Introduction
Rheumatoid arthritis (RA) is a chronic, systemic
autoimmune disease that is characterized by erosive arthrosynovitis
(1). The incidence of RA in females
is more than that in males, at a ratio of 1:3 (2). The mortality rate of RA in worldwide
populations varies from 0.01–0.05%, and prevalence rate is
0.18–1.07% (3). RA is also an
impactful disease, resulting in labor loss and disability in China
(2). According to US epidemiological
investigation over the last 40 years, the difference in mortality
rate between RA patients and the general population has increased
(4). The risk of cognitive
impairment for RA patients is higher than that of the general
population (5). The etiology of RA
is unclear. Previous studies suggest that RA is associated with
genetic factors, infection, immunity and endocrine function
(6,7).
The main pathological characteristics of RA are
hyperplasia of synovial cells and T cell accumulation during
inflammation of synovial tissues, accompanied by pannus formation,
followed by damage to the cartilage and bone (8). Finally, RA causes joint deformity and
functional loss (9). These
pathological changes may result from a combination of genetic
mutations, activation of protooncogenes, lesions of synoviocytes,
release of proinflammatory cytokines by inflammatory cells during
infiltration of synovial tissues, chemotactic factors and enzymatic
degradation of stromal proteins, amongst other factors (10). The etiology of RA is unclear, but it
is universally believed to be a multifactorial disease, associated
with genetic, environmental and infective factors (11). Autoimmunity may arise due to genetic
factors, or as an aberrant reaction to infection by pathogenic
agents. Numerous previous studies have reported that inflammatory
factors have an important role in the development of RA. Despite
this, the etiology of this disease remains uncertain (6,12,13).
Tripterygium is an ingredient in traditional
Chinese medicine with anti-inflammatory effects that is sourced
from Anhui, Zhejiang, Hunan, Guangxi, Guizhou, Yunnan and Sichuan
provinces. (5R)-5-hydroxytriptolide (LLDT-8) is the extract of
Tripterygium leaves, the main ingredients of which have a
number of anti-inflammatory and immunoregulatory functions
(14). Due to its anti-inflammatory
and immunosuppressive effects, LLDT-8 has an important role in the
treatment of autoimmune diseases and immunorejection reactions
following kidney transplantation (15). The applications of LLDT-8 are
diverse. It has been confirmed by pharmacological and clinical
research that LLDT-8 has anti-inflammatory, antineoplastic and
immunoregulatory functions (16).
Consequently, it is widely employed in the treatment of abnormal
immunity diseases such as RA, nephrotic syndrome, systemic lupus
erythematosus, immunorejection reactions following organ
transplantation, amongst others (14,17). In
the present study, the ability of LLDT-8 to prevent
collagen-induced arthritis (CIA), a model of RA, and the role of
osteoprotegerin (OPG)/receptor activator of nuclear factor κB
(RANK)/RANK ligand (RANKL) signaling in its prevention was
evaluated in a collagen-induced arthritis model.
Materials and methods
Animals and grouping
Male Sprague-Dawley (SD) rats (weight, 260±20 g;
Charles River Laboratories International, Inc., Wilmington, MA,
USA) were used, with access to food and water ad libitum.
Rats were maintained at 23–24°C, a humidity level of 55–60% and
under a 12:12 h light-dark cycle. All experiments were approved by
the Bioethics Committee of Beijing Friendship Hospital, Capital
Medical University, Beijing, China. All SD rats were randomly
divided into five groups, as follows: Control, RA model, RA treated
(0.5, 1 or 2 mg/kg LLDT-8) once daily for 1 week (n=10 rats per
group). These rats were sacrificed by an overdose of anesthetic (1%
pentobarbital) after 1 week of treatment.
Induction and assessment of
arthritis
CIA was used to mimic RA, and has previously been
confirmed as a valid model of this (18). Type II collagen was obtained from the
Tauto Biotech Co., Ltd, (Shanghai, China) and dissolved in 0.1 M
acetic acid. SD rats were injected at the tail base with 2 mg/ml
bovine type II collagen in Freund's complete adjuvant for 1 week.
In all subsequently described experiments, rats were sacrificed and
arthritic joint tissue samples were used. Clinical arthritic scores
were graded and analyzed by scoring each limb as follows: An
absence of symptoms, 0; erythema or swelling of ≥1 digit, 1;
erythema and moderate swelling extending from the ankle to the
mid-foot (tarsals), 2; severe and extensive swelling and erythema
from the ankle to the metatarsal joints, 3; and complete erythema
and swelling encompassing the ankle, foot and digits, resulting in
deformity and/or ankylosis, 4. Arthritic joint tissues were lysed
by homogenization in radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Haimen, China) on ice for 30
min.
Measurement of inflammation
Arthritic tissue samples were obtained from every
group and these were used to assay the levels of interleukin
(IL)-1β, IL-6 and nuclear factor (NF)-κB, determined using
enzyme-linked immunosorbent assay kits (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), performed according to
the manufacturer's instructions.
Western blot analysis
Arthritic tissue samples were gathered from each
group and lysed using 1 nM phenylmethane sulfonyl fluoride
containing sodium dodecyl sulfate (SDS) (Tauto Biotech Co., Ltd) on
ice for 30 min. Protein concentration was determined using a
bicinchoninic acid kit (Beyotime Institute of Biotechnology). A
total of 40 g of protein from each sample was loaded onto 10%
SDS-polyacrylamide gels and subsequently transferred to
polyvinylidene difluoride membranes (Merck Millipore, Darmstadt,
Germany). The membranes were blocked with 5% fat-free dry milk in
Tris-buffered saline for 2 h at room temperature and incubated
overnight at 4°C with shaking, using antibodies as follows:
Anti-induced nitric oxide synthase (iNOS; cat. no. 13120; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-matrix
metalloprotease (MMP)-13 (cat. no. sc-30073; 1:1,000; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) and anti-β-actin (cat. no.
BB-2101-2; 1:5,000; BestBio Science Biotechnology Co., Ltd.,
Shanghai, China), all raised in rabbit. Antibody binding was
analyzed with enhanced chemiluminescence (Beyotime Institute of
Biotechnology), following treatment with goat anti-rabbit
horseradish peroxidase-labelled secondary antibodies (cat. no.
