Introduction
The morbidity of type 2 diabetes mellitus (T2DM),
particularly in elderly people, is increasing in China due to
changes in lifestyle that have occurred in recent years. The
prevalence of diabetes in individuals >60 years old is ~20.4%
(1,2). However, blood glycemic excursion (BGE)
is poorly understood in this particular population.
Traditional treatment strategies for newly diagnosed
elderly diabetics include one or more oral medications in addition
to diet and exercise therapy, selected according to the level of
blood glucose. Insulin injections remain the most potent therapy
for uncontrolled T2DM. Multiple daily insulin injections can result
in decreased variability of 24 h glucose levels (3) and delay the onset or progression of
associated microvascular complications (4). However, hypoglycemia is a frequent side
effect during insulin treatment, particularly in elderly patients.
Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, increases
endogenous glucagon-like peptide 1 (GLP-1) secretion and improves
β-cell function and glycemic excursions without inducing
hypoglycemia in patients with T2DM (5,6). DPP-4
inhibitors provide a beneficial effect on body weight, episodes of
hypoglycemia, and total adverse events as determined via
meta-analysis (7). Furthermore, the
effect of DPP-4 inhibitors on glycated hemoglobin A1c
(GHbA1c) has been suggested to be greater in older
adults than in younger adults with T2DM (8), which makes DPP-4 inhibitors a good
choice for the clinical treatment of elderly patients with
T2DM.
The present randomized case-controlled study was
designed to examine the characteristics of glycemic excursion,
incretins and pancreatic hormone secretion in elderly patients with
newly diagnosed T2DM and to investigate the effects of sitagliptin
on glycemic excursions in such patients.
Materials and methods
Subjects
A total of 129 patients newly diagnosed with T2DM
from Qilu Hospital of Shandong University (Jinan, China) were
enrolled in this study from March 2012 to August 2013. Inclusion
criteria were as follows: i) Results from an oral glucose tolerance
test in accordance with the standards set by the World Health
Organization in 1999 (9) for
patients with newly diagnosed T2DM; ii) aged between 45 and 80
years; iii) negative plasma glutamic acid decarboxylase antibody,
islet cell antibody and insulin autoantibody test results; and iv)
no previous use of hypoglycemic drugs. The exclusion criteria were
as follows: i) Fasting blood glucose >16.7 mmol/l or
GHbA1c >10%; ii) acute diabetic complications such as
ketoacidosis; iii) severe hepatic, renal, cerebral-cardiovascular
or gastrointestinal co-morbidities; or iv) allergies to metformin
hydrochloride, sitagliptin phosphate or glimepiride.
Patients were divided into two age groups: Aged ≥65
years (NEDM group, n=86) and middle-aged group (45–65 years old;
NMDM group, n=43). The elderly subjects were randomly assigned to
receive sitagliptin phosphate (Januvia; Hangzhou MSD Pharmaceutical
Co., Ltd., Hangzhou, China) combined with metformin (Glucophage;
Sino-American Shanghai Squibb Pharmaceuticals Co., Ltd., Shanghai,
China) and were defined as the SIG group or took glimepiride
[Sanofi (Beijing) Pharmaceuticals Co., Ltd., Beijing, China]
combined with metformin and were defined as the GLM group. The dose
of sitagliptin was 100 mg once daily, and the initial dose of
glimepiride was 1 mg once daily, and then titrated to 4 mg once
daily, according to the patient's glucose level. The initial dose
of metformin was 0.25 g thrice daily and then increased to 0.5 g
thrice daily according to the patient's glucose level. The study
was approved by the institutional ethical review committee of Qilu
Hospital of Shandong University (Approval ID: KLS12185), and
informed written consent was obtained from each patient prior to
participation.
Clinical and laboratory
parameters
Clinical data including gender, age, height, weight,
waist measurement, systolic blood pressure (SBP), diastolic blood
pressure (DBP), body mass index (BMI) and past medical history were
collected. Fasting fingertip blood glucose, GHbA1c,
total serum cholesterol (TC), triglyceride (TG), low-density
lipoprotein-cholesterol (LDL-C), high-density
lipoprotein-cholesterol (HDL-C) and uric acid (UA) levels were
evaluated using fully automatic biochemical analysis
instruments.
Continuous blood glucose
monitoring
Subjects who agreed to use a blood glucose
monitoring system (NEDM, n=41; NMDM, n=21) used a continuous
glucose monitor (CGMS System Gold; Medtronic MiniMed, Inc.,
Northridge, CA, USA) for 72 h. The patient maintained a normal
amount of diet and exercise during monitoring to evaluate glucose
variability. The fluctuant indices of glucose included mean blood
glucose (MBG), standard deviation (SD) of the average blood
glucose, intraday mean amplitude of glucose excursion (MAGE), and
mean of daily differences (MODD) of interday blood glucose.
