Introduction
Aortico-left ventricular tunnel (ALVT) is a rare
congenital cardiac malformation, which accounts for only 0.12% of
the incidence of congenital heart disease (1). ALVT refers to the abnormal channel
between the ascending aorta and the left ventricular and located at
the side of ascending aorta valve. However, due to the unclear
display of inlet and inside structure of the channel, missed
diagnoses and misdiagnosis happened frequently in clinical
treatment. The disease was first reported by Edwards in 1961
(1) and was termed ALVT by Levy
et al in 1963 (2).
Subsequently, few ALVT cases have been reported worldwide (3–7).
However, it has been found that transthoracic
echocardiography (TTE) is a more effective way to display the inlet
and inside structure of the channel clearly and correctly which
plays an important role in diagnosing the ALVT (6,7). In the
present study, echocardiogram data of 6 ALVT patients in Xiehe
Hospital Affiliated to Tongji Medical College (Hubei, China) have
been analyzed to investigate the value of TTE on diagnosing
ALVT.
Materials and methods
Subjects
Six patients with ALVT (4 males and 2 females, aged
3–41 years, with a median age of 26 years) were treated at the
Xiehe Hospital Affiliated to Tongji Medical College hospital from
June, 2007 to January, 2012. Clinical manifestations include heart
murmur, chest tightness during activities, heart palpitations and
diastolic murmur. The six patients underwent echocardiographic
examination. One patient underwent a transesophageal
echocardiography and another patient underwent a multi-slice CT.
The surgery confirmed all manifestations.
The study was approved by the ethics committee of
Tongji Medical College. Signed written informed consent was
obtained from all the participants prior to the study.
Instruments and methods
The Philips iE33, GE Vivid 7 (GE Healthcare, Little
Chalfont, Buckinghamshire, UK) and GE E9 color ultrasonic
diagnostic apparatus (GE Healthcare) with the probe frequency of
3.5–7.5 MHz were used to conduct the study. The section was scanned
with a two-dimensional ultrasound and color Doppler flow imaging
(CDFI) and the parasternal left ventricle was observed in the
long-axis view, the cardiac-base short-axis view of the aorta, the
five-chamber view of the heart and the apical long axis view of the
left ventricle, and the origin of the regurgitation beam was
investigated.
Results
According to Hovaguimian et al, type casting,
of the six confirmed cases, two were type I cases, two type III
cases, one type IV cases, and one type II case (tunnel
recanalization after ALVT operation) (3). The ultrasound results are shown in
Table I.
 | Table I.Echocardiographic features of 6 cases
of ALVT. |
Table I.
Echocardiographic features of 6 cases
of ALVT.
| No. | Gender | Age (years) | Symptoms | AOP | AE | VSE | RVOTO | LVEF (%) | CL |
|---|
| 1 | Male | 21 | Chest distress | ARCS | − | − | − | 61 | AR, MR, VSD |
| 2 | Male | 40 | No | AJRLCS | − | − | − | 65 | BAV, AS |
| 3 | Female | 31 | Chest distress | ARCS | + | − | − | 40 | − |
| 4 | Male | 3 | No | ARCS | − | + | − | 62 | − |
| 5 | Male | 41 | Chest distress | ARCS | − | + | − | 61 | − |
| 6 | Female | 12 | No | AJRLCS | + | + | − | 57 | AR, AVP |
According to the ALVT form, the ultrasound results
of the six patients were divided into four types. Type I (two
cases): i) from the two-dimensional echocardiography, we saw a
tunnel structure in the aortic sinus (one case above the right
coronary sinus, the other case above the junction of the left and
right coronary sinus) of the parasternal left ventricle in the
long-axis view, the cardiac-base short-axis view of the aorta, the
five-chamber view of the heart and the apical long axis view of the
left ventricle. It is connected to the aortic lumen through a
narrow break (a case with the inner diameter of 0.65 cm, the other
with inner diameter of 0.8 cm). The left ventricle tunnel flows
through a break under the aortic valve ring down into the left
ventricular outflow tract; ii) the CDFI and continuous Doppler
ultrasound showed that a fine beam of regurgitation signal at a
high speed could be seen during the period of tunnel diastole; iii)
the aortic valve of one patient moved like a bicuspid with slight
limitation of the normal opening and closing. The aortic valve of
another case was normal in form and activities; iv) the ascending
aorta was significantly widened or expanded and the left ventricle
became bigger; and v) left and right coronary arteries had normal
origins.