BB-2202-1; 1:5,000; BestBio Science Biotechnology Co., Ltd.) for
1–2 h at 37°C. The protein bands were detected using ImageLab
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Arthritic tissue samples were collected from each
group, as above, and combined in Trizol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. Total RNA was extracted and 1 µg of
total RNA was used to transcribe cDNA using the RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific Inc.). SYBR
Green (Invitrogen; Thermo Fisher Scientific, Inc.), 1 µM of each
primer and 100–200 ng cDNA per group were used to perform qPCR
within a 7900HT PCR cycler (Applied Biosystems; Thermo Fisher
Scientific). The OPG primer sequences used were as follows:
5′-TTGGCTGAGTGTTCTGGT-3′ and 5′-TTGGGAAAGTGGTATGCT-3′; the RANKL
primer sequences were as follows: 5′-CATCGGGTTCCCATAAAG-3′ and
5′-GAAGCAAATGTTGGCGTA-3′; and the β-actin primer sequences were as
follows: 5′-CTATCGGCAATGAGCGGTTCC-3′ and
5′-TGTGTTGGCATAGAGGTCTTTACG-3′. The PCR cycling reaction was
performed as follows: Denaturation at 95°C for 1 min, followed by
40 PCR cycles of denaturation at 94°C for 30 sec, annealing at 58°C
for 45 sec, and extension at 72°C for 30 sec; subsequently, samples
were held at 4°C until use. The relative gene expression was
detected using the ΔΔCq method (19)
and is plotted as the fold change compared with the control
groups.
Statistical analyses
Data are presented as the mean ± standard error of
the mean. A one-way analysis of variance, then Student's t test was
used to determine the difference between two groups. P<0.05 was
considered to represent a statistically significant difference.
Results
LLDT-8 reduces clinical arthritis
scores
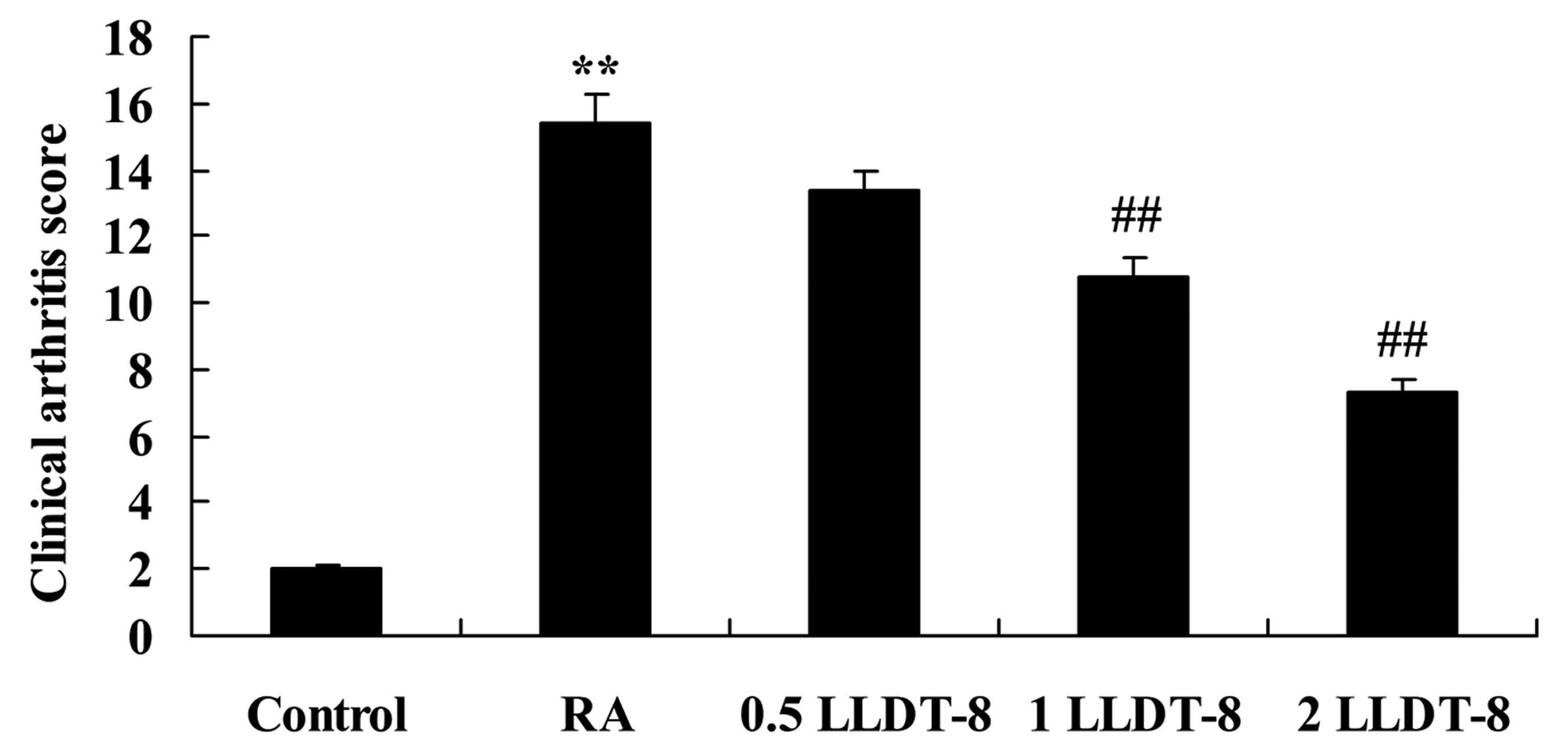
To determine the effects of LLDT-8 in a rat model of
CIA, clinical arthritis scores were reported (Fig. 1). CIA caused clinical arthritis
scores to be significantly higher than those of the control group.
Treatment with >1 mg/kg LLDT-8 significantly reduced the
clinical arthritis scores in rat model of CIA in a dose-dependent
manner (P=0.0092 and 0.0057 in 1 and 2 mg/kg LLDT-8 vs. RA groups,
respectively).