Oral glucose tolerance test (OGTT) and
pancreatic hormone measurements
An OGTT was performed at the beginning and end of
the study. Serum glucose (glucose oxidase method) (10), insulin (chemiluminescence assay;
#03649928; Siemens Healthcare Diagnostics, Inc., Tarrytown, NY,
USA), C-peptide (C-P; #03649933; Siemens Healthcare Diagnostics,
Inc.), glucagon [GLC enzyme-linked immunosorbent assay (ELISA);
R&D Systems, Inc., Minneapolis, MN, USA] and glucagon-like
peptide-1 (GLP-1; ELISA; R&D Systems, Inc.) were evaluated at
0, 30 and 120 min during the OGTT. The areas under the curve (AUC)
for blood glucose, insulin, glucagon and GLP-1 were calculated
using the following formula: 0.25 × [(FX + (4 × 30 min X) + (3 ×
120 min X)]. FX, 30 min × and 120 min X are the values of blood
glucose at 0 min, insulin at 30 min, and glucagon or GLP-1 at 120
min, respectively, during the OGTT. The homeostatic model
assessment of β cell function (HOMA-β) was calculated from fasting
blood glucose (FBG) and fasting insulin (FINS; measured at 0 min
during the OGTT) levels as follows: 20 × FINS/(FBG − 3.5).
HOMA-insulin resistance (HOMA-IR) was calculated as follows: (FBG ×
FINS)/22.5. The early phase of HOMA-β was evaluated as the ratio of
the net increments of insulin and blood glucose levels after 30 min
of sugar loading: ∆Ins30/∆Glu30 (∆Ins30 and ∆Glu30 are the
increases in insulin and blood glucose levels, respectively, at 30
min of the OGTT) (11).
Follow-up visits
For the follow-up assessment, all patients were
evaluated at the clinic at 4, 8, 12 and 24 weeks after enrollment.
The follow-up visits were completed in all 129 patients. Blood
glucose variation was measured using a fingertip micro blood
glucose instrument, and treatment plans were adjusted according to
the treatment guidelines for elderly patients with diabetic
mellitus (12). Hypoglycemia was
recorded. A self-monitored blood glucose level <3.9 mmol/l was
defined as hypoglycemia, and patients with hypoglycemic symptoms
were defined as having symptomatic hypoglycemia, and those
requiring management with emergency treatment were defined as
having severe hypoglycemia. The onset and related hospitalization
of severe cardiocerebral diseases including myocardial infarction,
heart failure and stroke were also recorded.
Statistical analysis
Data are presented as the mean ± SD. SPSS software
(version 16.0; SPSS, Inc., Chicago, IL, USA) was used for all
statistical analysis. Data from experiments were analyzed using
Student's t–test or Chi-square tests, wherever appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical parameters and blood glucose
fluctuation in the NEDM and NMDM groups
As shown in Table I,
age, SBP and LDL-C (P<0.05) levels were significantly different
in the NEDM group compared with the NMDM group. There was no
significant difference in other indices, including waist
measurement, BMI, DBP and TG, TC, HDL-C and UA levels between the
two groups.
 | Table I.Clinical characteristics of the two
age groups. |
Table I.
Clinical characteristics of the two
age groups.
| Characteristics | NMDM | NEDM |
|---|
| No. (M/F) | 43 (23/20) | 86 (45/41) |
| Age (years) | 49.2±4.5 | 68.6±5.2a |
| BMI
(kg/m2) | 27.80±3.16 | 28.22±3.42 |
| Waist (cm) | 85.44±6.84 | 87.03±7.18 |
| SBP (mmHg) | 130.7±14.6 |
138.5±14.2b |
| DBP (mmHg) | 74.1±9.8 | 72.4±10.5 |
| TG (mmol/l) | 2.62±0.60 | 2.47±0.66 |
| TC (mmol/l) | 5.82±2.01 | 5.68±1.53 |
| HDL-C (mmol/l) | 1.28±0.55 | 1.14±0.42 |
| LDL-C (mmol/l) | 3.51±0.53 |
4.43±0.67b |
| UA (µmol/l) | 390.75±91.05 | 383.06±75.29 |
| GHbA1c
(%) | 7.59±1.60 | 7.21±1.24 |
| MBG
(mmol/l)c | 11.03±0.52 | 12.16±0.35 |
| SD
(mmol/l)c | 1.85±0.12 |
3.29±0.17a |
| MAGE
(mmol/l)c | 4.83±0.25 |
8.06±0.38a |
| MODD
(mmol/l)c | 1.70±0.06 |
2.62±0.08b |
Although the GHbA1c levels were not
different between the two groups (P>0.05), the CGMS results
showed that the FBG level in the NEDM group was slightly elevated,
and the blood glucose level increased postprandially and then
dropped sharply. Glycemic excursion parameters including the SD,
MAGE and MODD in the NEDM group were greater than those in the NMDM
group (P<0.05). However, the MBG of the two groups exhibited no
significant difference (Table
I).
