Introduction
Cervical cancer is highly common in women worldwide,
with an estimated global incidence of 470,000 new cases each year
(1). Currently, radiotherapy remains
the most effective therapeutic method for cervical cancer,
particularly for the patients at an advanced stage, and can achieve
relatively satisfactory outcome in clinical practice (2). However, a range of side effects are
associated with conventional high-dose radiotherapy, and thus
numerous patients ultimately discontinue radiotherapy due to the
severe discomfort involved (3,4). By
contrast, low-dose radiotherapy exhibits the advantages of reduced
collateral damage, increased safety and easier acceptance by
patients, and may therefore offer a promising approach in the field
of radiotherapy (5). However, it
remains unclear how to guarantee a satisfactory therapeutic effect
when diminishing the dose of irradiation (6). Sensitizing cervical cancer cells to
irradiation has been proved to be a viable approach, mainly based
on recent molecular biotechnology that can modulate corresponding
genes to the end of promoting cancer cell radiosensitivity
(7).
MicroRNAs (miRNAs) are a class of non-coding RNAs
that regulate protein expression by inducing mRNA degradation or
interfering with translation, and have been shown to play important
role in cancer suppression or carcinogenesis (8). miR-145 has been verified to be
important in cancer suppression (9).
Downregulation of miR-145 has been widely observed in cervical
cancer and several other cancer types (9,10).
Artificially promoting the expression of miR-145 by plasmid
transfection shows obvious growth inhibition on cancer cells
(11,12). However, few studies have been
published documenting the role of miR-145 in modulating the
radiosensitivity of cancers.
Octamer-binding transcription factor 4 (OCT4) is a
stem-related transcription factor, a group of proteins that was
initially identified as being involved in the self-renewal and
differentiation of embryonic stem cells. However, a large number of
clinical reports suggesting that higher expression levels of OCT4
may be associated with higher grades of cancer suggest the function
of OCT4 in cancers (13–15). Subsequent research has shown that
OCT4 expression in oral cancer is positively correlated with cancer
cisplatin resistance, invasion and proliferation (16). OCT4 downregulation via RNA
interference in head neck squamous cell carcinoma causes an
increase in radiosensitivity and a loss of metastatic potential
(17). These result further indicate
the importance of OCT4 contributing to the development of cancers
in a number of ways (18,19).
The aim of the present study was to investigate the
role of miR-145 in the modulation of cervical cancer cell
radiosensitivity, using transfection with miR-145 mimics to
upregulate miR-145 in cervical cancer tera cells. Furthermore, we
investigated cell viability, apoptosis rate and migration rate
after these cells were exposed to low-dose irradiation. Our study
and others' (20) have indicated
that OCT4 is an important target of miR-145. Thus, in the present
study cervical cancer tera cells in the round were co-transfected
with miR-145 mimics and OCT4 expression vector to determine whether
OCT4 mediated miR-145 function. This study aimed to provide a
theoretical foundation for the modulation of the radiosensitivity
of cervical cancer tera cells during low-dose radiotherapy.
Materials and methods
Cell lines
The cervical cancer Tera cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA) and
were maintained as exponentially growing monolayers in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Grand Island, NY, USA) containing 10% fetal bovine serum (HyClone;
GE Healthcare Life Sciences, Chalfont, UK) in a 37°C incubator with
5% CO2.
Irradiation
Tera cells were trypsinized (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and seeded into six-well
plates with ~1.0×105 cells per well. After 48 h of
incubation, the cells were cultured in the medium without fetal
bovine serum and irradiated at dose of 1, 2, 4 or 6 Gy on ice.
Based on our evaluation of cell viability and apoptosis rate after
irradiation, 1 Gy irradiation was selected and performed in
following formal test.
Cell survival ratio assay
A Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) assay was used to measure cell survival
ratio after irradiation. 0.5×104 cells were seeded in
each 96-well plate for 24 h, and incubated with CCK-8 reagents at a
final concentration of 10% for 1 h. The optical density in each
well was determined using an enzyme immunoassay analyzer at 490
nm.
Flow cytometry method
Apoptosis ratio after irradiation was analyzed in
vitro using a FACS Annexin V assay kit (BD Biosciences, San
Jose, CA, USA) according to the manufacturer's instructions.
Briefly, the harvested cells were washed and resuspended in 0.1 M
phosphate-buffered saline (PBS). Next, cells were fixed overnight
with 75% cold ethanol, washed twice with cold PBS, then incubated
in PBS buffer containing 50 µg/ml propidium iodide (PI) and 20
µg/ml RNase A for 30 min at 37°C. Next, cells were incubated with 5
µl Annexin V-FITC in 195 µl binding buffer in the dark for 10 min.
PI and forward light scattering were detected using a FACSCalibur
flow cytometer (BD Biosciences) equipped with the ModFit LT
software package (version 3.2; Verity Software House, Inc.,
Topsham, ME, USA).
Dual luciferase reporter assay
Dual luciferase vector pRL-TK was purchased from
Promega Corporation (Madison, WI, USA. An oligonucleotide duplex
containing the predicted binding site of miR-145 (miRNA response
element; MRE) present in the 3′-UTR of OCT4 was inserted into
pRL-TK to construct an miR-145 MRE luciferase reporter (pRL-TK-OCT4
3′-UTR). This reporter and negative control were then transfected
into tera cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's recommendations.
Firefly luciferase and Renilla reniformis signals were
measured 48 h after transfection using GloMax 20/20n luminometer
(Promega Corporation).
