Introduction
Type 1 diabetes is an autoimmune disease, and the
prominent population affected by the disease is teenagers. In
recent years, the number of individuals diagnosed with the disease
has increased. Once affected by the disease, the patient undergoes
lifelong insulin treatment, which can pose an economic burden to
the patient and family. Patients with type 1 diabetes suffer from
multiple complications, which can lead to disabling and lethal
factors (1).
To the best of our knowledge, few studies are
available on the exact pathogenesis of type 1 diabetes, the
variation of patient blood and the pancreatic tissue immune
microenvironment (2–4). Non-obese diabetic (NOD) mice can
spontaneously generate autoimmune disease, and their pathogenic
process is similar to that of type 1 diabetes of human beings.
Consequently, a NOD rodent animal model should be established to
probe into morbidity and develop a treatment intervention mechanism
for human type 1 diabetes (5).
Exendin-4 is a type of polypeptide hormone separated from Gila
monster saliva. This hormone is a broad application prospect in
treating type 2 diabetes (6,7). However, there is little research or
studies on exendin-4 as an intervention mechanism in type 1
diabetes treatment. In order to determine the influence of
exendin-4 intervention on NOD mouse blood and the pancreatic tissue
immune microenvironment, a NOD mouse model was established for the
experiments. A variation in T-cell subset CD8, CD4 and CD25 in NOD
mouse peripheral blood and pancreatic tissue, as well as in the
contents of cell factors IL-2, IFN-γ and IL-10 after low-dose,
medium-dose and high-dose exendin-4 intervention was observed.
Materials and methods
Materials
A total of 40 NOD/Lt mice were purchased from the
Institute of Experimental Animals of the Chinese Academy of Medical
Sciences. There were 20 male and 20 female mice, with a weight of
17–25 g. Exendin-4 reagent (Kangtai Biotechnology, Beijing, China);
hematoxylin and eosin (H&E) dye liquor (Beijing Solarbio
Science and Technology, Co., Ltd., Beijing, China); IL-2, IL-10 and
IFN-γ reagent kits (Shanghai Biotechnology, Shanghai, China);
monoclonal CD4 rabbit antibody (Abcam, Cambridge, MA, USA; catalog
no.: ab133616; dilution, 1:100) and monoclonal CD8 rabbit antibody
(Abcam; catalog no.: ab22378; dilution, 1:100); a flow cytometer
(Beijing Kexue Yingye Science and Technology Development, Beijing,
China); microplate reader (Jinan Guangyao Medical Equipment, Jinan,
China); centrifugal machine (Shanghai Puyuan Instrument, Shanghai,
China); and an optical microscope (Shenzhen Final Technology,
Shenzhen, China) were used in the study.
The present study was approved by the ethics
committee of Fujian Medical University (Fujian, China).
Methods
A random number table was used to divide 40 clean
and healthy NOD mice into 4 groups (n=10/group) as follows: One
blank control group D with normal saline intervention, and the
remaining three groups with exendin-4 intervention. According to
the different exendin-4 doses of 2, 4 and 8 µg/kg/day, they were
called the low-dose group A, medium-dose group B and high-dose
group C, respectively. The four groups of mice were intervened for
8 weeks, after which they were sacrificed by cervical dislocation.
An inner canthus method was used to take ~1 ml of whole blood,
which was then centrifuged at 5,000 × g for 10 min at 4°C. Serum
was kept at −20°C to test serum IL-2, IL-10 and IFN-γ. Pancreatic
tissues were selected to conduct T-cell subset determination, while
other subsets continued to be observed.
Observation of pancreatitis
infiltration degree
Pancreatic samples were embedded with paraffin, and
then turned into tissue sections with H&E dyeing to be observed
under a light microscope (Olympus, Tokyo, Japan). Pathology
personnel used a double-blind method to determine the infiltration
degree of pancreatitis. The grade of pancreatitis infiltration
degree was divided as follows: i) Grade 0: Pancreas islet was
complete without lymphocyte infiltration; ii) grade 1: lymphocytes
infiltrated around the pancreas islet or <25% pancreas islet
area was affected; iii) grade 2: 25–50% pancreas islet area was
affected; and iv) grade 3: >50% pancreas islet area was
affected. According to the above grading standard, the pancreatic
sections of the four groups were observed under a ×5-x400
microscope.
Assessment of local expression of
pancreatic IL-10
Immunohistochemistry was used to observe the
expression of the IL-10 gene in local pancreatic samples.
Brownish granules in cytoplasm were considered positive cells.
Positive cell percentage and dyeing intensity were calculated. The
positive cell percentage was calculated as: i) <1% was 0; ii)
1–10% was 1; iii) 11–50% was 2; iv) 51–80% was 3; and v) >80%
was 4. The dyeing intensity of granules was calculated as: i) No
dyeing was 0; ii) faint yellow was 1; iii) yellow was 2; and iv)
brown was 3. The product of positive cell percentage and dyeing
intensity determined the immunohistochemistry score as follows: 0
score (−); 1–4 scores (+); 5–8 scores (++); and 9–12 scores
(+++).