Characteristics of incretins in the
NEDM group
During the OGTT, blood glucose levels at 0 and 120
min were lower and those at 30 min were higher in the NEDM group
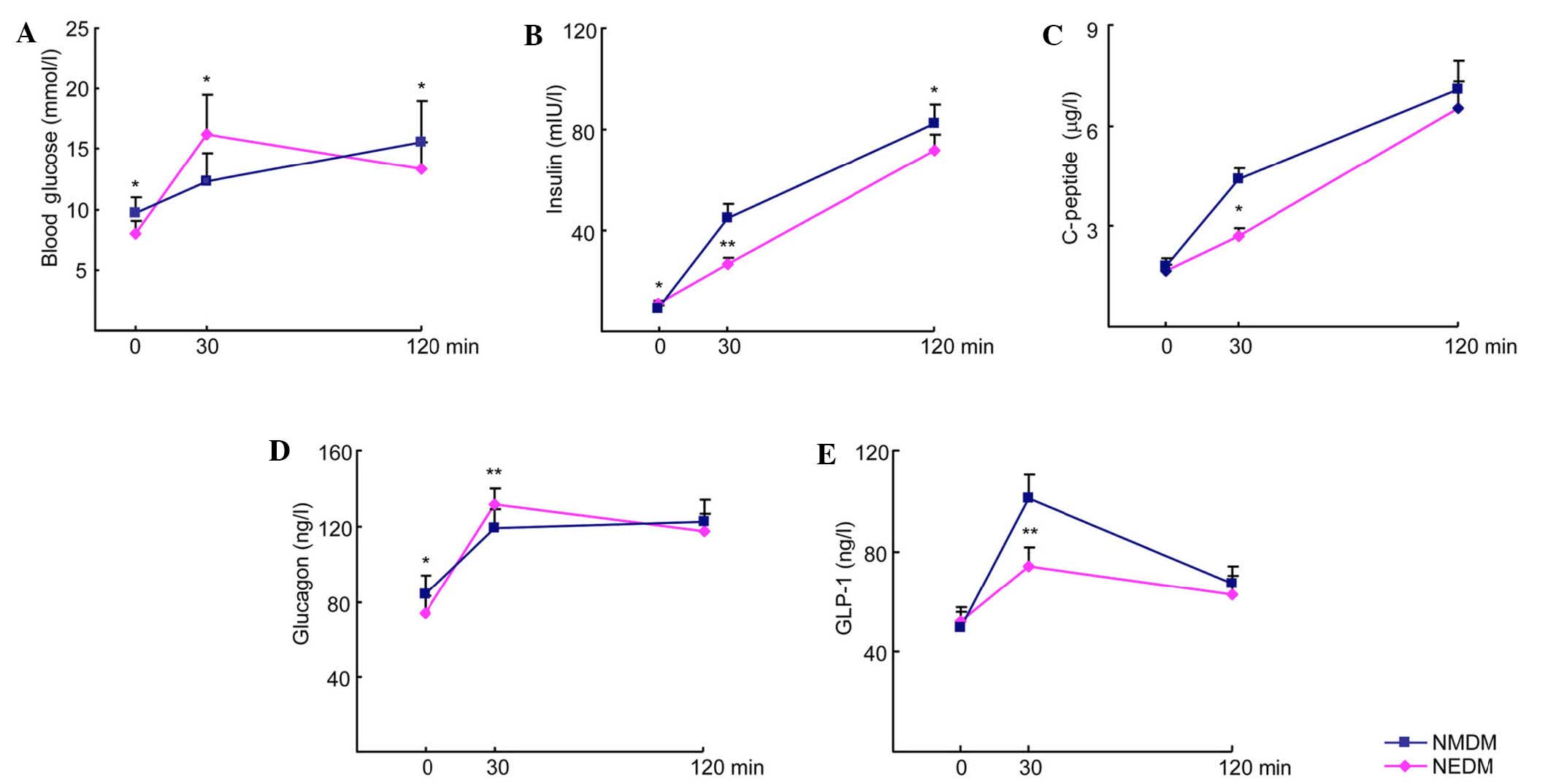
compared with the NMDM group (P<0.05; Fig. 1). The insulin levels at 0, 30 and 120
min and the C-P and GLP-1 levels at 30 min were also lower in the
NEDM group than in the NMDM group (8.09 vs 9.86 mIU/L; P<0.05).
The glucagon level at 0 min was lower and at 30 min higher in the
NEDM group than in the NMDM group (P<0.05; Fig. 1). The AUCins,
∆Ins30/∆Glu30 and AUCglp-1 in the NEDM group were lower
than those the NMDM group (P<0.05; Table II). These results indicate that the
secretion of GLP-1 and insulin decreased in the early phase and was
accompanied by increased secretion of glucagon by α cells during
this time.
 | Table II.AUCs and indices of the two age
groups. |
Table II.
AUCs and indices of the two age
groups.
| Variable | NMDM | NEDM |
|---|
|
AUCBG | 26.75±2.42 | 28.64±2.75 |
|
AUCIns | 107.27±9.20 |
82.33±7.26a |
|
AUCGlc | 324.61±30.33 | 335.28±36.59 |
|
AUCGLP-1 | 169.11±15.46 |
139.52±14.07a |
| HOMA-β | 37.05±3.20 | 41.02±3.76 |
| HOMA-IR | 3.48±0.25 | 3.54±0.26 |
| ΔIns30/ΔGlu30 | 7.15±0.58 |
2.88±0.22b |
Effect of sitagliptin on glucose
excursion and incretins in the NEDM group
No significant differences in clinical
characteristics, GHbA1C or CGMS glucose fluctuation
indices were observed between the GLM and SIG groups prior to
treatment (P>0.05; Table III).
However, after 24 weeks of treatment, these parameters improved in
both the SIG and GLM groups (P<0.05). Moreover, the MAGE and
MODD in the SIG group were significantly lower compared with those
in the GLM group (P<0.05; Table
IV).
 | Table III.Clinical characteristics of the GLM
and SIG groups of NEDM subjects at baseline. |
Table III.