Transfection treatment
Overexpression of miR-145 in tera cells was achieved
by transfection with miR-145 mimics (GenePharma, Co., Ltd,
Shanghai, China) using Lipofectamine 2000 according to the
manufacturer's instructions. OCT4 expression vector, the
full-length OCT4-coding sequence was amplified and cloned into a
pEGFP-C1 expression vector (Invitrogen). Co-transfection of miR-145
mimics and OCT4 expression vector into tera cells was performed
using Lipofectamine 2000. Total RNA and protein were extracted from
tera cells for subsequent polymerase chain reaction (PCR) and
western blot analyses for detecting the mRNA and protein expression
levels of miR-145 and OCT4.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol reagent (Invitrogen) was used to extract
total RNA from tera cells. Reversing transcribed RNA (1 µg) into
cDNA was performed using a MiScript Reverse Transcription Kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's instructions. Gene expression of miR-145 was
assessed using a Power SYBR® Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
amplification parameters were used: 95°C for 10 min, followed by 50
cycles of 95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec. The
following primers were used: miR-145, forward
5′-GTCCTCACGGTCCAGTTT-3′ and reverse 5′-TTTGGCACTAGCACATT-3′; U6,
forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-AACGCTTCACGAATTTGCGT-3′. The assay was repeated three times, and
gene expression levels were normalized against U6, and calculated
using the 2−ΔCt method (21). Replacing RNA or cDNA with equal
quantities of deionized water was used as the negative control.
Western blot analysis
Cells were lysed on ice in lysis buffer (50 mM
Tris-HCl, pH 7.4; 150 mM NaCl; 2 mM EDTA; 1% NP-40; and 0.1% SDS).
A total of 20 µg protein extracted from cell lysis was separated
using 10% SDS-PAGE and transferred onto a nitrocellulose membrane
(Merck Millipore, Billerica, MA, USA). The membrane was then
blocked with 5% bovine serum albumin (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at room temperature for 1 h, and incubated
with the following primary murine monoclonal antibodies at 4°C
overnight: Anti-OCT4 (1:500; sc-9081; Santa Cruz Biotechnology,
Inc.), anti-cyclin D1 (1:500; #2926; Cell Signaling Technology,
Inc., Danvers, MA, USA) and anti-β-actin (1:500; sc-47778; Santa