Determination of pancreatic tissue
T-cell subset
After the mice were sacrificed, they were sterilized
with alcohol. Pancreatic tissues were separated through an aseptic
technique. Nearby connective tissues and pancreatic tissues were
removed and rinsed twice in Hanks' liquid. Ophthalmic scissors were
used to cut the tissues into pasty sections. A tip sucker was used
to blow and disperse pancreatic cells, while a 300-mesh net was
used to implement infiltration. A lymphocyte-separating medium was
used to extract lymphocytes at rotating speeds of 400 × g. They
were then centrifuged for 20 min. The suspended layer of
lymphocytes was extracted after a 2 ml pH 7.4 phosphate buffer
saline (PBS) working solution was added. The lymphocytes were
centrifuged for 5 min at 800 × g. The supernatant was discarded,
and the cell concentration was adjusted to 2×109
cells/l. Under a microscope, 0.4% trypan blue was used to count
living cells. The cell survival rate was determined to be >95%.
A 100 µl pancreatic single-cell suspension at a concentration of
2×109/ml was measured at 25°C and kept in the dark. The
suspension was then incubated with 10 µl CD8-FITC and 10 µl CD4-PE
fluorescent antibody for 15 min. A 2-m erythrocyte lysate buffer
was added to the suspension to incubate it for 10 min. A 2-ml PBS
solution was then added to conduct centrifugation at 800 × g for 5
min. PBS washing was then repeated twice (the method used before),
the supernatant was removed, and 200 µl PBS solution was added to
each tube to conduct FCM detection. Macintosh (Apple, Inc.,
Cupertino, CA, USA) was the data processing system used. Cells were
analyzed using CellQuest software (BD Biosciences, San Jose, CA,
USA). Grating was conducted on lymphocytes on forward and lateral
angular scattering diagrams. A logarithmic method was used to
acquire fluorescence signals before 1×104 cells were
taken and analyzed under the same software. Serum of IL-2, IL-10
and IFN-γ were determined using ELISA. Operating steps were
performed with ELISA reagent kits of IL-2, IL-10 and IFN-γ. The
samples were determined in the same batch.
Statistical analysis
SPSS 21.0 statistical software (IBM SPSS, Armonk,
NY, USA) was used to conduct statistical analysis. Experimental
data were presented as mean ± standard deviation, t-test or
t'-test. A χ2 test was conducted on experimental
results. P<0.05 was considered to indicate a statistically
significant difference.
Results