Clinical characteristics of the GLM
and SIG groups of NEDM subjects at baseline.
| Variable | GLM group | SIG group |
|---|
| No. (M/F) | 18 (10/8) | 23 (13/10) |
| Age (years) | 69.1±6.5 | 68.7±6.3 |
| BMI
(kg/m2) | 27.92±3.87 | 28.34±3.81 |
| Waist (cm) | 87.81±7.67 | 88.16±7.66 |
| SBP (mmHg) | 137.3±13.6 | 139.7±14.1 |
| DBP (mmHg) | 71.8±10.5 | 72.5±10.8 |
| TG (mmol/l) | 2.55±0.71 | 2.39±0.69 |
| TC (mmol/l) | 5.87±1.64 | 5.59±1.65 |
| HDL-C (mmol/l) | 1.09±0.37 | 1.16±0.46 |
| LDL-C (mmol/l) | 4.18±0.49 | 4.53±0.64 |
| UA (µmol/l) | 381.56±74.37 | 385.44±76.22 |
 | Table IV.Glucose excursion parameters between
the SIG and GLM groups. |
Table IV.
Glucose excursion parameters between
the SIG and GLM groups.
|
| GLM group | SIG group |
|---|
|
|
|
|
|---|
| Parameter | Pretreatment | After
treatment | Pretreatment | After
treatment |
|---|
| GHbA1c
(%) | 7.27±1.15 |
6.47±0.70a | 7.32±1.01 |
6.25±0.62a |
| MBG (mmol/l) | 11.89±1.12 |
8.82±0.85a | 12.16±1.35 |
8.35±0.79b |
| SD (mmol/l) | 3.18±0.45 |
1.63±0.23a | 3.29±0.47 |
1.57±0.21a |
| MAGE (mmol/l) | 8.27±1.21 |
6.01±0.77a | 8.06±1.18 |
4.42±0.46b, c |
| MODD (mmol/l) | 3.70±0.46 |
2.63±0.37a | 3.62±0.48 |
1.74±0.21a, d |
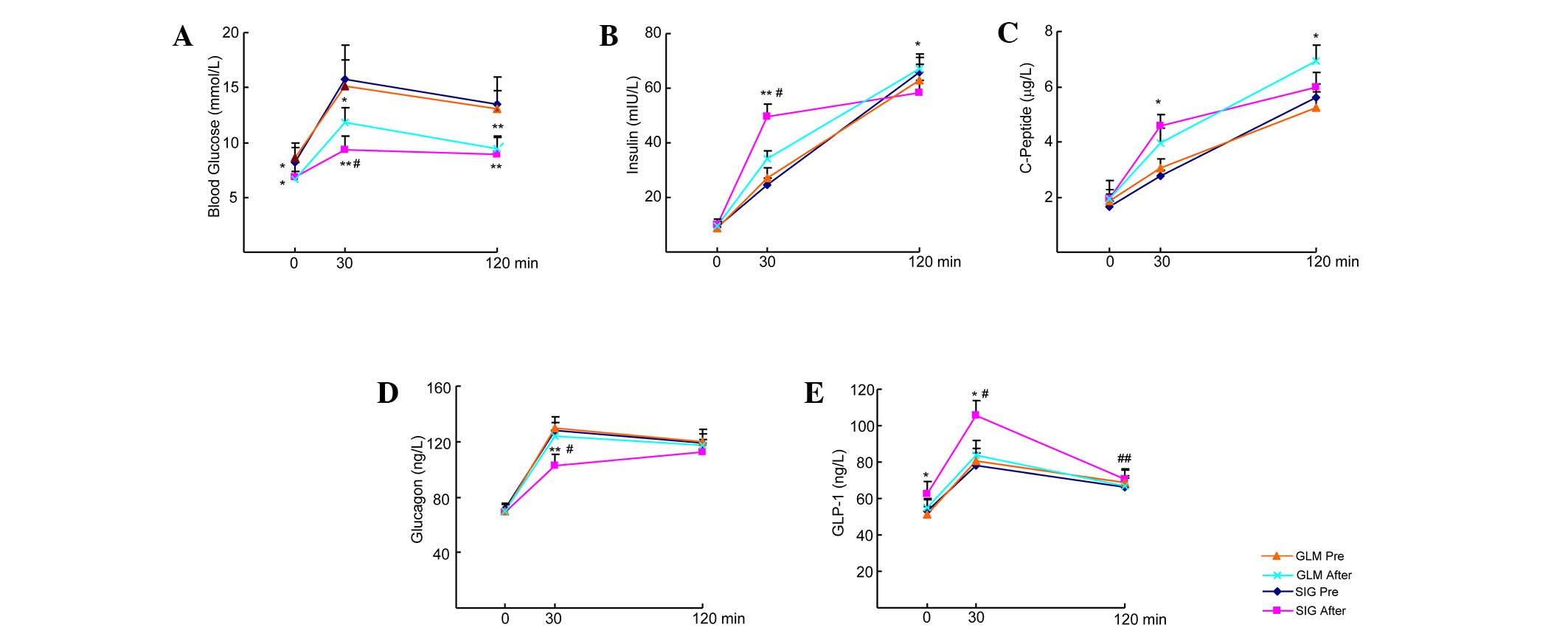
No significant difference existed between the GLM
and SIG groups for glucose, insulin, C-P, glucagon and GLP-1 during
the OGTT prior to treatment (P>0.05). However, these parameters
significantly improved in the two groups after treatment
(P<0.05). Furthermore, with regard to OGTT results in the SIG
group, the levels of insulin and C-P at 30 min and of GLP-1 at 0
and 30 min significantly increased (P<0.05), whereas the
glucagon level at 30 min dropped sharply (P<0.01) compared with
pre-treatment levels. In the GLM group, the levels of insulin and
C-P at 120 min were significantly increased (P<0.05), whereas
the glucagon and GLP-1 levels were maintained (P>0.05) compared
with pre-treatment levels. In the SIG group post-treatment, glucose
and glucagon levels at 30 min were lower compared with those in the
GLM group (P<0.05), whereas insulin and GLP-1 levels at 30 min
were higher compared with those in the GLM group (P<0.05;
Fig. 2).