Cruz Biotechnology, Inc.). In the following steps, membrane
underwent at least three washes with 0.1 M PBST before incubation
with horseradish peroxidase-conjugated secondary antibodies
(1:10,000; sc-2004 and sc-2005; Santa Cruz Biotechnology, Inc.) for
2 h at room temperature. Bands were detected using an enhanced
chemiluminesence detection kit (Pierce Protein Biology; Thermo
Fisher Scientific, Inc.). Relative quantification was determined
with the AlphaView system (version 3.4.0.729; ProteinSimple, Santa
Clara, CA, USA), using β-actin as the loading control.
Wound healing assay
Cells were trypsinized and seeded in equal numbers
(1×105 cells/well) into six-well tissue culture plates,
and allowed to grow to confluence (85%; ~24 h). A 100-µl pipette
tip was used to create an artificial wound by scratching a
homogenous line on the cell monolayer. After scratching, the cells
were washed and cultured in serum-free medium. The microscopic
images of same area were collected immediately after a wound was
inflicted to the cell and at time point 24 h. Migration rates were
calculated using the following equation: (Initial distance – final
distance / initial distance) × 100.
Statistical analysis
SPSS statistical software, version 19.0 (IBM SPSS,
Armonk, NY, USA) was used for statistical analysis. One-way
analysis of variance with post-hoc t–testing was used for
multiple comparisons between each group. Data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
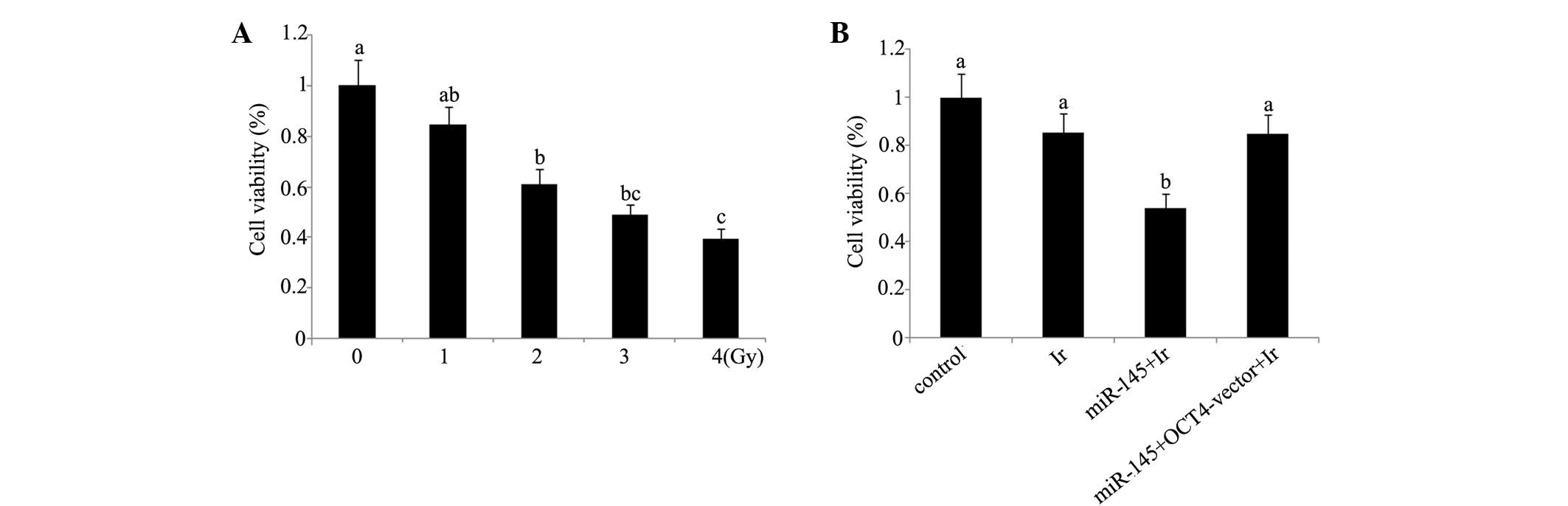
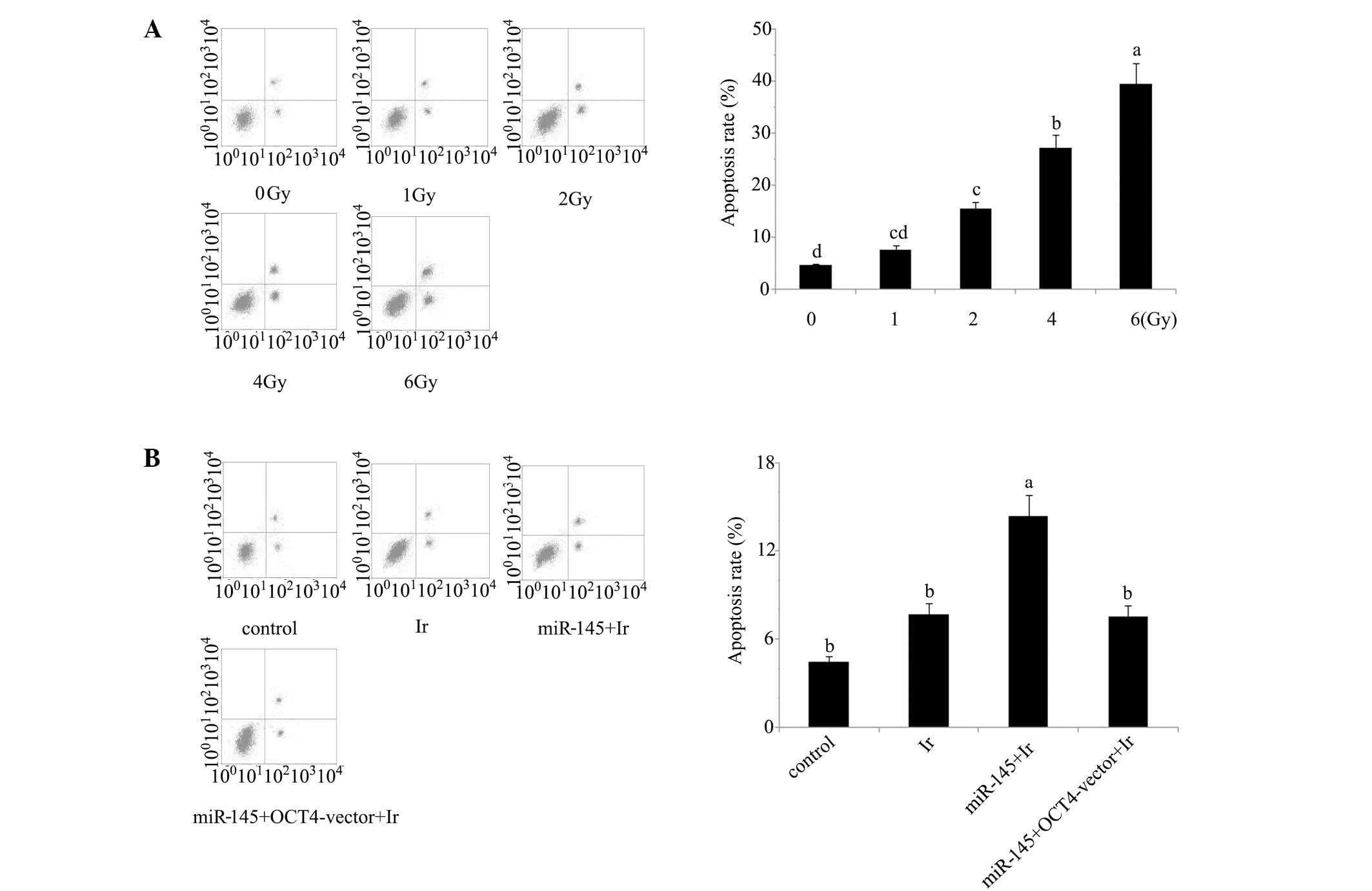
Cell viability and apoptosis analyses indicate that
1 Gy is an appropriate dose for modelling low-dose radiotherapy
in vitro. In our preliminary experiment, cervical cancer
tera cells were exposed to 1, 2, 4 and 6 Gy of irradiation. Based
on the evaluation of cell viability (Fig. 1) and apoptosis rate (Fig. 2) after irradiation (Figs. 1A and 2A), irradiation-induced cell damage was
increased with the elevation of the irradiation dose and reached
the most severe level at 6 Gy irradiation, with ~50% cell viability
and 45% apoptosis rate of the control cells, while there was no
significant damage inflicted by 1 Gy irradiation. All subsequent
experiments were performed using irradiation at dose of 1 Gy, as
this dosage was an appropriate model of low-dose radiotherapy for
enhancing the radiosensitivity.