LLDT-8 attenuates inflammatory
responses
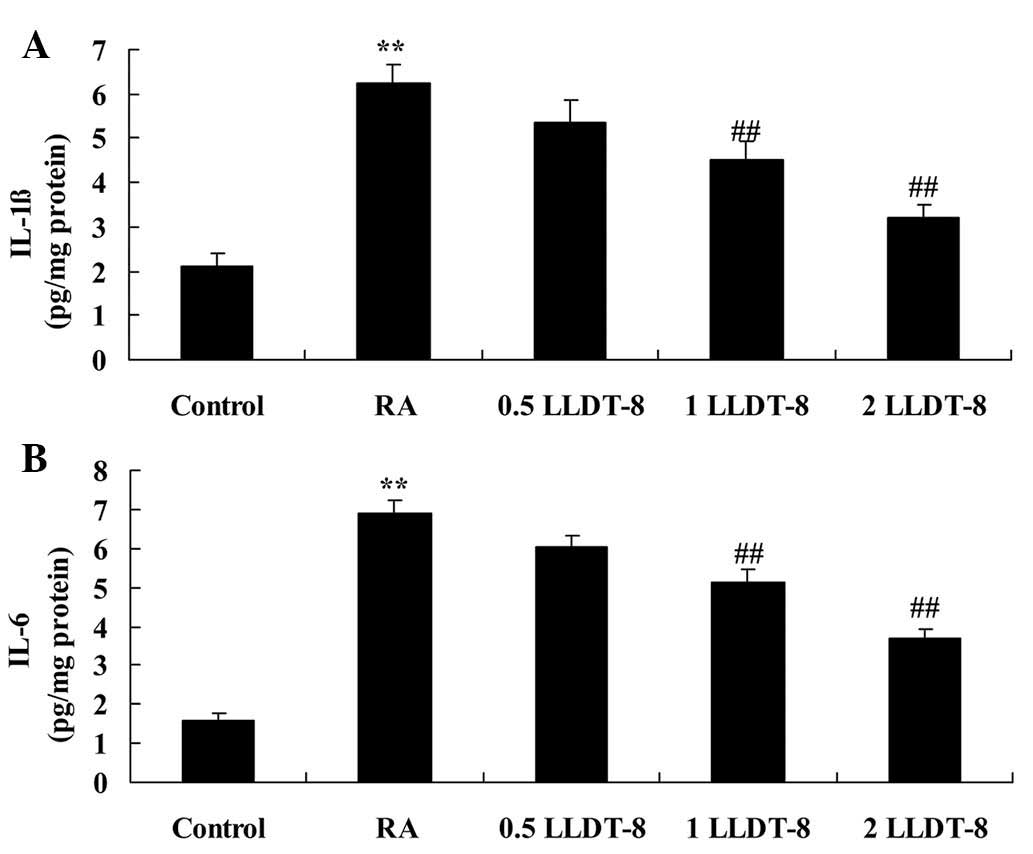
The effects of LLDT-8 on inflammatory responses in
the rat model of CIA were subsequently examined. As reported in
Fig. 2, the levels of IL-1β and IL-6
were significantly increased compared with the control group.
Comparatively, >1 mg/kg LLDT-8 treatment significantly
suppressed the CIA-induced IL-1β (P=0.0088 and 0.0049 in 1 and 2
mg/kg LLDT-8 vs. RA groups, respectively) and IL-6 increases
(P=0.0094 and 0.0061 in 1 and 2 mg/kg LLDT-8 vs. RA groups,
respectively) (Fig. 2).
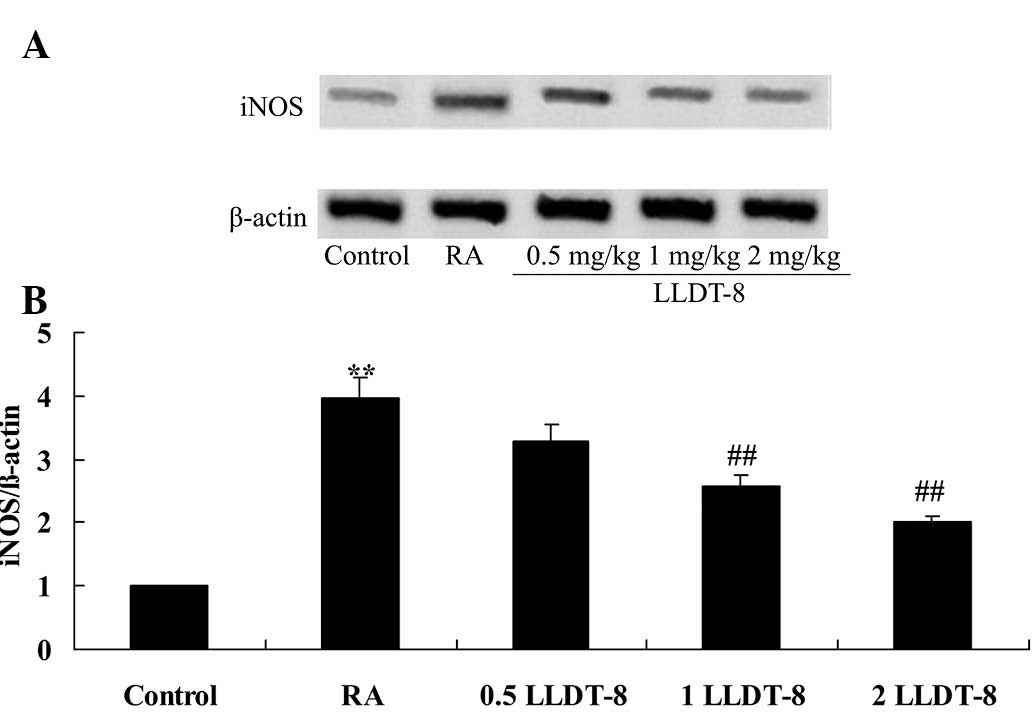
LLDT-8 reduces iNOS protein
expression
The effect of LLDT-8 on the protein expression of
iNOS in the rat model of CIA was detected using western blotting
analysis. Notably, the iNOS protein expression in the CIA rat was
significantly higher than that of the control group (Fig. 3). Furthermore, >1 mg/kg LLDT-8
significantly reduced the iNOS protein expression in arthritic rats
(Fig. 3; P=0.0071 and 0.0035 in 1
and 2 mg/kg LLDT-8 vs. RA groups, respectively).