As shown in Fig. 3,
the AUCs for glucose, insulin, glucagon and GLP-1, and the HOMA-β,
HOMA-IR and ∆Ins30/∆Glu30 indices in the SIG and GLM groups were
comparable prior to treatment (P>0.05). After treatment, all of
these indices significantly improved in the SIG group (P<0.05),
with the exception of the HOMA-IR. In the GLM group, the AUC for
glucose significantly decreased (P<0.05), whereas the AUC for
insulin, HOMA-β and ∆Ins30/∆Glu30 significantly increased
(P<0.05). In addition, the AUC for INS, GLP-1 and ∆Ins30/∆Glu30
in the SIG group were higher than those in the GLM group following
treatment (P<0.05), whereas the AUC for glucagon was lower
(P<0.05).
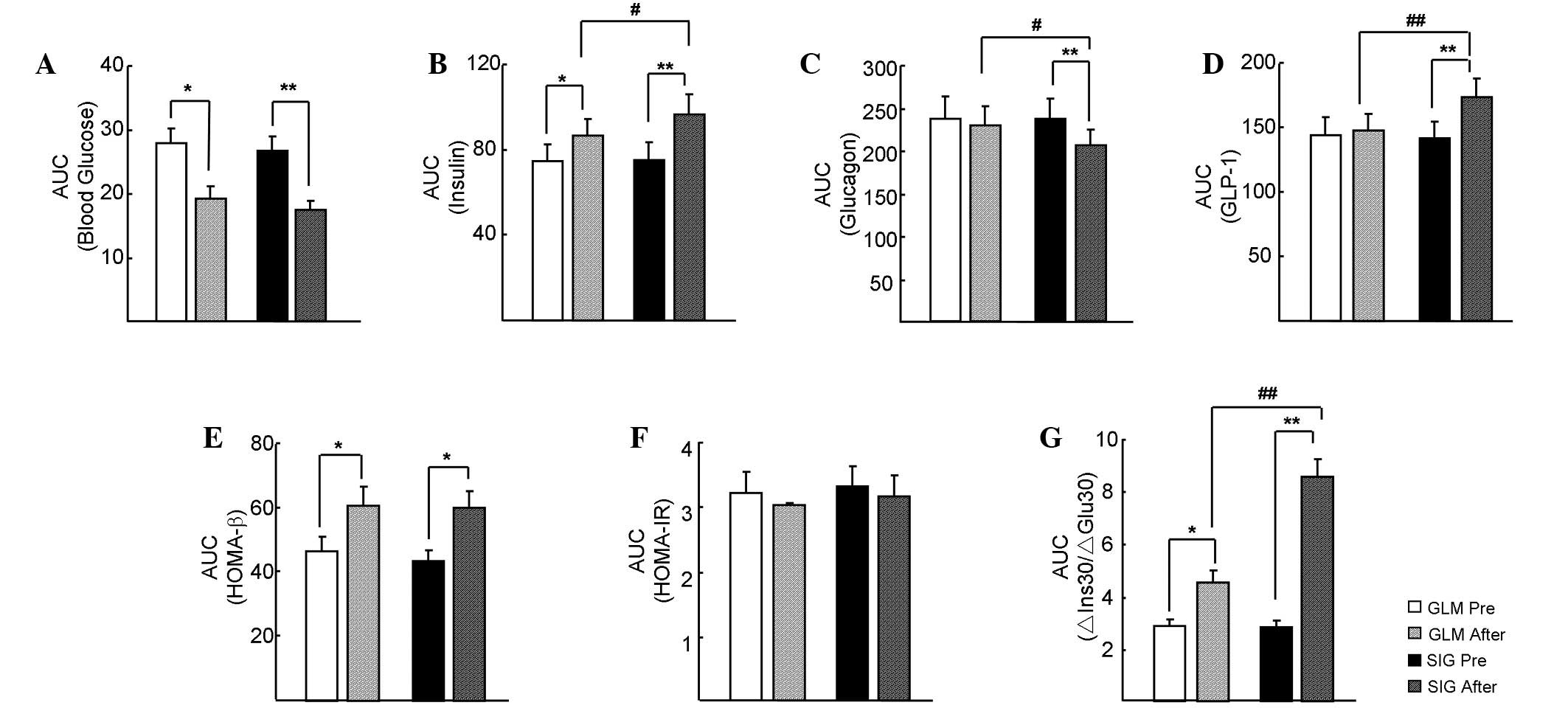 | Figure 3.Area under the curve (AUC) comparisons
during the OGTT after sitagliptin (SIG) or glimepiride (GLM)
treatment. (A) Glucose, (B) serum insulin, (C) C-peptide and (D)
GLP-1 AUCs were analyzed at different time points during the OGTT.