Pancreatitis infiltration degree
Pancreatitis of mice in control group D was mainly
at grade 2 and 3. Under a light microscope, it was observed that
pancreatic cell morphology was in disorder, and the size and
quantity of the pancreas was small. Mouse pancreatitis in exendin-4
low-dose group A, medium-dose group B and high-dose group C was
mainly at grade 0 and 1. Under the light microscope, it was
observed that pancreatic cell morphology improved, infiltration
degree of lymphocyte improved and pancreatic islet size was
restored somewhat.
Local expression of IL-10 in
pancreatic tissues
Under the light microscope, numerous brown granules
with deep color within pancreatic sample cells in exendin-4
low-dose group A, medium-dose group B and high-dose group C
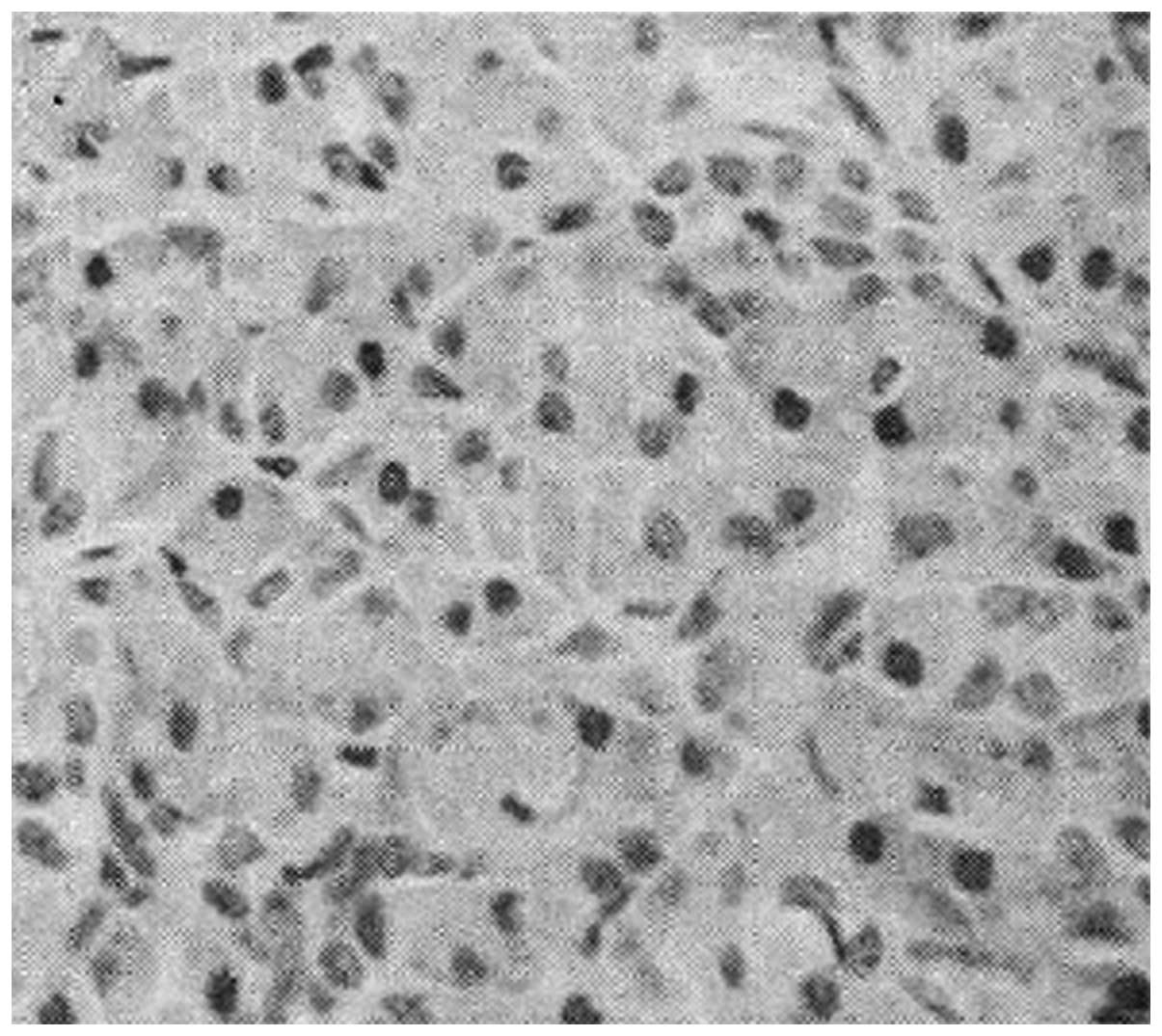
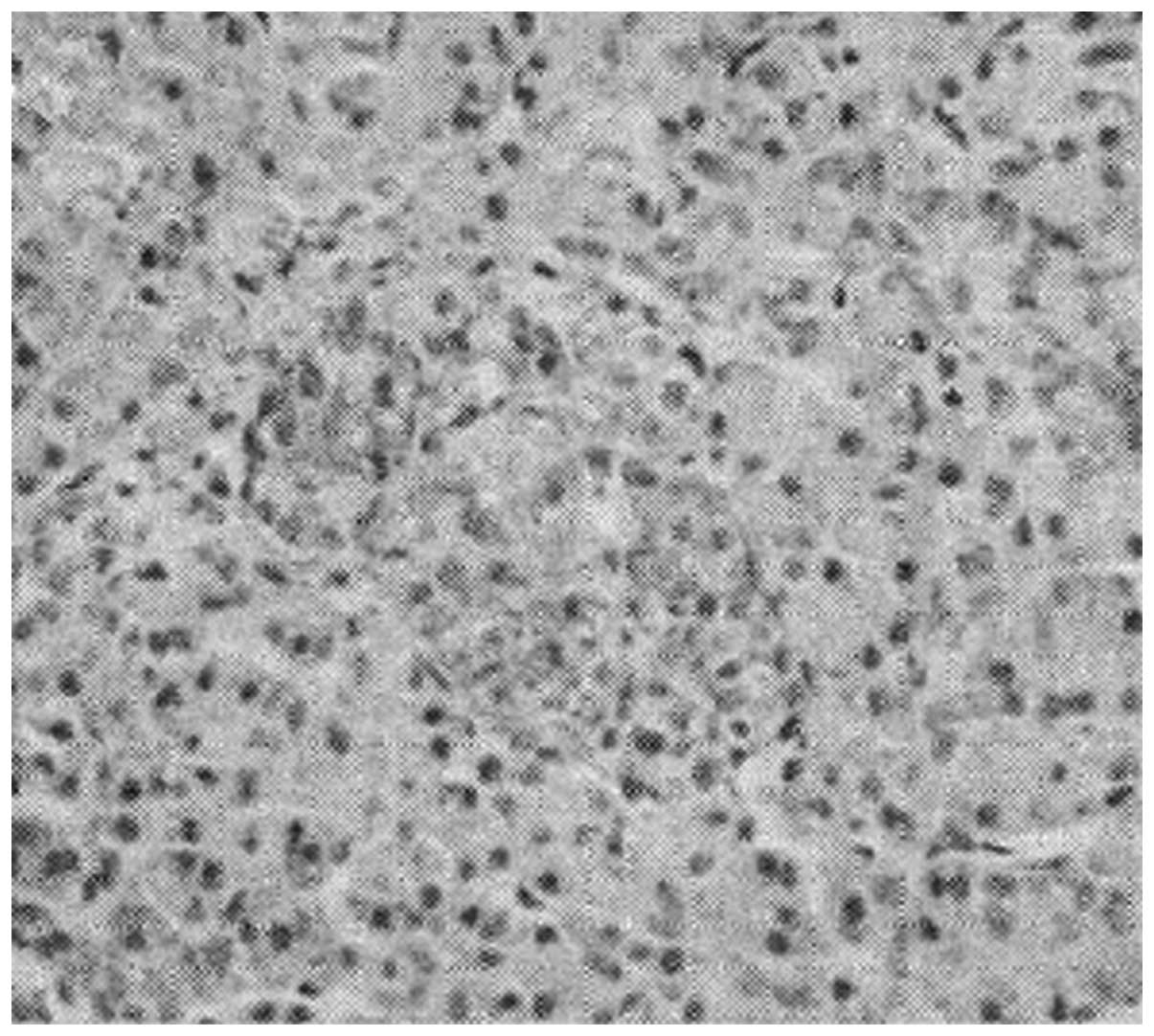
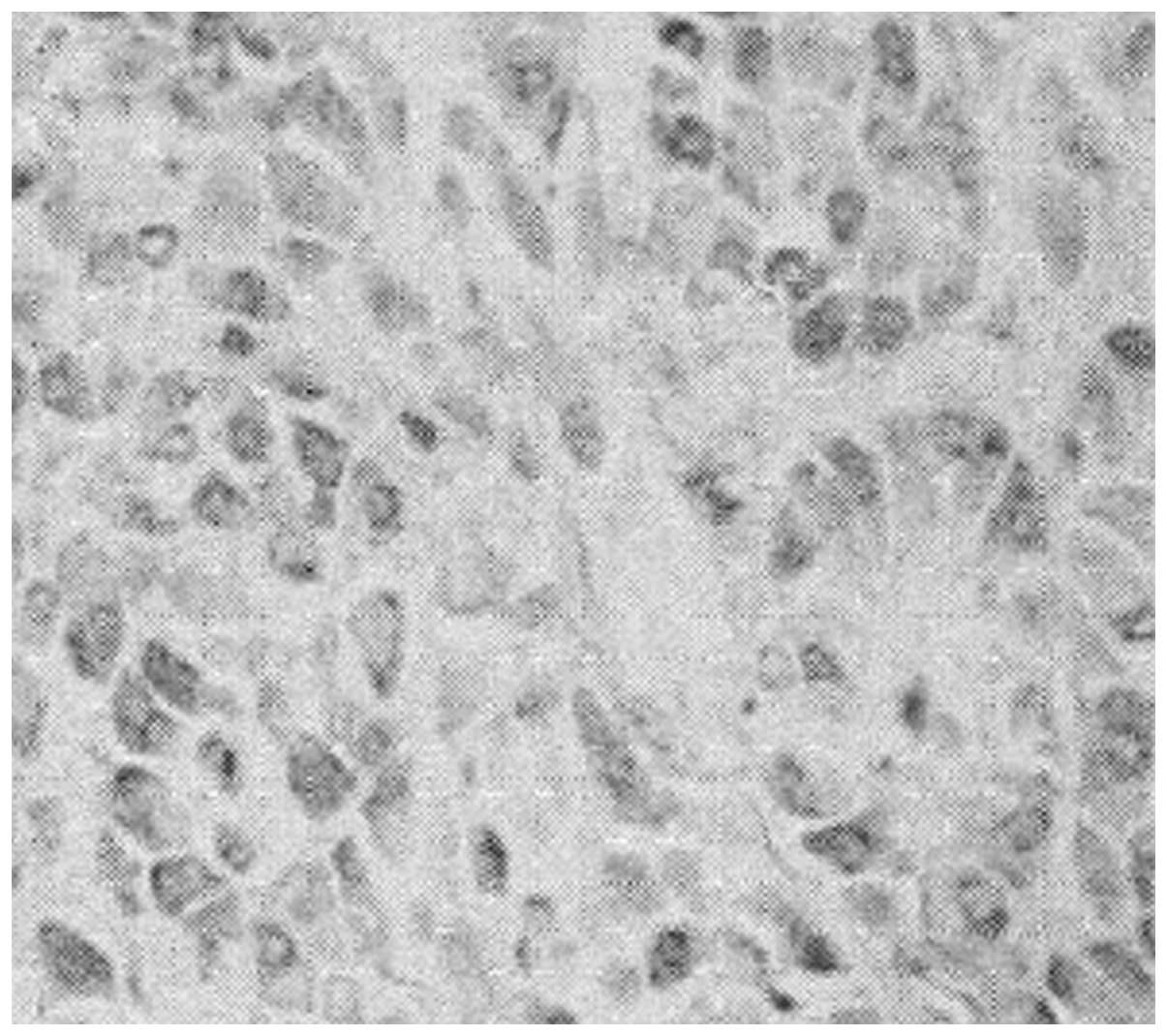
(Figs. 1–3) were observed. There were a few brown
granules within pancreatic sample cells in control group D
(Fig. 4). IL-10 immunohistochemistry
scores in low-dose group A, medium-dose group B and high-dose group
C were 3.82±0.72, 4.34±0.86 and 4.81±0.94, respectively, all of
which were higher than the immunohistochemistry score of 2.25±0.63
in group D.