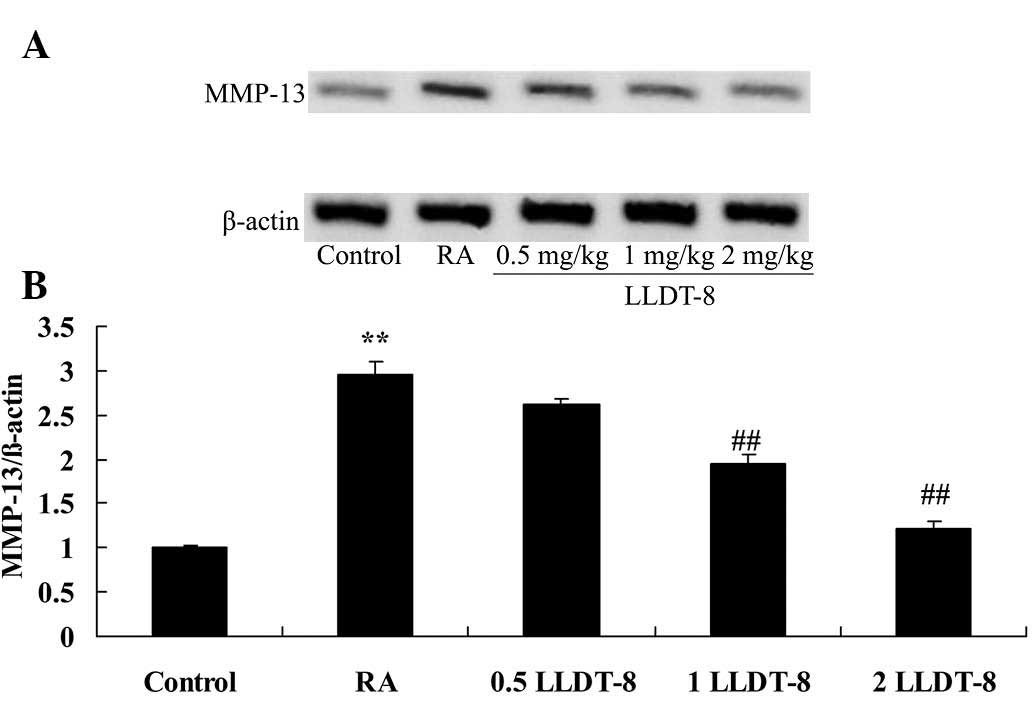
LLDT-8 reduces MMP-13 protein
expression
To examine the effect of LLDT-8 on MMP-13 in the rat
model of CIA, western blotting analysis was used to analyze the
MMP-13 protein expression. Compared with the control group, there
was a significant increase in MMP-13 protein expression in
arthritic rats (Fig. 4). By
contrast, >1 mg/kg LLDT-8 prevented the increase in MMP-13
associated with the CIA rat model (Fig.
4; P=0.0068 and 0.0028 in 1 and 2 mg/kg LLDT-8 vs. RA groups,
respectively).
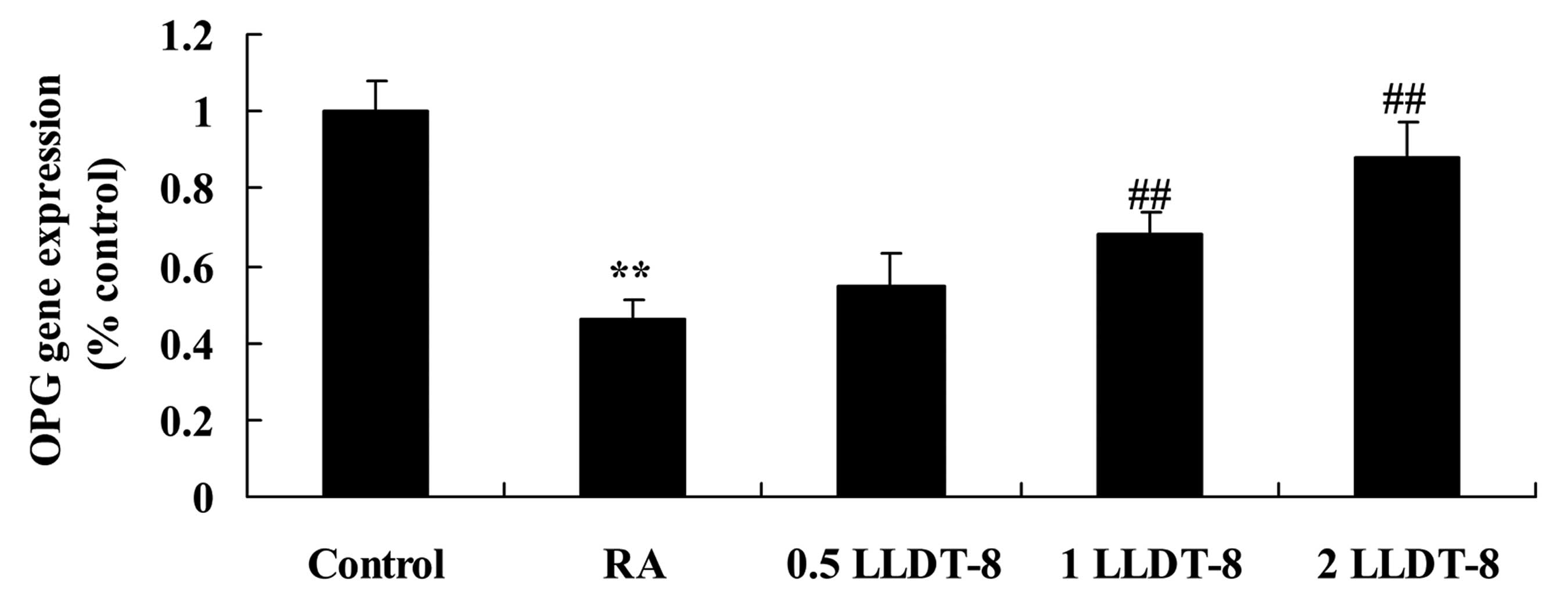
LLDT-8 affects OPG gene
expression
To investigate the effect of LLDT-8 on OPG gene
expression, this was detected using RT-qPCR. In the present study,
the gene expression of OPG in the CIA rat model was significantly
lower than that of the control group (Fig. 5). Treatment with >1 mg/kg LLDT-8
significantly increased OPG gene expression in CIA rat models
(Fig. 5; P=0.0046 and 0.0011 in 1
and 2 mg/kg LLDT-8 vs. RA groups, respectively).