(D) The AUC of GLP-1 was significantly increased after SIG
treatment, but not after GLM treatment. (E) The SIG and GLM groups
showed similar increases in HOMA-β AUC during treatment, with no
significant difference between groups. (F) Neither sitagliptin nor
glimepiride affected the HOMA-IR. (G) The AUC of ∆Ins30/∆Glu30
increased in the two groups, although a greater increase occurred
in the SIG group. OGTT, oral glucose tolerance test; GLP-1,
glucagon-like peptide-1; HOMA-β, homeostatic model assessment of β
cell function; HOMA-IR, HOMA-insulin resistance; ΔIns30/ΔGlu30,
ratio of increments of insulin and blood glucose levels after 30
min of sugar loading. *P<0.05 and **P<0.01 vs. pre-treatment;
#P<0.05 and ##P<0.01 vs. the GLM
group. |
Other effects
The average body weight significantly decreased in
the SIG group following treatment (78.2±4.5 kg at baseline vs.
75.8±4.0 kg at the end of treatment; P<0.05), whereas there was
no significant change in the average body weight (79.0±4.6 kg at
baseline vs. 78.1±4.3 kg at the end of treatment; P>0.05) in the
GLM group. The body weights were significantly different in the SIG
group compared with the GLM group at the end of treatment
(P<0.05). The incidence of symptomatic hypoglycemia in the SIG
and GLM groups was 2.9 and 4.7%, respectively (P<0.05). No
severe hypoglycemia was observed in any subject.
No myocardial infarction, heart failure, and stroke
occurred during the follow up.
Discussion
The amplitude of glucose variability increases from
subjects with normal glucose tolerance (NGT) to those in patients
with T2DM. When compared with individuals with NGT and impaired
glucose regulation, subjects with NMDM show more predominant intra-
and interday glucose variability and postprandial glucose excursion
(13). However, to the best of our
knowledge, there has been no case-control study regarding the
characteristics of glycemic excursion in NEDM patients. In the
present study, glucose variability was observed as a slight
increase in FBG level and a sharp increase in the postprandial
glucose level in NEDM patients. The CGMS glucose excursion
parameters such as MAGE and MODD were also observed to increase and
exhibited extreme fluctuations.
The feature of glucose fluctuation in T2DM is
closely associated with the number and function of β cells and the
secretion level and function of insulin, glucagon and GLP-1.
Previous studies have already verified that aging has a negative
effect on islet function (14,15). In
hyperglycemic clamp experiments, first and second phase insulin
secretion in people with NGT normally decrease by 0.7% per year,
with a greater reduction in the first phase compared with the
second phase (16). A study
involving Chinese individuals with NGT also demonstrate that
insulin secretion and the ability of islets to compensate decrease
with age for basal glucose levels and after oral glucose loading
(17). Age-related IGT in healthy
elderly people is not caused by a reduction in β cell number but
rather the secretion and function of insulin (18), as β cells in all age groups including
healthy, aged and very aged remain stable (19,20).
Patients with IGT and elderly diabetes mellitus (EDM) subjects have
a 40 and 50% deficit, respectively, in the number of β cells
compared with those in healthy controls (21,22).
This deficit results from decreased proliferation and replication
along with increased apoptosis and necrosis of β cells with aging
(20). However, β cell secretion in
EDM patients gradually decreases as diabetes mellitus progresses,
with urinal C-P decreasing 6% per year (23). Glucagon and GLP-1 secretion levels in
the early phase for EDM patients remain unclear. In the present
study, the ∆Ins30/∆Glu30, and the AUCIns and GLP-1 level
at 30 min were lower in the NEDM group than in the NMDM group, and
the glucagon level increased at 30 min in the NEDM group. These
results indicate that the synthesis of glycogen by the liver
significantly decreases under the action of insulin, whereas the
breakdown of liver glycogen into glucose is accelerated by
glucagon, resulting in the highly undulating glycemic excursions in
NEDM subjects. The results of the present study demonstrate that
aging impairs GLP-1 secretion, which contributes to high
post-prandial glycemia, and these data are in accordance with
results of a recent study (24).
DPP-4 inhibitors and sulfonylureas are important
second-line anti-diabetic agents used with metformin or as a
monotherapy. DPP-4 degrades endogenous GLP-1 following its
secretion. Sitagliptin is a highly selective DPP-4 inhibitor that
inhibits ≥80% of plasma DPP-4 activity and augments the level of
active GLP-1 following an OGTT (5).
Animal experiments have indicated that GLP-1 reduces β cell
apoptosis, improves β cell replication and regeneration, and
inhibits α cell oversecretion (25).
A clinical study has found that sitagliptin phosphate used as a
monotherapy or in combination with other antidiabetic drugs can
help improve GLP-1 secretion and β cell function, resulting in
improved glycemic control (26).
Rauch et al administered a 7-week monotherapy of linagliptin
to T2DM subjects aged from 18 to 80 years and found that the
treatment significantly increased the GLP-1 level, suppressed
glucagon and improved the FBG and HbA1c levels (27). In the present study, after 24 weeks
of metformin combined with sitagliptin phosphate treatment in the
NEDM group, it was found that insulin secretion in the early phase
and GLP-1 secretion was improved, and the abnormal secretion of
glucagon was suppressed. However, metformin combined with
glimepiride did not change the GLC and GLP-1 levels, suggesting
that the combination of metformin with sitagliptin phosphate has a
synergistic effect for the treatment of NEDM.
In a previous study, the FBG and 2 h postprandial
blood glucose levels in newly diagnosed NEDM subjects decreased
after 24 weeks of sitagliptin monotherapy (6). Sitagliptin monotherapy treatment
significantly and rapidly improves glycemic measures and is well
tolerated in patients with T2DM aged ≥65 years (28). In the present study, sitagliptin
improved early phase insulin secretion and GLP-1 levels and
suppressed the reverse secretion of α cells in the NEDM group.