Type III (2 cases): i) from the two-dimensional
echocardiography, we can see a small gap between the aortic root
and the annulus in the cardiac-base short-axis view of the aorta
and the five-chamber view of the heart. The gap was connected to a
break between the aortic lumens on the aortic valve ring and
entered through the lower aortic valve ring into the left
ventricular outflow tract. According to the five-chamber view of
the heart, the ventricular septum of the tunnel protruded like a
tumor into the right ventricle. No continuous breaks were seen on
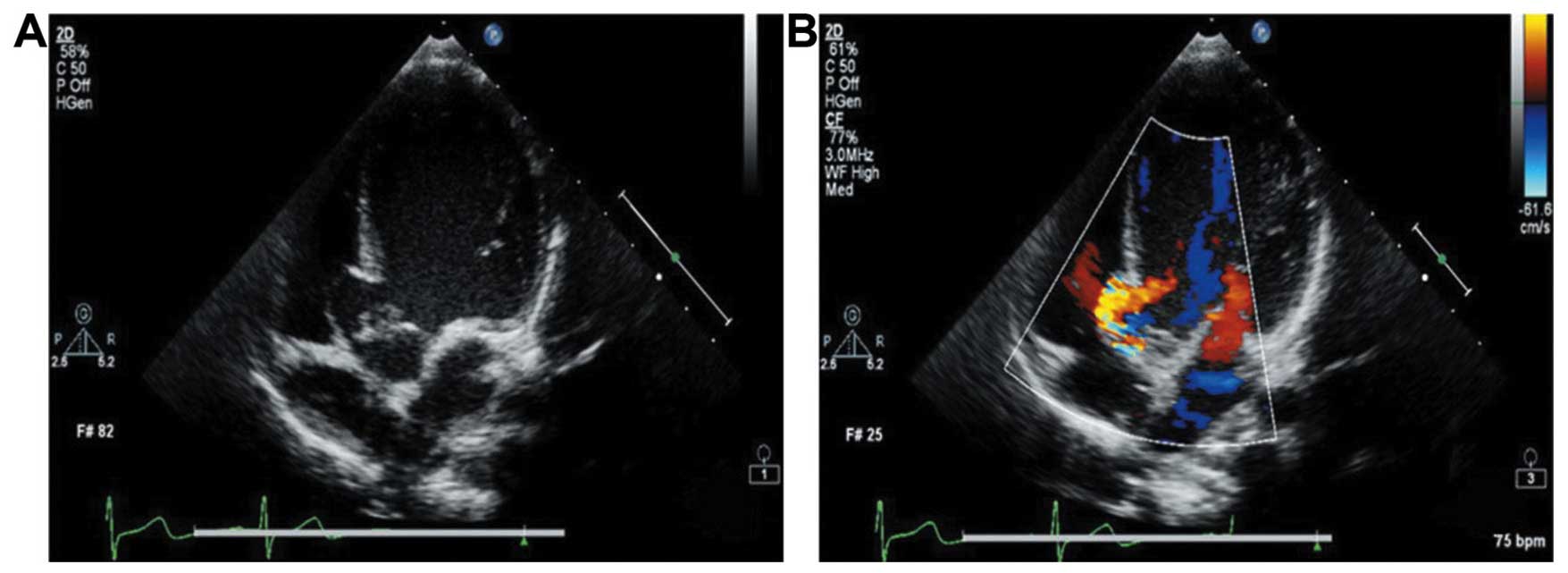
the tumor wall (Fig. 1A); ii) the
CDFI and continuous Doppler ultrasound showed a high-speed
regurgitation signal entered through the tunnel into the left
ventricular outflow tract (Fig. 1B)
along the septal aneurysm tunnel wall; iii) the aortic valve of two
cases was normal; iv) the blood in the right ventricular outflow
tract flowed smoothly; v) the ascending aorta had widened or
expanded and the left ventricle became bigger; and vi) left and
right coronary arteries had healthy origins.