LLDT-8 decreases RANKL gene expression
and increases the ratio of OPG to RANKL in a CIA rat model
In order to investigate the effect of LLDT-8 on
RANKL gene expression and the OPG to RANKL ratio, OPG and RANKL
gene expression was detected using RT-qPCR. A significant increase
in RANKL gene expression was observed in CIA rats compared with the
control group (Fig. 6A). However,
>1 mg/kg LLDT-8 significantly decreased the RANKL gene
expression (P=0.0087 and 0.0045 in 1 and 2 mg/kg LLDT-8 vs. RA
groups, respectively) and increased the ratio of OPG to RANKL
(P=0.0062 and 0.0027 in 1 and 2 mg/kg LLDT-8 vs. RA groups,
respectively) in the rat model of CIA (Fig. 6).
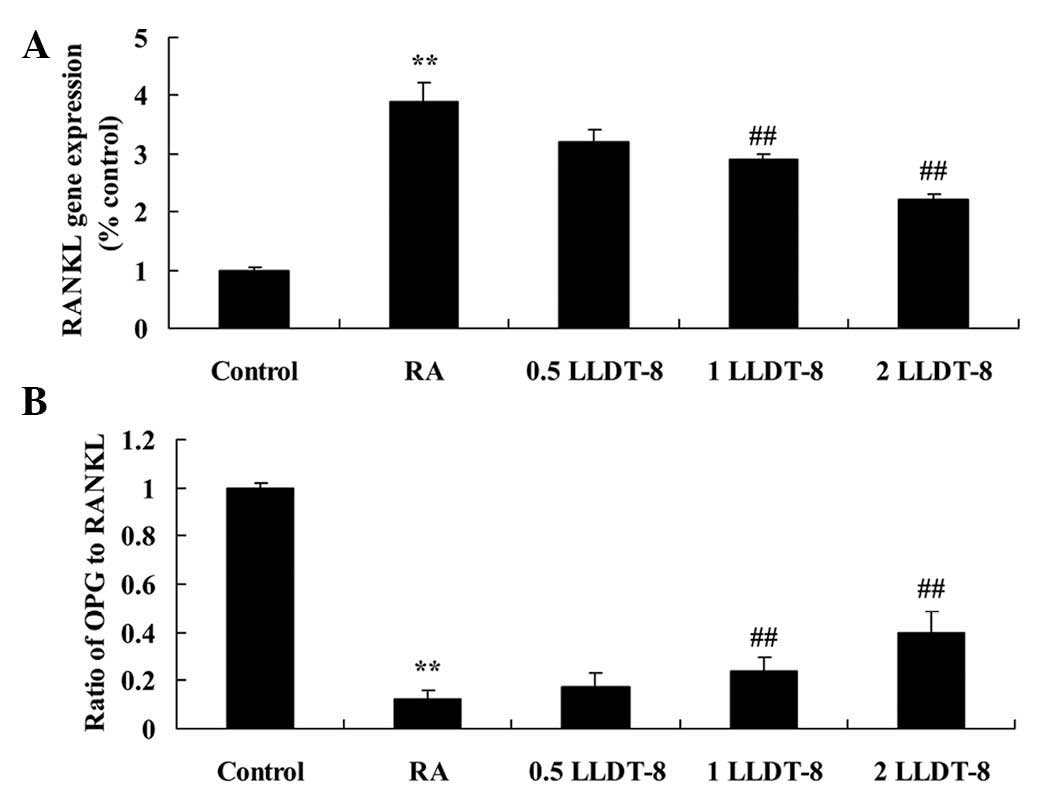 | Figure 6.LLDT-8 (A) decreases the RANKL gene
expression and (B) increases the ratio of OPG to RANKL in a rat
model of CIA. RA, CIA-induced rheumatoid arthritis model; 0.5
LLDT-8, 0.5 mg/kg LLDT-8; 1 LLDT-8, 1 mg/kg LLDT-8; 2 LLDT-8, 2
mg/kg LLDT-8. **P<0.01 vs. control group, ##P<0.01
vs. RA group. RANK, receptor activator of nuclear factor κB; RANKL,
RANK ligand; OPG, osteoprotegerin; LLDT-8,
(5R)-5-hydroxytriptolide; CIA, collagen-induced arthritis. |
LLDT-8 inhibits RANKL-induced NF-κB
expression
To assess the mechanism by which LLDT-8 affects the
inflammatory response via RANKL, NF-κB expression was determined in
arthritic rats. Compared with the control group, NF-κB expression
was significantly increased in the CIA rat model (Fig. 7). However, >1 mg/kg LLDT-8
significantly inhibited this RANKL-induced increased NF-κB
expression in CIA rats (Fig. 7;
P=0.0047 and 0.0015 in 1 and 2 mg/kg LLDT-8 vs. RA groups,
respectively).