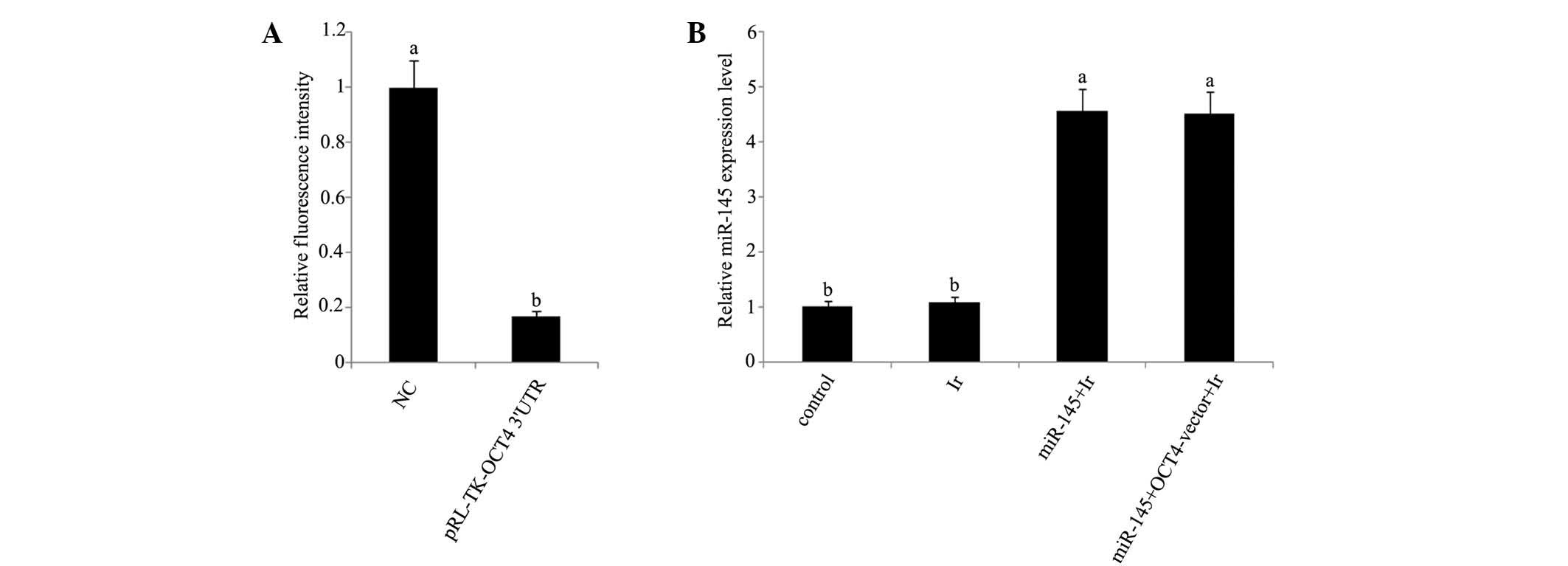
mRNA and protein expression levels. In dual
luciferase reporter assay, a dual luciferase vector that was
ligated to a fragment corresponding to the predicted target site of
miR-145 in OCT4 3′-UTR reduced by 83% fluorescence (Fig. 3A). Furthermore, RT-qPCR analysis
showed that miR-145 was significantly upregulated in tera cells
transfected with miR-145 mimics or co-transfected with miR-145
mimics and OCT4 expression vector before cell exposure to 1 Gy
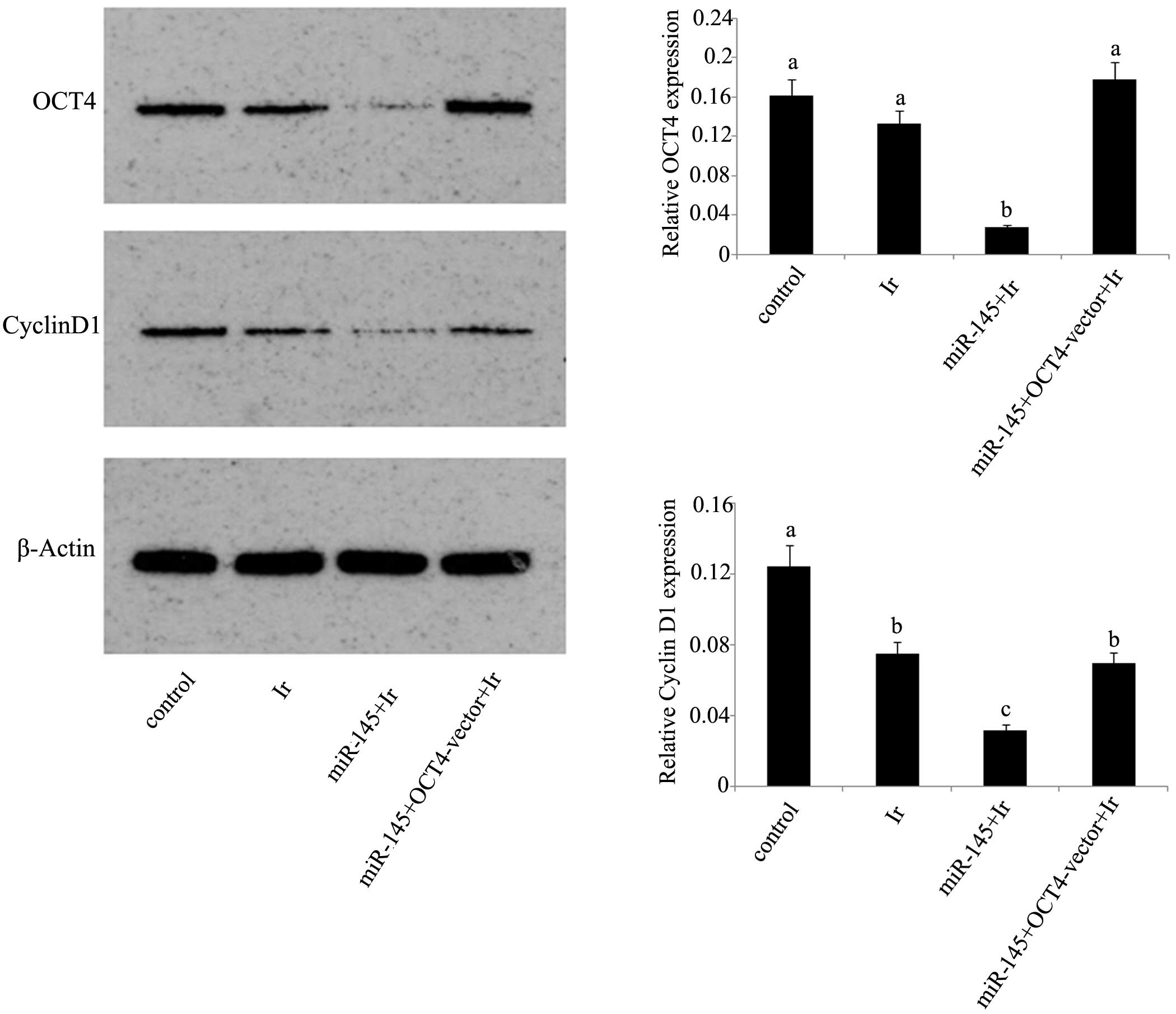
irradiation (Fig. 3B). Exposure to 1
Gy irradiation resulted in the significant reduction of cyclin D1
protein expression (P<0.05), but not of OCT4 protein expression
(Fig. 4). After irradiation, tera
cells that were initially transfected with miR-145 mimics showed
marked inhibition of their protein expression levels of OCT4 and