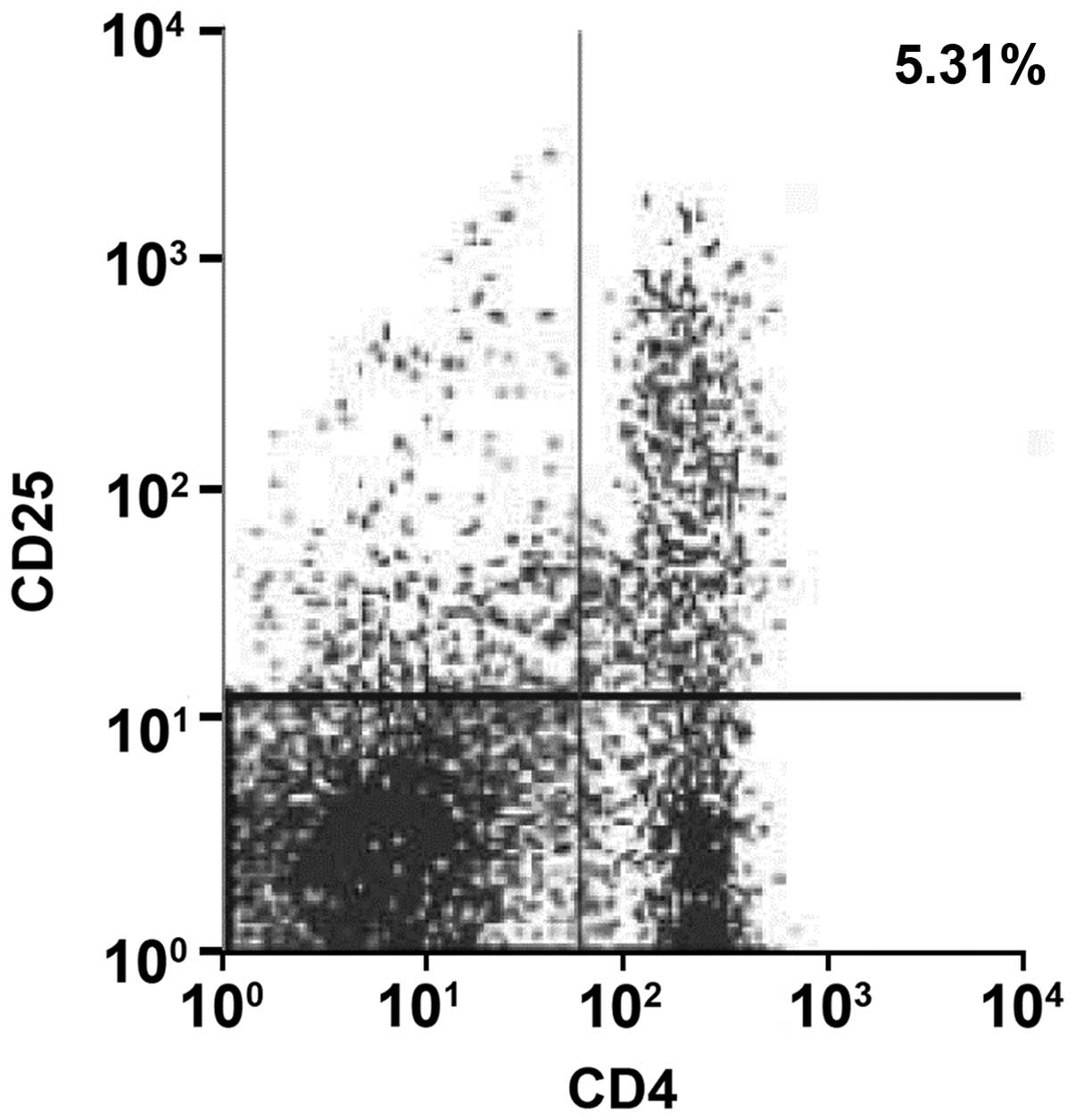
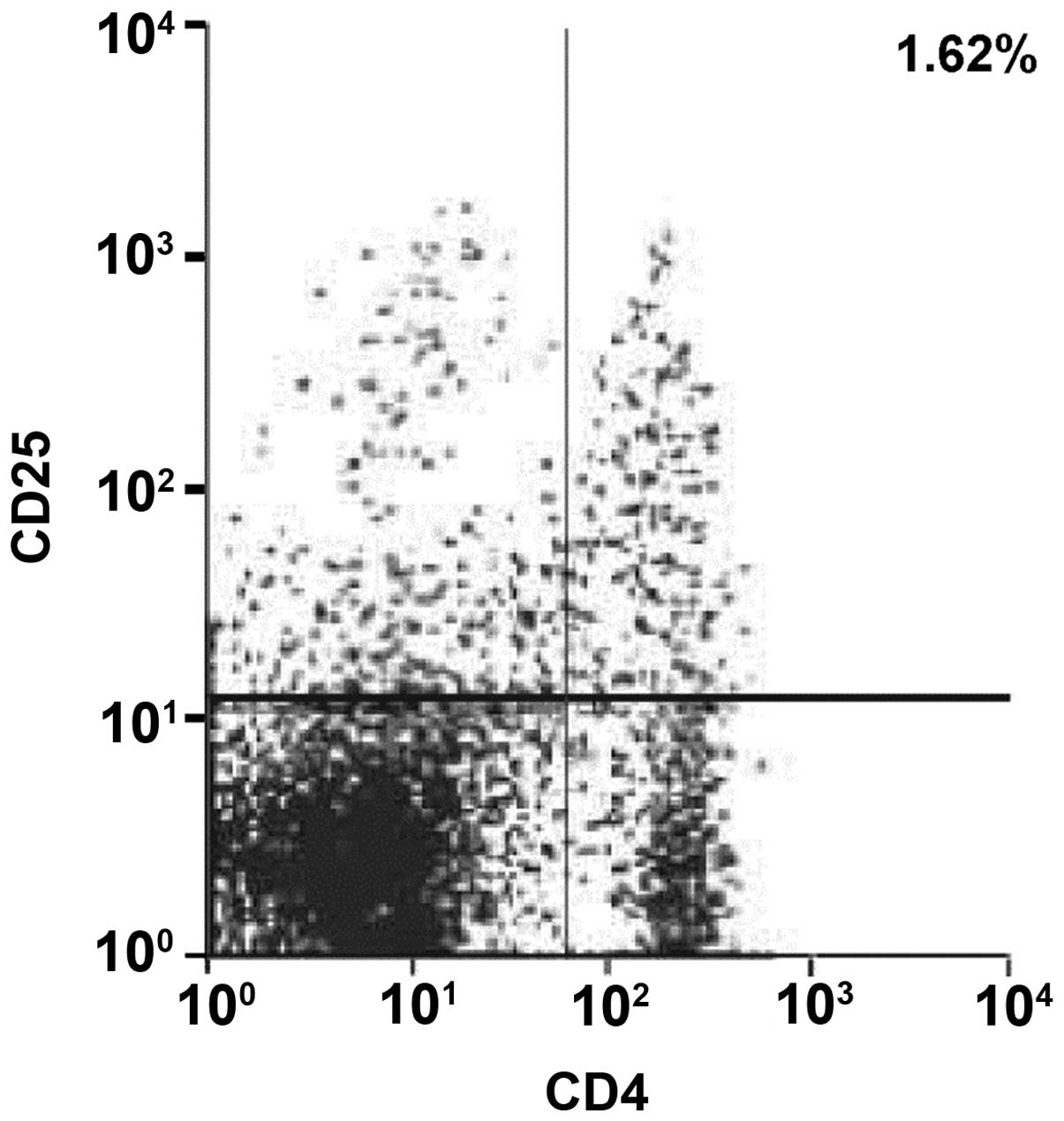
Test results of FCM
The CD4+CD25+T-cell proportions in pancreatic
tissues in exendin-4 low-dose group A, medium-dose group B and
high-dose group C were 5.31, 5.53 and 5.74%, respectively, all of
which were higher than the CD4+CD25+T-cell proportions of 1.62% in
group D, as shown in Figs.
5–8 (right upper quadrant of
each figure was a CD4+CD25+T cell subset). The proportions of
CD4+CD25high T-cells in CD4+T cells increased in groups
A, B and C.
Comparison of serum IL-2, IL-10 and
IFN-γ
Compared with control group D, serum IL-10 levels in
exendin-4 low-dose group A, medium-dose group B and high-dose group
C increased (P<0.05), while IL-2 and IFN-γ levels decreased
(P<0.05). The difference of serum IL-10, IL-2 and IFN-γ levels
existing in IFN-γ was of statistical significance (P<0.05)
(Table I).
 | Table I.Comparison of serum IL-2, IL-10 and
IFN-γ in the four groups. |
Table I.
Comparison of serum IL-2, IL-10 and
IFN-γ in the four groups.
| Group | No. | IL-2 (pg/ml) | IL-10 (pg/ml) | IFN-γ (pg/ml) |
|---|
| Low-dose group A | 10 |
61.12±9.48a |
195.67±20.15a |
321.36±28.26a |
| Medium-dose group
B | 10 |
58.36±8.97a,b |
213.92±21.84a,b |
303.46±25.37a,b |
| High-dose group
C | 10 |
53.15±8.26a–c |
238.72±23.34a–c |