Type IV (1 case): i) the dimensional and
transesophageal echocardiography showed that in the cardiac-base
short-axis view of the aorta and the five-chamber view of the
heart, an abnormal tunnel between the aorta and the left
ventricular outflow tract was evident. The aortic end was at the
ventricle-artery connection of the left and right coronary sinus
junction. It looked similar to a tumor protruding outside the heart
and was connected to the break with the inner diameter of 0.8 cm in
the aortic lumen. The left ventricular end of the tunnel was in the
left ventricular outflow tract under the aortic valve. The
ventricular septum of the tunnel protruded to the right ventricle
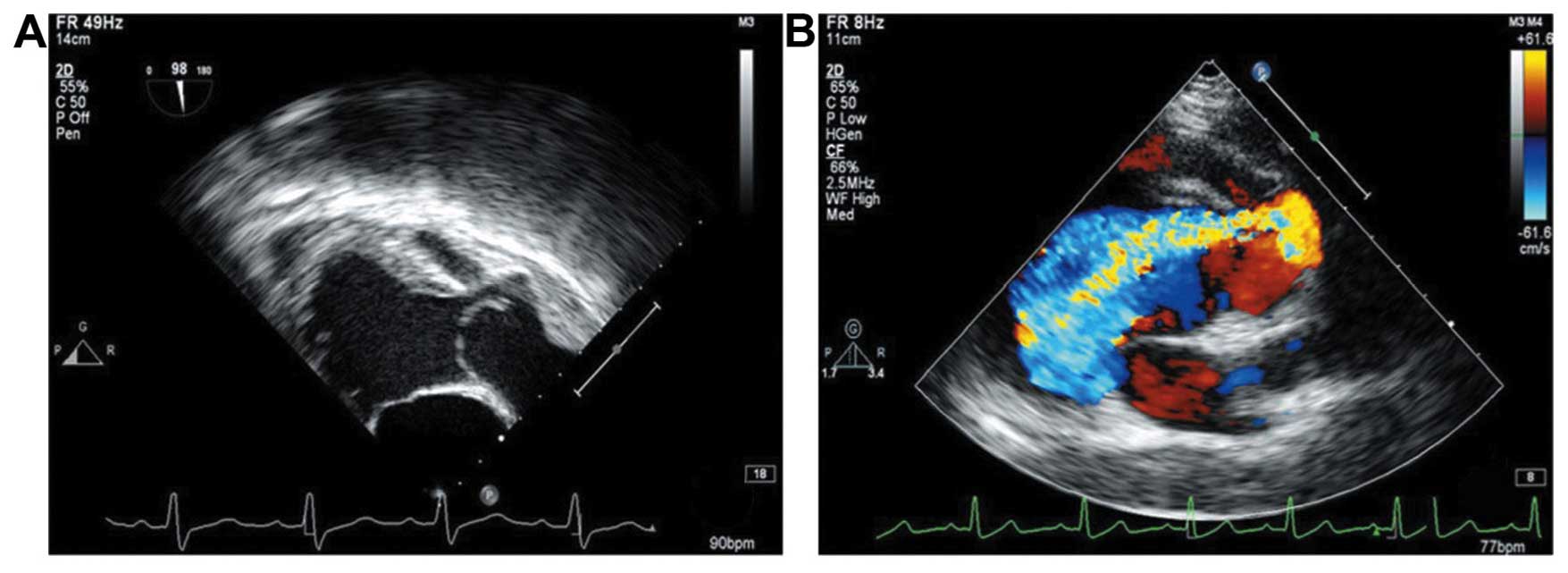
resembling a tumor (Fig. 2A); ii)
the CDFI and continuous Doppler showed that many high-speed
regurgitation signals entered into the left ventricular outflow
tract alongside the aortic aneurysm by the lateral wall aortic
tumor of the tunnel through the aortic valve and along the septal
aneurysm wall of the tunnel (Fig.
2B); iii) the right coronary aortic valve protruded to the left
ventricular outflow tract during the diastole period. It opened
normally but closed incorrectly; iv) the blood circulation was
smooth in the right ventricular outflow tract; v) the ascending
aorta was greatly widened and the left ventricle increased in size;
and vi) left and right coronary arteries had normal origins.
Type II (1 case): this case was misdiagnosed as
aortic regurgitation before surgery and later underwent an
echocardiography exam because of heart failure and was diagnosed
with ALVT. With surgery to repair the ALVT and replace the aortic
valve, the tunnel achieved recanalization one year later. The
ultrasonographs of the tunnel recanalization revealed the
following: i) from the two-dimensional echocardiography, we could
see that the aorta was connected to the left ventricle outflow
tract by the tunnel in the parasternal left ventricle in the
long-axis view, the cardiac-base short-axis view of the aorta, the
five-chamber view of the heart and the apical long axis view of the
left ventricle. The aortic side of the tunnel had a large opening
and the aortic wall protruded outside the heart like tumors. The
left ventricular end of the tunnel was connected to the left
ventricular outflow tract under the aortic valve ring through a
small gap; ii) the CDFI and continuous Doppler ultrasound showed
that an appropriate amount of high-speed diastolic regurgitation
signals entered into the left ventricular outflow tract through the
tunnel along the aortic tumor wall; iii) the strong echo of the
artificial metal aortic valve at the position of the aortic valve
could be heard. Its form and activities were normal; iv) the
ascending aorta was widened and the left ventricle became larger.
The left ventricular blood ejection fraction measured by M-mode
echocardiography was at 40%; and v) the origins of the left and
right coronary arteries were normal.
The six patients were diagnosed correctly and
confirmed by surgery. The postoperative echocardiography showed
that, except for one case of recanalization, the re-examination one
month after the surgery witnessed no or only a small amount of
residual regurgitation signals. The left ventricular blood ejection
fraction remained normal.
