enhances osseous healing in a rat calvarial defect
model. Arch Oral Biol. 57:1545–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fellah BH, Gauthier O, Weiss P, Chappard D
and Layrolle P: Osteogenicity of biphasic calcium phosphate
ceramics and bone autograft in a goat model. Biomaterials.
29:1177–1788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mah J, Hung J, Wang J and Salih E: The
efficacy of various alloplastic bone grafts on the healing of rat
calvarial defects. Eur J Orthod. 26:475–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doggrell SA: Clinical efficacy and safety
of zoledronic acid in prostate and breast cancer. Expert Rev
Anticancer Ther. 9:1211–1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lipton A: The safety of zoledronic acid.
Expert Opin Drug Saf. 6:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jakobsen T, Baas J, Kold S, Bechtold JE,
Elmengaard B and Søballe K: Local bisphosphonate treatment
increases fixation of hydroxyapatite-coated implants inserted with
bone compaction. J Orthop Res. 27:189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jakobsen T, Baas J, Bechtold JE,
Elmengaard B and Søballe K: Soaking morselized allograft in
bisphosphonate can impair implant fixation. Clin Orthop Relat Res.
463:195–201. 2007.PubMed/NCBI
|
|
12
|
Ayan M, Dolanmaz D, Mihmanli A, Ayan A and
Kürkcü M: The effect of systemically administrated zoledronic acid
on the osseointegration of dental implants. Oral Dis. 18:802–808.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruggiero SL: Bisphosphonate-related
osteonecrosis of the jaw (BRONJ): Initial discovery and subsequent
development. J Oral Maxillofac Surg. 67(5 Suppl): S13–S18. 2009.
View Article : Google Scholar
|
|
14
|
Ruggiero SL, Mehrotra B, Rosenberg TJ and
Engroff SL: Osteonecrosis of the jaws associated with the use of
bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg.
62:527–534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yaman F, Atilgan S, Günes N, Agacayak S,
Günay A, Ucan MC, Bakir S, Erol B, Kose I and Atalay Y:
Phosphodiesterase-5 inhibitors may facilitate bone defect recovery.
Eur Rev Med Pharmacol Sci. 15:1301–1305. 2011.PubMed/NCBI
|
|
16
|
Ozdemir H, Ezirganli S, Isa Kara M,
Mihmanli A and Baris E: Effects of platelet rich fibrin alone used
with rigid titanium barrier. Arch Oral Biol. 58:537–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walsh JP, Ward LC, Stewart GO, Will RK,
Criddle RA, Prince RL, Stuckey BG, Dhaliwal SS, Bhagat CI,
Retallack RW, et al: A randomized clinical trial comparing oral
alendronate and intravenous pamidronate for the treatment of
Paget's disease of bone. Bone. 34:747–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wellington K and Goa KL: Zoledronic acid:
A review of its use in the management of bone metastases and
hypercalcaemia of malignancy. Drugs. 63:417–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodan GA and Fleisch HA: Bisphosphonates:
Mechanisms of action. J Clin Invest. 97:2692–2696. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altundal H and Gursoy B: The influence of
alendronate on bone formation after autogenous free bone grafting
in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
99:285–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menezes AM, Rocha FA, Chaves HV, Carvalho
CB, Ribeiro RA and Brito GA: Effect of sodium alendronate on
alveolar bone resorption in experimental periodontitis in rats. J
Periodontol. 76:1901–1909. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coxon FP, Thompson K and Rogers MJ: Recent
advances in understanding the mechanism of action of
bisphosphonates. Curr Opin Pharmacol. 6:307–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Knoch F, Jaquiery C, Kowalsky M,
Schaeren S, Alabre C, Martin I, Rubash HE and Shanbhag AS: Effects
of bisphosphonates on proliferation and osteoblast differentiation
of human bone marrow stromal cells. Biomaterials. 26:6941–6949.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaynak D, Meffert R, Günhan M, Günhan O
and Ozkaya O: A histopathological investigation on the effects of
the bisphosphonate alendronate on resorptive phase following
mucoperiosteal flap surgery in the mandible of rats. J Periodontol.
71:790–796. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Idris AI, Rojas J, Greig IR, Van'tHof RJ
and Ralston SH: Aminobisphosphonates cause osteoblast apoptosis and
inhibit bone nodule formation in vitro. Calcif Tissue Int.
82:191–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Myoung H, Park JY and Choung PH: Effects
of a bisphosphonate on the expression of bone specific genes after
autogenous free bone grafting in rats. J Periodontal Res.
36:244–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue QY, Ji Q, Li HS, Zou XN, Egund N, Lind
M, Christensen FB and Bünger C: Alendronate treatment does not
inhibit bone formation within biphasic calcium phosphate ceramics
in posterolateral spinal fusion: An experimental study in porcine
model. Chin Med J (Engl). 122:2770–2774. 2009.PubMed/NCBI
|
|
28
|
Srisubut S, Teerakapong A, Vattraphodes T
and Taweechaisupapong S: Effect of local delivery of alendronate on
bone formation in bioactive glass grafting in rats. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 104:e11–e16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pampu AA, Dolanmaz D, Tüz HH and
Karabacakoglu A: Experimental evaluation of the effects of
zoledronic acid on regenerate bone formation and osteoporosis in
mandibular distraction osteogenesis. J Oral Maxillofac Surg.
64:1232–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yildiz A, Esen E, Kürkcü M, Damlar I,
Dağlioğlu K and Akova T: Effect of zoledronic acid on
osseointegration of titanium implants: An experimental study in an
ovariectomized rabbit model. J Oral Maxillofac Surg. 68:515–523.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tatli U, Ustün Y, Kürkcü M, Erdoğan O,
Gürbüz CC, Ozgür H and Polat S: Effects of zoledronic acid on
healing of mandibular fractures: An experimental study in rabbits.
J Oral Maxillofac Surg. 69:1726–1735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen T, Berenson J, Vescio R, Swift R,
Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, et al:
Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer
patients with bone metastases. J Clin Pharmacol. 42:1228–1236.
2002. View Article : Google Scholar : PubMed/NCBI
|