285.48±22.65a–c |
| Control group D | 10 | 81.34±10.27 | 162.66±18.37 | 363.57±31.28 |
Discussion
Previous findings have shown that the occurrence of
type 1 diabetes involves phases such as cellular imbalance,
inflammatory cell recruitment and inflammatory cell effect
(8). The classical immunoregulation
imbalance theory states that, the imbalance of Th1/Th2 cell subset
and disorder of body immunologic functions are key factors inducing
type 1 diabetes (9,10). IL-2, IFN-γ and others are common Th1
cell factors that can upregulate pro-inflammatory factors, thus
generating damage on pancreatic β cells. IL-10, IL-4 and others
belong to Th2 cell factors, which can downregulate the activity of
Th1 cells and have a preventative effect on type 1 diabetes
(11). The present study has shown
that compared with control group D, serum IL-10 levels in exendin-4
low-dose group A, medium-dose group B and high-dose group C
increased (P<0.05), while IL-2 and IFN-γ levels decreased
(P<0.05). The difference of the serum IL-10, IL-2 and IFN-γ
levels existing among IFN-γ were of statistical significance
(P<0.05). Thus, it can be seen that exendin-4 intervention can
reduce the expression of NOD mouse serum IL- 2 and IFN-γ, elevate
the expression of serum IL-10 and exert a protective effect on
pancreatic β cells.