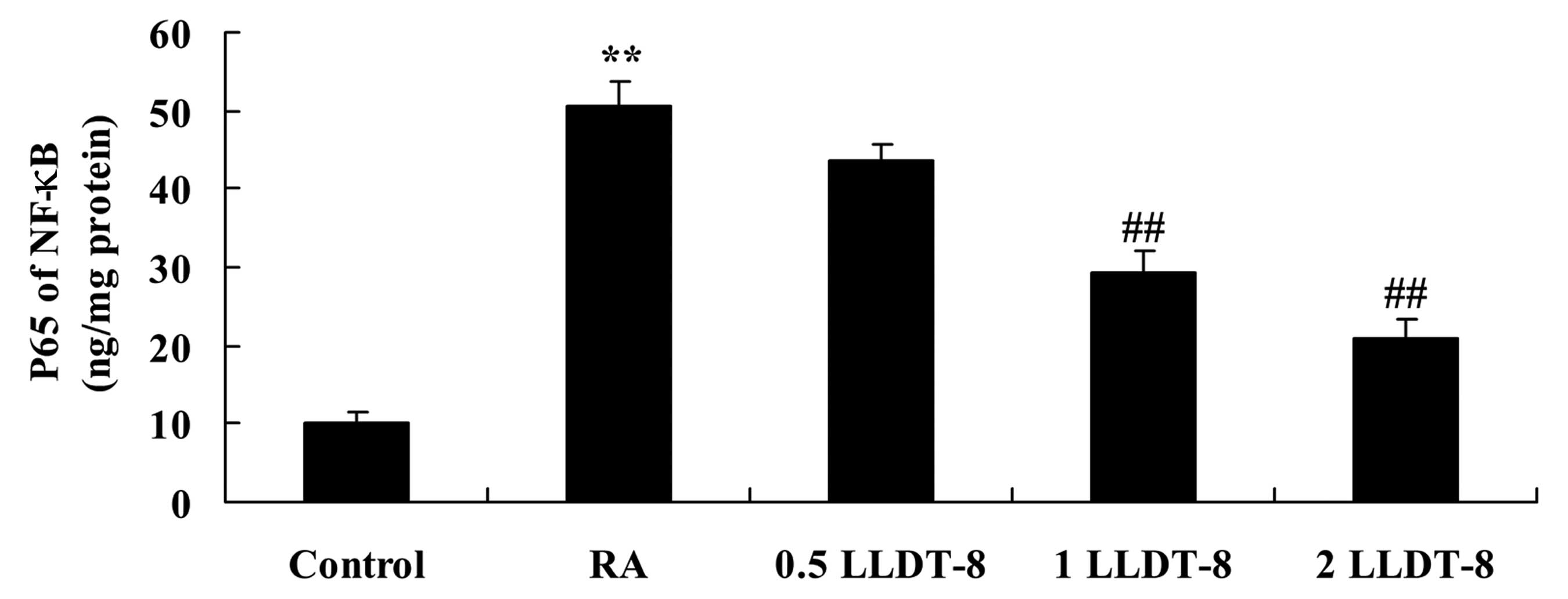 | Figure 7.LLDT-8 inhibits RANKL-induced NF-κB
activation in a rat model of CIA. RA, CIA-induced rheumatoid
arthritis model; 0.5 LLDT-8, 0.5 mg/kg LLDT-8; 1 LLDT-8, 1 mg/kg
LLDT-8; 2 LLDT-8, 2 mg/kg LLDT-8. **P<0.01 vs. control group,
##P<0.01 vs. RA group. NF-κB, nuclear factor κB;
RANK, receptor activator of nuclear factor κB; RANKL, RANK ligand;
LLDT-8, (5R)-5-hydroxytriptolide; CIA, collagen-induced
arthritis. |
Discussion
RA is a chronic autoimmune disease, the major
manifestation of which is bilateral and progressive polyarthritis
(20). The mortality rate in
patients with RA in China is 0.32–0.36% which is slightly lower
than that of the global average (5).
Due to its chronic nature and high disability rates, 75% patients
with RA would become disabled in 3 years without effective and
timely treatment (21). In the
present study, treatment with >1 mg/kg LLDT-8 significantly
reduced the clinical arthritis scores in a rat model of CIA.
Therefore, LLDT-8 may be a potential drug in RA treatment.
RA is an autoimmune disease resulting from
dysfunction of the immune system, but its exact etiology has yet to
be elucidated (22). In order to
investigate the pathogenesis of the disease, it is therefore
necessary to generate animal models (23). Animal models of arthritis and
autoimmune arthritis are available, amongst which CIA is the best
studied (24). The clinical symptoms
and pathological characteristics of this CIA model, particularly
inflammation of the joints, are similar or identical to RA
(25). As a result, CIA is an ideal
and commonly used animal model (26). In the current study, LLDT-8 treatment
significantly suppressed the CIA-induced increase in IL-1β and IL-6
expression in the arthritic rat. These observations suggested that
LLDT-8 possesses anti-inflammatory properties (14).
Increasingly, studies have reported upon the
pathogenesis of osteoarthritis (OA), and on the roles of
inflammatory cytokines and biomarkers in the occurrence and
development of these diseases (27).
Nitric oxide (NO) is a lipid-soluble inorganic molecule that
diffuses rapidly through the plasma membrane (28). NO is a notable pathogenic molecule in
the development of OA, with a half-life of 3–5 sec, and it
indirectly and directly affects cartilage metabolism. Nitric oxide
synthase (NOS) is a key enzyme in the generation of NO (29). During the development of OA,
cartilage cells are impaired, caused by a release of inflammatory
factors, which leads to the generation of NO (30). Released NO inhibits cartilage cell
proliferation and induces apoptosis (31). This would interfere with cell
signaling, accelerate the degradation of the cartilage matrix,
specifically the proteoglycan (32).
Finally, this would aggravate cartilage injury (33). The present results demonstrated that
LLDT-8 significantly reduced the CIA-induced iNOS protein
expression in the arthritic rat. Zhou et al (15) previously reported that LLDT-8
inhibited iNOS in interferon-gamma- and bacterial
lipopolysaccharide-stimulated macrophages.
Previous studies have revealed that expression of
MMP-1 and MMP-13 in cartilage and synovium were significantly
higher than those of control group (34). With increasing time, expression of
MMP-1 increased (34). However, the
expression of MMP-13 was markedly decreased in the current study.
MMP-13 had an important role in the early and intermediate stages
of OA development, but MMP-1 had a continuous role in its
pathogenesis (35).
Previous studies indicated that RANKL, RANK and OPG
are key regulatory factors in the generation, growth, activation
and maturation of osteoclasts (36).