cyclin D1 compared with those in non-treated tera cells. However,
this inhibition was not observed in tera cells co-transfected with
miR-145 mimics and OCT4 expression vector.
Cell viability and apoptosis rate. Tera cells
transfected with miR-145 mimics exhibited a significant reduction
in post-irradiation cell viability and increase of post-irradiation
apoptosis rate (P<0.05). By contrast, similar reductions were
not observed in tera cells co-transfected with miR-145 mimics and
OCT4 expression vector (Figs. 1B and
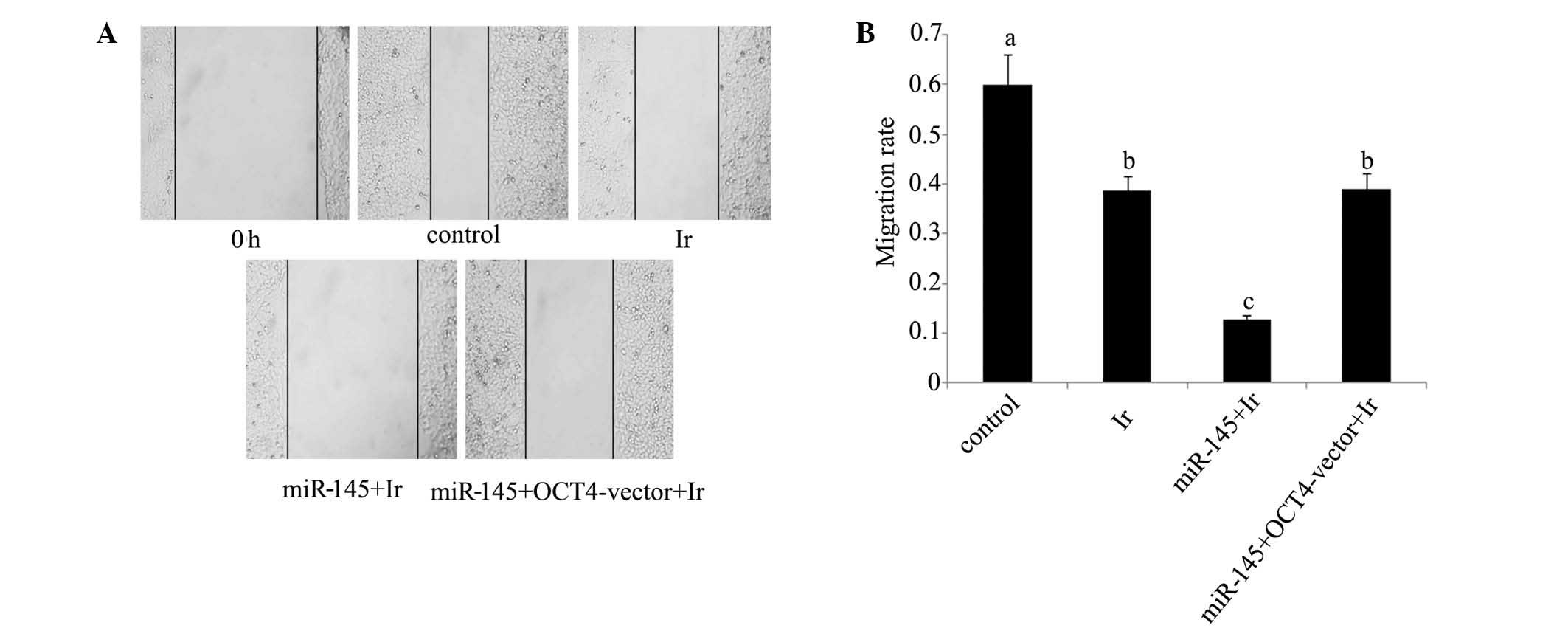
2B). In the would healing assay
(Fig. 5), the cell migration rate
exhibited a significant reduction following cell exposure to 1 Gy
irradiation (P<0.05). Transfection with miR-145 mimics before
irradiation rendered cell migration rate further attenuated
(P<0.05 vs. cell exposure to irradiation only; P<0.01 vs.