T lymphocytes are closely related to diabetes
pancreatitis. The inflammatory reaction induced by cell factors
that are released by body CD4+T lymphocytes and cell toxic reaction
mediated by body CD8+T lymphocytes are considered the main forms of
cellular immunity induced by T lymphocytes (12–14). If
CD4+T lymphocyte subsets gradually increase, then multiple types of
cell factors can be released. While these cell factors, may enhance
the inflammatory reaction of lymphocyte infiltration, form lethal
effects on antigens and generate adverse effects, they also can
facilitate cellular immunity and humoral immunity to a certain
degree, and then promote effective removal of virus in vivo.
Regulatory T-cells (CD4+CD25high Foxp3+T reg) are a type
of T-cell subset with immunomodulating functions that has been
verified in recent years. It can maintain self-stabilization of an
immune system while regulating the immune response (15–17). The
present study has shown that CD4+CD25+T-cell proportions in
pancreatic tissues of exendin-4 low-dose group A, medium-dose group
B and high-dose group C were 5.31, 5.53 and 5.74%, respectively,
all of which were higher than the CD4+CD25+T-cell proportion of
1.62% in control group D. CD4+CD25high T-cells in groups
A, B and C occupied an increasing proportion in CD4+T-cells.
Studies have indicated that CD4+CD25high T increased in
NOD tumor infiltration lymphocytes under exendin-4 intervention,
which may be associated with the fact that it exerted an
immunosupressive effect by inhibiting the lethal effects of CD8+T
cells through contact among cells (18).
Previous studies reported that, IL-10 can reduce the
accumulated morbidity of diabetes, lower the inflammation degree of
pancreas islets and relieve or prevent the occurrence and
development of type 1 diabetes (19,20).
Notably diabetes is rarely diagnosed in an early phase. The
lymphocyte infiltration degree may be serious once the patient is
diagnosed with diabetes, and there are quite a few residual
pancreatic β cells (21). The
present findings have shown that pancreatitis of mice in control
group D was mainly at grade 2 and 3, and under a light microscope,
it was observed that pancreatic cell morphology was in disorder and
the size and quantity of the pancreas was small. Pancreatitis in
exendin-4 low-dose group A, medium-dose group B and high-dose group
C was mainly at grade 0 and 1, and light microscopy showed that
pancreatic cell morphology and lymphocyte infiltration improved,
and the size of the pancreas was recovered. Additionally, a few
brown granules with deep color were found within the pancreatic
sample cells in control group D. IL-10 immunohistochemistry scores
in low-dose group A, medium-dose group B and high-dose group C were
(3.82±0.72), (4.34±0.86) and (4.81±0.94), respectively, all of
which were higher than that in control group D (2.25±0.63). We
conjectured that exendin-4 intervention elevates IL-10 levels in
pancreatic tissues, downregulates bioactivity of Th1 cell factors
through overexpression of IL-10, is regulated in the in vivo
immune microenvironment and prevents the occurrence and development
of diabetes.
In summary, NOD mouse body immune function is low,
while the regulatory mechanism of its body immunity is in a state
of disorder. Pancreatic tissue necrosis and inflammatory
infiltration are serious. Exendin-4 intervention can exert certain
regulatory effects on the IL-2, IFN-γ, IL-10 and T lymphocyte
subset of NOD mice. By reducing the expression of serum IL- 2 and
IFN-γ, it elevates IL-10 expression in serum and pancreatic tissues
and increases the quantity of CD8+, CD4+ and CD25+ T cells in
pancreatic tissues, thus eliminating inflammatory factors, boosting
pancreatic tissue recovery and improving lymphocyte infiltration
degree. Thus, the results of the current study, revealed the
mechanism involved in exendin-4 on NOD mouse blood and the
pancreatic tissue immune microenvironment.
Acknowledgements
The present study was funded by the Natural Science
Foundation of Fujian province (grant no. 2016J01650).
References
|
1
|
Lind K, Hühn MH and Flodström-Tullberg M:
Immunology in the clinic review series; focus on type 1 diabetes
and viruses: The innate immune response to enteroviruses and its
possible role in regulating type 1 diabetes. Clin Exp Immunol.
168:30–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spinale FG and Stolen CM: Biomarkers and
heart disease: What is translational success? J Cardiovasc Transl
Res. 6:447–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diana J and Lehuen A: Macrophages and
β-cells are responsible for CXCR2-mediated neutrophil infiltration
of the pancreas during autoimmune diabetes. EMBO Mol Med.