Increased early phase insulin secretion promotes glucose intake and
metabolism in cells while increased serum GLP-1 may improve the
early phase secretion of β cells in NEDM patients.
Notably, a recent study has shown that glucose
fluctuations during the acute phase of acute myocardial infarction
affect the myocardial salvage index (MSI), and the plasma GLP-1
level is positively correlated with the MSI. Since coronary artery
diseases (CADs) are common in elderly people with T2DM, this
finding indicates that patients with CAD may obtain greater
benefits after sitagliptin treatment (29).
Animal experiments and clinical research have found
that the hypoglycemic effect of sitagliptin presents glucose
dependence (30,31). Notably, the prevalence of
cerebral-cardiovascular disease in EDM patients is much higher than
in other groups (32), so safety is
particularly important. There are previous reports concerning the
association of sitagliptin with heart failure (33,34),
however, in the present study, no severe cardiovascular diseases
occurred. This study demonstrates that the combination of metformin
with sitagliptin is a relatively safe treatment for NEDM
patients.
This study has several limitations. First, the
sample size was low. Second, not all subjects underwent continuous
blood glucose monitoring due to the cost of this system. Third,
this study was a small-size clinical observation, and the mechanism
was not investigated. More studies are required to explain the
underlying mechanism.
In conclusion, in NEDM patients, blood glucose
fluctuations occur due to defects in the first phase of insulin and
incretin secretion and the excessive secretion of glucagon by
pancreatic α cells. Sitagliptin phosphate combined with metformin
effectively and safely improves glycemic excursion and carbohydrate
metabolism in NEDM patients by promoting the first phase of insulin
and incretin secretion and inhibiting the secretion of
glucagon.
Acknowledgements
This study was supported by grants from special
funds for scientific research projects of clinical medicine of the
Chinese Medical Association (grant no. 13060990484), the Medicine
Health Care Science and Technology Development Project Program of
Shandong Province (grant no. 2013WSC02036), Science Foundation of
Qilu Hospital of Shandong University (grant no. 2015QLMS11) and
Fundamental Research Funds of Shandong University
(26010175616012).
References
|
1
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Testa MA, Gill J, Su M, Turner RR, Blonde
L and Simonson DC: Comparative effectiveness of basal-bolus vs.
premix analog insulin on glycemic variability and patient-centered
outcomes during insulin intensification in type 1 and type 2
diabetes: A randomized, controlled, crossover trial. J Clin
Endocrinol Metab. 97:3504–3514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wake N, Hisashige A, Katayama T, Kishikawa
H, Ohkubo Y, Sakai M, Araki E and Shichiri M: Cost-effectiveness of
intensive insulin therapy for type 2 diabetes: A 10-year follow-up
of the Kumamoto study. Diabetes Res Clin Pract. 48:201–210. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herman GA, Bergman A, Stevens C, Kotey P,
Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, et al:
Effect of single oral doses of sitagliptin, a dipeptidyl
peptidase-4 inhibitor, on incretin and plasma glucose levels after
an oral glucose tolerance test in patients with type 2 diabetes. J
Clin Endocrinol Metab. 91:4612–4619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenstock J, Aguilar-Salinas C, Klein E,
Nepal S, List J and Chen R: Effect of saxagliptin monotherapy in
treatment-naive patients with type 2 diabetes. Curr Med Res Opin.
25:2401–2411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Hong J, Chi J, Gu W, Ning G and
Wang W: Head-to-head comparison of dipeptidyl peptidase-IV
inhibitors and sulfonylureas - a meta-analysis from randomized
clinical trials. Diabetes Metab Res Rev. 30:241–256. 2013.
View Article : Google Scholar
|
|
8
|
Monami M, Cremasco F, Lamanna C,
Marchionni N and Mannucci E: Predictors of response to dipeptidyl
peptidase-4 inhibitors: Evidence from randomized clinical trials.
Diabetes Metab Res Rev. 27:362–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gabir MM, Hanson RL, Dabelea D, Imperatore
G, Roumain J, Bennett PH and Knowler WC: Plasma glucose and
prediction of microvascular disease and mortality: Evaluation of
1997 American Diabetes Association and 1999 World Health
Organization criteria for diagnosis of diabetes. Diabetes Care.
23:1113–1118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lott JA and Turner K: Evaluation of
Trinder's glucose oxidase method for measuring glucose in serum and
urine. Clin Chem. 21:1754–1760. 1975.PubMed/NCBI
|
|
11
|
Bonora E, Targher G, Alberiche M,
Bonadonna RC, Saggiani F, Zenere MB, Monauni T and Muggeo M:
Homeostasis model assessment closely mirrors the glucose clamp
technique in the assessment of insulin sensitivity: Studies in
subjects with various degrees of glucose tolerance and insulin
sensitivity. Diabetes Care. 23:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tuerk PW, Mueller M and Egede LE:
Estimating physician effects on glycemic control in the treatment
of diabetes: Methods, effects sizes and implications for treatment
policy. Diabetes Care. 31:869–873. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Lv L, Yang Y, Chen D, Liu G, Chen
L, Song Y, He L, Li X, Tian H, et al: Glucose fluctuations in
subjects with normal glucose tolerance, impaired glucose regulation
and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol
(Oxf). 76:810–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cnop M, Igoillo-Esteve M, Hughes SJ,
Walker JN, Cnop I and Clark A: Longevity of human islet α-and
β-cells. Diabetes Obes Metab (13 Suppl 1). 39–46. 2011. View Article : Google Scholar
|
|
15
|
Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa
MB, Sayyed F, van de Laar L, Gunter JH, de Koning EJ, Walls GV,
Gray DW, et al: The long lifespan and low turnover of human islet
beta cells estimated by mathematical modelling of lipofuscin
accumulation. Diabetologia. 53:321–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szoke E, Shrayyef MZ, Messing S, Woerle
HJ, van Haeften TW, Meyer C, Mitrakou A, Pimenta W and Gerich JE:
Effect of aging on glucose homeostasis: Accelerated deterioration
of beta-cell function in individuals with impaired glucose
tolerance. Diabetes Care. 31:539–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu HQ, Yang ZJ, Zhang B, Xiao JZ and Yang
WY: Ageing related changes of insulin secretion and insulin
sensitivity among normal glucose tolerance individuals in China.