control). Co-transfection with miR-145 mimics and OCT4 expression
vector before irradiation restored cell migration rate close to
that of cell exposure to irradiation only (P<0.05 vs.
control).
Discussion
miRNAs are characterized by negatively regulating
the expression levels of numerous key proteins involved in
psychological and pathological processes, and have been associated
with regulating various hallmarks of cancer (9,10).
However, there uncertainty remains regarding the functional effects
of each miRNA in specific cancer types. The present results suggest
that miR-145 promotes the radiosensitivity of cervical cancer tera
cells, as demonstrated by the finding that miR-145 overexpression
via the transfection with miR-145 mimics significantly decreased
post-irradiation cell viability of tera cells and enhanced its
post-irradiation apoptosis rate.
miR-145 is documented to be suppressive to cell
growth of cancer cells (9,10). It has been revealed that miR-145
overexpression correlates with breast cancer MCF-7 cell growth
inhibition (11). The loss of
miR-145 serves as a selective advantage for the growth of colon,
cervical and bladder cancers (12).
However, the function of miR-145 as a cancer growth inhibitor does
not necessarily mean that miR-145 can enhance the radiosensitivity
of cancers, which involves numerous mechanisms responsible for
death-inducing effects after radiation damage (22). It has been demonstrated that exposure
to radiation may result in the generation of substantial oxidative
free radicals which have harmful effects on DNA via deteriorating
its original molecule structures (23). DNA injury is a strongly positive
signal for the initiation of apoptosis, and cells that are less
sensitive to radiation are observed to evade cell death
predominantly by blocking and interfering apoptosis signals and/or
immediately repairing injured DNA (4). The present results showed that tera
cells with elevated miR-145 had much lower cell viability and a
higher apoptosis rate after cell exposure to low-dose irradiation,
indicating that miR-145 enhances the sensitivity of cervical cancer
cells to radiation.
The present study, and prior experiments (20), showed that the endogenous OCT4
protein level was significantly downregulated in tera cells
transfected with miR-145 mimics, suggesting that OCT4 expression is
negatively regulated by miR-145 in tera cells. Further experiments
involving the co-transfection of tera cells with miR-145 mimics and
an OCT4 expression vector, to remove the inhibitory effect of
miR-145 on OCT4 expression, showed that miR-145 significantly
decreased post-irradiation cell viability and that the enhanced
post-irradiation apoptosis rate was abrogated. These data
collectively indicate that miR-145 enhancing radiosensitivity
occurs primarily via silencing of OCT4.
Previous results indicate that OCT4 facilitates cell
proliferation and inhibits apoptosis. It has been reported that
OCT4 promotes the proliferation of esophageal squamous cell
carcinoma by positively regulating the expression of survivin,
which is an important member of the inhibitors of the apoptotic
gene family (24). Furthermore, OCT4
has been shown to influence survival signal pathways, including
those mediated by Tcl1/Akt1, signal transducer and activator of
transcription 3 and tumor protein p53 in various cancer types
(25–27). Previous data suggest that OCT4
directly induces expression of miR-125b, which inhibits its target,
Bcl-2 antagonist/killer 1, leading to the suppression of cervical
cancer cell apoptosis (28). OCT4
harboring anti-apoptosis property may to some extent takes the
responsibility that OCT4 attenuates the radiosensitivity.
Cyclin D1 is also a key mediator that contributes to
reduce cancer cell radiosensitivity via an established mechanism
that facilitates G1-S cell cycle transition to improve cell
self-renew and proliferation after irradiation (29–31). In
the present study, cyclin D1 downregulation was observed in cells
exposed to irradiation and cells transfected with miR-145 mimics
was associated with considerable reduction of cell migration rate
in wound healing assay. Wound healing assays may be used to detect
cellular self-repairing capability following wound. A lower cell
migration rate indicates slower proliferation and weaker
self-repairing capability, which in turn suggests a higher
radiosensitivity (32). Furthermore,
previous studies have reported that upregulated cyclin D1 was
associated a high incidence of cervical lymph node metastasis of
squamous cell carcinoma (33). In
addition, prior experiments suggest that cyclin D1 serves a crucial
function in processes leading to an increase in metastatic
potential, such as migratory and invasive properties, potentially
through increasing matrix metalloproteinase activity and cellular
motility (34,35). It is widely accepted that cancer
metastasis facilitates cancer cells to evade irradiation (36). Thus, the present observation of
downregulated cyclin D1 and lower cell migration rate indicates
potential utility in clinical radiotherapy. However, there is
limited evidence that cyclin D1 is directly regulated by miR-145.