6:1090–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muller O, Ntalianis A, Winjs W, Delrue L,
Dierickx K, Auer R, Rodondi N, Mangiacapra F, Trana C, Hamilos M,
et al: Association of biomarkers of lipid modification with
functional and morphological indices of coronary stenosis severity
in stable coronary artery disease. J Cardiovasc Transl Res.
6:536–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skyler JS: Prevention and reversal of type
1 diabetes - past challenges and future opportunities. Diabetes
Care. 38:997–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaminitz A, Mizrahi K, Ash S, Ben-Nun A
and Askenasy N: Stable activity of diabetogenic cells with age in
NOD mice: Dynamics of reconstitution and adoptive diabetes transfer
in immunocompromised mice. Immunology. 142:465–473. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Savinov AY and Strongin AY: Targeting the
T-cell membrane type-1 matrix metalloproteinase-CD44 axis in a
transferred type 1 diabetes model in NOD mice. Exp Ther Med.
5:438–442. 2013.PubMed/NCBI
|
|
8
|
Chen YG, Forsberg MH, Khaja S, Ciecko AE,
Hessner MJ and Geurts AM: Gene targeting in NOD mouse embryos using
zinc-finger nucleases. Diabetes. 63:68–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rachmiel M, Bloch O, Bistritzer T,
Weintrob N, Ofan R, Koren-Morag N and Rapoport MJ: TH1/TH2 cytokine
balance in patients with both type 1 diabetes mellitus and asthma.
Cytokine. 34:170–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nikoopour E, Schwartz JA, Huszarik K,
Sandrock C, Krougly O, Lee-Chan E and Singh B: Th17 polarized cells
from nonobese diabetic mice following mycobacterial adjuvant
immunotherapy delay type 1 diabetes. J Immunol. 184:4779–4788.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hill T, Krougly O, Nikoopour E, Bellemore
S, Lee-Chan E, Fouser LA, Hill DJ and Singh B: The involvement of
interleukin-22 in the expression of pancreatic beta cell
regenerative Reg genes. Cell Regen (Lond). 2:Apr 4–2013.(Epub ahead
of print). doi: 10.1186/2045-9769-2-2. PubMed/NCBI
|
|
12
|
Vong AM, Daneshjou N, Norori PY, Sheng H,
Braciak T, Sercarz EE and Gabaglia CR: Spectratyping analysis of
the islet-reactive T cell repertoire in diabetic NOD Igµ(null) mice
after polyclonal B cell reconstitution. J Transl Med. 9:1–10. 2011.
View Article : Google Scholar
|
|
13
|
Costa N, Pires AE, Gabriel AM, Goulart LF,
Pereira C, Leal B, Queiros AC, Chaara W, Moraes-Fontes MF,
Vasconcelos C, et al: Broadened T-cell repertoire diversity in
ivIg-treated SLE patients is also related to the individual status
of regulatory T-cells. J Clin Immunol. 33:349–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ablamunits V, Henegariu O, Hansen JB,
Opare-Addo L, Preston-Hurlburt P, Santamaria P, Mandrup-Poulsen T
and Herold KC: Synergistic reversal of type 1 diabetes in NOD mice
with anti-CD3 and interleukin-1 blockade: Evidence of improved
immune regulation. Diabetes. 61:145–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim J, Shon E, Kim CS and Kim JS: Renal
podocyte injury in a rat model of type 2 diabetes is prevented by
metformin. Experimental Diabetes Research. 2012 Sep 27–2012.(Epub
ahead of print). doi: 10.1155/2012/210821. View Article : Google Scholar
|
|
16
|
Kachapati K, Bednar KJ, Adams DE, Wu Y,
Mittler RS, Jordan MB, Hinerman JM, Herr AB and Ridgway WM:
Recombinant soluble CD137 prevents type one diabetes in nonobese
diabetic mice. J Autoimmun. 47:94–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suryani S, Evans K, Richmond J and Lock
RB: Evaluation of the Bcl-2 inhibitor ABT-199 in xenograft models
of acute lymphoblastic leukemia by the pediatric preclinical
testing program. Cancer Res. 75:32762015. View Article : Google Scholar
|
|
18
|
Marino E, Villanueva J, Walters S,
Liuwantara D, Mackay F and Grey ST: CD4(+)CD25(+) T-cells control
autoimmunity in the absence of B-cells. Diabetes. 58:1568–1577.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP and
Li HB: Impacts of gut bacteria on human health and diseases. Int J
Mol Sci. 16:7493–7519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zechner D, Spitzner M, Bobrowski A, Knapp
N, Kuhla A and Vollmar B: Diabetes aggravates acute pancreatitis
and inhibits pancreas regeneration in mice. Diabetologia.
55:1526–1534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Attinkara R, Mwinyi J, Truninger K, Regula
J, Gaj P, Rogler G, Kullak-Ublick GA and Eloranta JJ: Swiss IBD
Cohort Study Group: Association of genetic variation in the NR1H4
gene, encoding the nuclear bile acid receptor FXR, with
inflammatory bowel disease. BMC Res Notes. 5:1–12. 2012. View Article : Google Scholar : PubMed/NCBI
|