Zhonghua Yi Xue Za Zhi. 92:1948–1953. 2012.(In Chinese). PubMed/NCBI
|
|
18
|
Basu R, Breda E, Oberg AL, Powell CC, Man
C Dalla, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, et al:
Mechanisms of the age-associated deterioration in glucose
tolerance: Contribution of alterations in insulin secretion, action
and clearance. Diabetes. 52:1738–1748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saisho Y, Butler AE, Manesso E, Elashoff
D, Rizza RA and Butler PC: β-cell mass and turnover in humans:
Effects of obesity and aging. Diabetes Care. 36:111–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kushner JA: The role of aging upon β cell
turnover. J Clin Invest. 123:990–995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Butler AE, Janson J, Bonner-Weir S, Ritzel
R, Rizza RA and Butler PC: Beta-cell deficit and increased
beta-cell apoptosis in humans with type 2 diabetes. Diabetes.
52:102–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maclean N and Ogilvie RF: Quantitative
estimation of the pancreatic islet tissue in diabetic subjects.
Diabetes. 4:367–376. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bushhouse SA, Goetz FC, Jacobs DR Jr,
Bender AP, French LR, Oestreich PG and Geisser MS: C-peptide
response to meal challenge in nondiabetic and diabetic adults
living in Wadena, Minnesota. Diabetes Care. 15:1335–1347. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geloneze B, de Oliveira Mda S, Vasques AC,
Novaes FS, Pareja JC and Tambascia MA: Impaired incretin secretion
and pancreatic dysfunction with older age and diabetes. Metabolism.
63:922–929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stoffers DA, Kieffer TJ, Hussain MA,
Drucker DJ, Bonner-Weir S, Habener JF and Egan JM: Insulinotropic
glucagon-like peptide 1 agonists stimulate expression of
homeodomain protein IDX-1 and increase islet size in mouse
pancreas. Diabetes. 49:741–748. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solis-Herrera C, Triplitt C,
Garduno-Garcia JJ, Adams J, DeFronzo RA and Cersosimo E: Mechanisms
of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin
when used alone or with metformin in type 2 diabetes: A
double-tracer study. Diabetes Care. 36:2756–2762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rauch T, Graefe-Mody U, Deacon CF, Ring A,
Holst JJ, Woerle HJ, Dugi KA and Heise T: Linagliptin increases
incretin levels, lowers glucagon and improves glycemic control in
type 2 diabetes mellitus. Diabetes Ther. 3:102012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barzilai N, Guo H, Mahoney EM, Caporossi
S, Golm GT, Langdon RB, Williams-Herman D, Kaufman KD, Amatruda JM,
Goldstein BJ and Steinberg H: Efficacy and tolerability of
sitagliptin monotherapy in elderly patients with type 2 diabetes: A
randomized, double-blind, placebo-controlled trial. Curr Med Res
Opin. 27:1049–1058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teraguchi I, Imanishi T, Ozaki Y, Tanimoto
T, Ueyama M, Orii M, Shiono Y, Shimamura K, Ishibashi K, Yamano T,
et al: Acute-phase glucose fluctuation is negatively correlated
with myocardial salvage after acute myocardial infarction. Circ J.
78:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takihata M, Nakamura A, Tajima K, Inazumi
T, Komatsu Y, Tamura H, Yamazaki S, Kondo Y, Yamada M, Kimura M and
Terauchi Y: Comparative study of sitagliptin with pioglitazone in
Japanese type 2 diabetic patients: The COMPASS randomized
controlled trial. Diabetes Obes Metab. 15:455–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyre D, Froguel P, Horber FF and Kral JG:
Comment on: Valette et al. Melanocortin-4 receptor mutations and
polymorphisms do not affect weight loss after bariatric surgery.
PLoS One. 9:e933242014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alexander CM, Landsman PB, Teutsch SM and
Haffner SM: Third National Health and Nutrition Examination Survey
(NHANES III); National Cholesterol Education Program (NCEP):
NCEP-defined metabolic syndrome, diabetes, and prevalence of
coronary heart disease among NHANES III participants age 50 years
and older. Diabetes. 52:1210–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH,
Chen SJ, Yeh CM, Chen TJ, Lin SJ and Chiang CE: Sitagliptin and the
risk of hospitalization for heart failure: A population-based
study. Int J Cardiol. 177:86–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weir DL, McAlister FA, Senthilselvan A,
Minhas-Sandhu JK and Eurich DT: Sitagliptin use in patients with
diabetes and heart failure: A population-based retrospective cohort
study. JACC Heart Fail. 2:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|