As cyclin D1 exhibited similar variations in protein expression
with OCT4 in the present test, cyclin D1 was hypothesized to be
under the positive regulation of OCT4. Contradictorily, previous
results suggest that cyclin D1 is negatively regulated by OCT4 in
human embryonic stem cells (37).
This difference may be due to the different types of cells used in
each experiment, or it is possible that cyclin D1 is under more
complicated regulation than our hypothesized mechanism.
In summary, a dual luciferase reporter assay
verified that OCT4 is an important target of miR-145 in cervical
cancer tera cells. Transfection with miR-145 mimics repressed OCT4
expression and promoted radiosensitivity of cervical cancer tera
cells. However, co-transfection of miR-145 mimic and OCT4
expression vector removed the inhibition of miR-145 to OCT4 and
abrogated the enhancement of miR-145 to radiosensitivity,
suggesting that the miR-145-associated increase in the
radiosensitivity of cervical cancer cells is a result of OCT4
silencing. In addition, cyclin D1 was inhibited by miR-145, but
co-transfection with miR-145 mimics and OCT4 expression vector that
restored OCT4 expression and led to the recovery of cyclin D1
expression. Thus, it is speculated that cyclin D1 is under the
positive regulation of OCT4. However, this concept is contradictory
to previous research (37). Further
research investigating the mechanism by which cyclin D1 is
regulated by miR-145 and/or OCT4 are required.
Acknowledgements
The authors thank Professor Jun Yuan for his
valuable suggestions and critical reading of the manuscript.
References
|
1
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:25572008. View Article : Google Scholar
|
|
2
|
Mongula J, Slangen B, Lambregts D, Bakers
F, Mahesh S, Lutgens L, Van Gorp T, Vliegen R, Kruitwagen R and
Beets-Tan R: Predictive criteria for MRI-based evaluation of
response both during and after radiotherapy for cervical cancer. J
Contemp Brachytherapy. 8:181–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitahara O, Katagiri T, Tsunoda T, Harima
Y and Nakamura Y: Classification of sensitivity or resistance of
cervical cancers to ionizing radiation according to expression
profiles of 62 genes selected by cDNA microarray analysis.
Neoplasia. 4:295–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu SS, Chan KY, Leung RC, Law HK, Leung
TW and Ngan HY: Enhancement of the radiosensitivity of cervical
cancer cells by overexpressing p73alpha. Mol Cancer Ther.
5:1209–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu R, Wang X, Tian JH, Yang K, Wang J,
Jiang L and Hao XY: High dose rate versus low dose rate intracavity
brachytherapy for locally advanced uterine cervix cancer. Cochrane
Database Syst Rev. 9:CD0075632014.
|
|
6
|
Hareyama M, Sakata K, Oouchi A, Nagakura
H, Shido M, Someya M and Koito K: High-dose-rate versus
low-dose-rate intracavitary therapy for carcinoma of the uterine
cervix: A randomized trial. Cancer. 94:117–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui H, Qin Q, Yang M, Zhang H, Liu Z, Yang
Y, Chen X, Zhu H, Wang D, Meng C, et al: Bortezomib enhances the
radiosensitivity of hypoxic cervical cancer cells by inhibiting
HIF-1α expression. Int J Clin Exp Pathol. 8:9032–9041.
2015.PubMed/NCBI
|
|
8
|
Reshmi G and Pillai MR: Beyond HPV:
Oncomirs as new players in cervical cancer. FEBS Lett.
582:4113–4116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue M, Zhao L, Yang F, Li Z and Li G:
MicroRNA-145 inhibits the malignant phenotypes of gastric carcinoma
cells via downregulation of fascin 1 expression. Mol Med Rep.
13:1033–1039. 2016.PubMed/NCBI
|
|
10
|
Han T, Yi XP, Liu B, Ke MJ and Li YX:
MicroRNA-145 suppresses cell proliferation, invasion and migration
in pancreatic cancer cells by targeting NEDD9. Mol Med Rep.
11:4115–4120. 2015.PubMed/NCBI
|
|
11
|
Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng
L, Zhou H and Zhao RC: miR-145 inhibits breast cancer cell growth
through RTKN. Int J Oncol. 34:1461–1466. 2009.PubMed/NCBI
|
|
12
|
Ostenfeld MS, Bramsen JB, Lamy P,
Villadsen SB, Fristrup N, Sørensen KD, Ulhøi B, Borre M, Kjems J,
Dyrskjøt L and Orntoft TF: miR-145 induces caspase-dependent and
-independent cell death in urothelial cancer cell lines with
targeting of an expression signature present in Ta bladder tumors.