RANKL belongs to the tumor necrosis factor superfamily, acting as a
ligand for the receptors RANK and OPG (37). RANK is located on the plasma membrane
of osteoclast precursor cells, and the binding of RANKL to RANK
promotes the differentiation and maturity of osteoclasts (38). The binding capacity of OPG to RANKL
is higher than that of RANK to RANKL, which competitively binds
RANKL, thereby competitively inhibiting its binding to RANK
(39). Consequently, OPG may inhibit
the differentiation of osteoclasts (39). The present results suggested that
LLDT-8 increases OPG gene expression, decreases RANKL gene
expression, increases the ratio of OPG to RANKL and inhibits
RANKL-induced NF-κB expression in the current rat model of CIA.
Shen et al (36) previously
reported that LLDT-8 inhibits osteoclastogenesis through
RANKL/RANK/OPG signaling. The ratio of OPG/RANKL was significantly
increased and was observed alongside suppression of the
inflammatory response in the current study, which indicated that
the effect of LLDT-8 on RA may be associated with the OPG/RANKL
pathway. In conclusion, the present results indicated that LLDT-8
had an anti-arthritic effect by suppressing inflammation, and the
iNOS and OPG/RANKL pathways. However, the specific mechanisms by
which LLDT-8 affects RA remain to be elucidated.
References
|
1
|
Strand V, Kosinski M, Gnanasakthy A,
Mallya U and Mpofu S: Secukinumab treatment in rheumatoid arthritis
is associated with incremental benefit in the clinical outcomes and
HRQoL improvements that exceed minimally important thresholds.
Health Qual Life Outcomes. 12:312014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao J, Yue T, Liu W, Zhang Q, Zhou L, Xu
HJ and Dai SM: Secondary failure to treatment with recombinant
human IL-1 receptor antagonist in Chinese patients with rheumatoid
arthritis. Clin Rheumatol. 30:697–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang S, Lukey P, Beerahee M and Hoke F:
Population pharmacokinetics of losmapimod in healthy subjects and
patients with rheumatoid arthritis and chronic obstructive
pulmonary diseases. Clin Pharmacokinet. 52:187–198. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Qi H, Jiang D, Wang R, Chen A, Yan
Z and Xiao J: The new use of an ancient remedy: A double-blinded
randomized study on the treatment of rheumatoid arthritis. Am J
Chin Med. 41:263–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XX, Dai Q, Huang AB, Wu HX, Zhao DB,
Li XF, Hu SX, Yang NP, Tao Y, Xu JH, et al: A multicenter,
randomized, double-blind clinical trial of combination therapy with
Anbainuo, a novel recombinant human TNFRII: Fc fusion protein, plus
methotrexate versus methotrexate alone or Anbainuo alone in Chinese
patients with moderate to severe rheumatoid arthritis. Clin
Rheumatol. 32:99–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yadlapati S and Efthimiou P:
Autoimmune/inflammatory arthritis associated lymphomas: Who is at
risk? Biomed Res Int. 863–1061. 2016.
|
|
7
|
Eneljung T, Tengvall S, Jirholt P,
Henningsson L, Holmdahl R, Gustafsson K and Gjertsson I:
Antigen-specific gene therapy after immunisation reduces the
severity of collagen-induced arthritis. Clin Dev Immunol.
3450922013.PubMed/NCBI
|
|
8
|
Li J, Li J, Chen R and Cai G: Targeting
NF-kB and TNF-α activation by electroacupuncture to suppress
collagen-induced rheumatoid arthritis in model rats. Altern Ther
Health Med. 21:26–34. 2015.PubMed/NCBI
|
|
9
|
Liu F, Cheng W, Pappoe F, Hu X, Wen H, Luo
Q, Wang S, Deng F, Xie Y, Xu Y and Shen J: Schistosoma japonicum
cystatin attenuates murine collagen-induced arthritis. Parasitol
Res. 2016. View Article : Google Scholar
|
|
10
|
Li N, Wang JC, Liang TH, Zhu MH, Wang JY,
Fu XL, Zhou JR, Zheng SG, Chan P and Han J: Pathologic finding of
increased expression of interleukin-17 in the synovial tissue of
rheumatoid arthritis patients. Int J Clin Exp Pathol. 6:1375–1379.
2013.PubMed/NCBI
|
|
11
|
Fleischmann R, Kremer J, Tanaka Y, Gruben
D, Kanik K, Koncz T, Krishnaswami S, Wallenstein G, Wilkinson B,
Zwillich SH and Keystone E: Efficacy and safety of tofacitinib in
patients with active rheumatoid arthritis: Review of key Phase 2
studies. Int J Rheum Dis. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farzaei MH, Farzaei F, Abdollahi M,
Abbasabadi Z, Abdolghaffari AH and Mehraban B: A mechanistic review
on medicinal plants used for rheumatoid arthritis in traditional
Persian medicine. J Pharm Pharmacol. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kochi Y: Genetic background of tolerance
breakdown in rheumatoid arthritis. Nihon Rinsho Meneki Gakkai
Kaishi. 33:48–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang W and Zuo JP: Immunosuppressant
discovery from Tripterygium wilfordii Hook f: The novel triptolide
analog (5R)-5-hydroxytriptolide (LLDT-8). Acta Pharmacol Sin.
33:1112–1118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou R, Zheng SX, Tang W, He PL, Li XY,
Yang YF, Li YC, Geng JG and Zuo JP: Inhibition of inducible
nitric-oxide synthase expression by (5R)-5-hydroxytriptolide in
interferon-gamma- and bacterial lipopolysaccharide-stimulated
macrophages. J Pharmacol Exp Ther. 316:121–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren YX, Zhou R, Tang W, Wang WH, Li YC,
Yang YF and Zuo JP: (5R)-5-hydroxytriptolide (LLDT-8) protects
against bleomycin-induced lung fibrosis in mice. Acta Pharmacol
Sin. 28:518–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenthal KS, Mikecz K, Steiner HL, Glant
TT, Finnegan A, Carambula RE and Zimmerman DH: Rheumatoid arthritis
vaccine therapies: Perspectives and lessons from therapeutic ligand
epitope antigen presentation system vaccines for models of
rheumatoid arthritis. Expert Rev Vaccines. 14:891–908. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Xian PF, Yang L and Wang SX:
MicroRNA-21 promotes proliferation of fibroblast-like synoviocytes
through mediation of NF-kappaB nuclear translocation in a rat model
of collagen-induced rheumatoid arthritis. Biomed Res Int.