Oncogene. 29:1073–1084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu TT, Liu SY and Zheng PS: Cytoplasmic
NANOG-positive stromal cells promote human cervical cancer
progression. Am J Pathol. 181:652–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaiphei K, Sinha SK and Kochhar R:
Comparative analysis of Oct4 in different histological subtypes of
esophageal squamous cell carcinomas in different clinical
conditions. Asian Pac J Cancer Prev. 15:3519–3524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vargas TH, Pulz LH, Barra CN, Kleeb SR,
Xavier JG, Catão-Dias JL, Fukumasu H, Nishiya AT and Strefezzi RF:
Immunohistochemical expression of the pluripotency factor OCT4 in
canine mast cell tumours. J Comp Pathol. 153:251–255. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai LL, Yu CC, Chang YC, Yu CH and Chou
MY: Markedly increased Oct4 and Nanog expression correlates with
cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol
Med. 40:621–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo WL, Chien Y, Chiou GY, Tseng LM, Hsu
HS, Chang YL, Lu KH, Chien CS, Wang ML, Chen YW, et al: Nuclear
localization signal-enhanced RNA interference of EZH2 and Oct4 in
the eradication of head and neck squamous cell carcinoma-derived
cancer stem cells. Biomaterials. 33:3693–3709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo KK, Lee KT, Chen KK, Yang YH, Lin YC,
Tsai MH, Wuputra K, Lee YL, Ku CC, Miyoshi H, et al: Positive
feedback loop of OCT4 and c-JUN expedites cancer stemness in liver
cancer. Stem Cells. Jun 24–2016.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Lu CS, Shieh GS, Wang CT, Su BH, Su YC,
Chen YC, Su WC, Wu P, Yang WH, Shiau AL and Wu CL:
Chemotherapeutics-induced Oct4 expression contributes to drug
resistance and tumor recurrence in bladder cancer. Oncotarget. May
26–2016.(Epub ahead of print).
|
|
20
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2 and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dote H, Burgan WE, Camphausen K and
Tofilon PJ: Inhibition of hsp90 compromises the DNA damage response
to radiation. Cancer Res. 66:9211–9220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shuryak I and Brenner DJ: A model of
interactions between radiation-induced oxidative stress, protein
and DNA damage in Deinococcus radiodurans. J Theor Biol.
261:305–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Yan Y, Ji W, Bao L, Qian H, Chen L,
Wu M, Chen H, Li Z and Su C: OCT4 positively regulates Survivin
expression to promote cancer cell proliferation and leads to poor
prognosis in esophageal squamous cell carcinoma. PLoS One.
7:e496932012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu T, Liu S, Breiter DR, Wang F, Tang Y
and Sun S: Octamer 4 small interfering RNA results in cancer stem
cell-like cell apoptosis. Cancer Res. 68:6533–6540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X,
Zhou L, Liu C and Chen C: Aself-renewal and survival of embryonal
carcinoma cells. Mol Cell. 48:627–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Zhu Y, Lai Y, Wu X, Feng Z, Yu Y,
Bast RC Jr, Wan X, Xi X and Feng Y: Follicle-stimulating hormone
inhibits apoptosis in ovarian cancer cells by regulating the OCT4
stem cell signaling pathway. Int J Oncol. 43:1194–1204.
2013.PubMed/NCBI
|
|
28
|
Wang YD, Cai N, Wu XL, Cao HZ, Xie LL and
Zheng PS: OCT4 promotes tumorigenesis and inhibits apoptosis of
cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis.
4:e7602013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeselsohn R, Brown NE, Arendt L, Klebba I,
Hu MG, Kuperwasser C and Hinds PW: Cyclin D1 kinase activity is
required for the self-renewal of mammary stem and progenitor cells
that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell.
17:65–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimura T, Noma N, Oikawa T, Ochiai Y,
Kakuda S, Kuwahara Y, Takai Y, Takahashi A and Fukumoto M:
Activation of the AKT/Cyclin D1/Cdk4 survival signaling pathway in
radioresistant cancer stem cells. Oncogenesis. 1:e122012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu Q, Han N, Yuan X, Nie X, Wu H, Chen Y,
Guo M, Yu S and Wu K: DACH1 inhibits Cyclin D1 expression, cellular
proliferation and tumor growth of renal cancer cells. J Hematol
Oncol. 7:732014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin Q, Li X and Cao P: EphA2 modulates
radiosensitive of hepatocellular carcinoma cells via
p38/mitogen-activated protein kinase-mediated signal pathways.
Kaohsiung J Med Sci. 31:510–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suresh TN, Hemalatha A, Kumar ML Harendra
and Mohiyuddin SM Azeem: Evaluation of histomorphological and
immunohistochemical parameters as biomarkers of cervical lymph node
metastasis in squamous cell carcinoma of oral cavity: A
retrospective study. J Oral Maxillofac Pathol. 19:18–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hwang SJ, Lee HW, Kim HR, Song HJ, Lee DH,
Lee H, Shin CH, Joung JG, Kim DH, Joo KM and Kim HH: Overexpression
of microRNA-95-3p suppresses brain metastasis of lung
adenocarcinoma through downregulation of Cyclin D1. Oncotarget.
6:20434–20448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen YJ, Lee LY, Chao YK, Chang JT, Lu YC,
Li HF, Chiu CC, Li YC, Li YL, Chiou JF and Cheng AJ: DSG3
facilitates cancer cell growth and invasion through the
DSG3-plakoglobin-TCF/LEF-Myc/cyclin D1/MMP signaling pathway. PLoS
One. 8:e640882013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Atkinson RL, Zhang M, Diagaradjane P,
Peddibhotla S, Contreras A, Hilsenbeck SG, Woodward WA, Krishnan S,
Chang JC and Rosen JM: Thermal enhancement with optically activated
gold nanoshells sensitizes breast cancer stem cells to radiation
therapy. Sci Transl Med. 2:55ra792010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Card DA, Hebbar PB, Li L, Trotter KW,
Komatsu Y, Mishina Y and Archer TK: Oct4/Sox2-regulated miR-302
targets Cyclin D1 in human embryonic stem cells. Mol Cell Biol.
28:6426–6438. 2008. View Article : Google Scholar : PubMed/NCBI
|