92790782016.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanbe K, Oh K, Chiba J, Inoue Y, Taguchi M
and Yabuki A: Analysis of mitogen-activated protein kinases in bone
and cartilage of patients with rheumatoid arthritis treated with
abatacept. Clin Med Insights Arthritis Musculoskelet Disord.
9:51–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Chen W, Wang L, Li F, Zhang C and
Xu L: Tumor necrosis factor receptor-associated factor 6 promotes
migration of rheumatoid arthritis fibroblast-like synoviocytes. Mol
Med Rep. 11:2761–2766. 2015.PubMed/NCBI
|
|
22
|
Cordova KN, Willis VC, Haskins K and
Holers VM: A citrullinated fibrinogen-specific T cell line enhances
autoimmune arthritis in a mouse model of rheumatoid arthritis. J
Immunol. 190:1457–1465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park KS, Park MJ, Cho ML, Kwok SK, Ju JH,
Ko HJ, Park SH and Kim HY: Type II collagen oral tolerance;
mechanism and role in collagen-induced arthritis and rheumatoid
arthritis. Mod Rheumatol. 19:581–589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kakimoto K, Kojima Y, Ishii K, Onoue K and
Maeda H: The suppressive effect of gelatin-conjugated superoxide
dismutase on disease development and severity of collagen-induced
arthritis in mice. Clin Exp Immunol. 94:241–246. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Wang Y, Huang C, Xu J, Li Z, Xu L,
He L, Sun Y, Wang Y, Xu S, Zhao P, et al: Efficacy and safety of
Xinfeng capsule in patients with rheumatoid arthritis: A
multi-center parallel-group double-blind randomized controlled
trial. J Tradit Chin Med. 35:487–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi JH, Lee JH, Roh KH, Seo SK, Choi IW,
Park SG, Lim JG, Lee WJ, Kim MH, Cho KR and Kim YJ: Gallium nitrate
ameliorates type II collagen-induced arthritis in mice. Int
Immunopharmacol. 20:269–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varadé J, Lamas JR, Fernández-Arquero M,
Jover JA, de la Concha EG, Martínez A, Fernández-Gutierrez B and
Urcelay E: NO role of NOS2A susceptibility polymorphisms in
rheumatoid arthritis. Nitric Oxide. 21:171–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura T, Mogi C, Tomura H, Kuwabara A, Im
DS, Sato K, Kurose H, Murakami M and Okajima F: Induction of
scavenger receptor class B type I is critical for simvastatin
enhancement of high-density lipoprotein-induced anti-inflammatory
actions in endothelial cells. J Immunol. 181:7332–7340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abramson SB, Amin AR, Clancy RM and Attur
M: The role of nitric oxide in tissue destruction. Best Pract Res
Clin Rheumatol. 15:831–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang Q, Ju Y, Chen Y, Wang W, Li J, Zhang
L, Xu H, Wood RW, Schwarz EM, Boyce BF, Wang Y and Xing L:
Lymphatic endothelial cells efferent to inflamed joints produce
iNOS and inhibit lymphatic vessel contraction and drainage in
TNF-induced arthritis in mice. Arthritis Res Ther. 18:622016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salerno L, Sorrenti V, Di Giacomo C, Romeo
G and Siracusa MA: Progress in the development of selective nitric
oxide synthase (NOS) inhibitors. Curr Pharm Des. 8:177–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Markovics A, Ocskó T, Katz RS, Buzás EI,
Glant TT and Mikecz K: Immune recognition of citrullinated
proteoglycan aggrecan epitopes in mice with proteoglycan-induced
arthritis and in patients with rheumatoid arthritis. PLoS One.
11:e01602842016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarban S, Isikan UE, Kocabey Y and
Kocyigit A: Relationship between synovial fluid and plasma
manganese, arginase, and nitric oxide in patients with rheumatoid
arthritis. Biol Trace Elem Res. 115:97–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee YA, Choi HM, Lee SH, Hong SJ, Yang HI,
Yoo MC and Kim KS: Hypoxia differentially affects IL-1β-stimulated
MMP-1 and MMP-13 expression of fibroblast-like synoviocytes in an
HIF-1α-dependent manner. Rheumatology (Oxford). 51:443–450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee YA, Ji HI, Lee SH, Hong SJ, Yang HI,
Yoo M Chul and Kim KS: The role of adiponectin in the production of
IL-6, IL-8, VEGF and MMPs in human endothelial cells and
osteoblasts: Implications for arthritic joints. Exp Mol Med.
46:e722014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen Y, Jiang T, Wang R, He S, Guo M, Zuo
J and He D: (5R)-5-Hydroxytriptolide (LLDT-8) inhibits
osteoclastogenesis via RANKL/RANK/OPG signaling pathway. BMC
Complement Altern Med. 15:772015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Remuzgo-Martínez S, Genre F, López-Mejías
R, Ubilla B, Mijares V, Pina T, Corrales A, Blanco R, Martín J,
Llorca J and González-Gay MA: Expression of osteoprotegerin and its
ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep.
6:297132016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ho TY, Santora K, Chen JC, Frankshun AL
and Bagnell CA: Effects of relaxin and estrogens on bone remodeling
markers, receptor activator of NF-kB ligand (RANKL) and
osteoprotegerin (OPG), in rat adjuvant-induced arthritis. Bone.
48:1346–1353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng X, Lv C, Wang F, Gan K, Zhang M and
Tan W: Modulatory effect of 1,25-dihydroxyvitamin D 3 on IL1
β-induced RANKL, OPG, TNF α, and IL-6 expression in human
rheumatoid synoviocyte MH7A. Clin Dev Immunol. 2013:1601232013.
View Article : Google Scholar : PubMed/NCBI
|