Discussion
Cause of ALVT
The cause of ALVT is uncertain. Cooley et al
(4) argued that it may be similar to
the increased deposits of acid mucopolysaccharides in the arteries
of patients affected with Marfan syndrome. However, Terada et
al believed that the tunnel formation was the result of the
abnormal development of the right coronary artery based on three
fetal autopsies (5). Histological
sections showed that the aortic end of the tunnel was the arterial
structure and linked closely or even fused with the origin of the
right coronary artery. Levy et al also suspected that ALVT
belonged to abnormalities of the coronary artery (2). It was reported that as the coronary
artery originated from ALVT, that ALVT was a special type of
coronary artery fistula (6–8).
Clinical features of ALVT
According to the clinical data of 153 cases of ALVT
reported in relevant literature (9–14), ALVT
has the following characteristics: i) there are more male than
female patients (82/23), 1 day to 71 years of age; ii) the tunnel
is mostly single; iii) greatly closing aortic valves lead to
regurgitation while frequent closing leads to prolapse; iv) cardiac
malformations are often manifested in bicuspid valves and sometimes
in anomalous origins of the coronary artery (15–18) or a
single coronary artery; v) patients with no other cardiac
malformations and complications visit the doctor when they discover
a diastolic murmur during a medical examination. Patients with
small tunnels have no clinical manifestations at the early stage
but show palpitation, shortness of breath, and other symptoms of
cardiac insufficiency after activities due to an overload of the
left ventricular volume.
Comparison of the advantages and
disadvantages of angiography and echocardiography in the diagnosis
of ALVT
Before echocardiography is used, selective
angiography of the ascending aorta or aortic openings of the tunnel
is the golden standard for diagnosing this disease (19–21).
Successful angiography of the ascending aorta can diagnose the
disease, but the technology can only be dependent on indirect signs
from the contrast agent reflux. However, when the coexisting aortic
valves cannot close completely, the ALVT contrast agents reflux is
concealed. Thus, its clinical application has certain restrictions.
Echocardiography can clearly show ALVT in two-dimensional images in
real time, and CDFI can display the blood flow signal within ALVT.
Echocardiography is non-invasive, accurate, and simple to use and
has become the standard imaging exam for diagnosing ALVT (22–24). Of
the 153 cases reported, the accuracy rate of the ultrasound
diagnosis was 79.7%, the misdiagnosis rate was 17.0%, and missed
diagnosis rate was 3.3%.
Key points, symptoms, complicated
lesions and differential diagnosis of the ultrasound diagnosis of
ALVT
i) Key points and symptoms of the ultrasound
diagnosis of ALVT: a) common scanning sections include the
parasternal left ventricle in the long-axis view, the cardiac-base
short-axis view of the aorta, the apical five-chamber view of the
heart and the apical long axis view of the heart. To uncover aortic
valve regurgitation, great attention should be paid to the
multi-slice scanning to identify the origin of the regurgitation
beam; b) The position of aortic opening of ALVT is relatively
complex, and more common at the ventricle-artery connection (68.0%)
to which the right ventricular coronary valve attaches or perhaps
at the ventricle-artery connection to which the left coronary
aortic valve (10.4%) or non-coronary valve (5.9%) attaches, or the
triangle (13.1%) of active sinus bicuspid valves or in the
ascending aorta (2.6%); c) ALVT type casting: Hovaguimian et
al divided ALVT into four types (3). Type I is simply ALVT whereby the aortic
opening is small and cracked, and is not associated with aortic
valve damage. In type II, the aortic opening is oval and the
corresponding aortic sinus aneurysm wall expands like tumors with
or without aortic valve damage. As for type III, the septal heart
aneurysm of the tunnel expands with or without the right
ventricular outflow tract obstruction (25). Type IV is a mix of types II and III.
In this group, case 1 and 2 belong to type I, case 3 to type II,
case 4 and 5 to type III, case 6 to type IV. Ninety-three of the
153 cases reported here, can be categorized into 34 cases of type I
(36.62%), 40 cases of type II (43.0%), 14 cases of type III
(15.0%), 5 cases of type IV (5.4%); d) color and spectral Doppler
displays that high-speed regurgitation signal can be seen in the
tunnel between the aorta and the left ventricle during the diastole
period. The regurgitant beam starts from the aortic valve; e)
long-term high-speed blood flow impacting the aortic valve and the
left ventricular outflow tract easily leads to aortic valve
insufficiency and aortic valve prolapse; f) whether the origin of
the left and right coronary arteries is normal should be made
clear; g) of 153 cases reported here, there are only four cases of
recanalization after ALVT surgery. The ALVT ultrasonography of
partial recanalization is similar to perivalvular leakage.
ii) According to the clinical data of the 153 cases,
there are 51 cases (33.3%) with aortic regurgitation and 19 cases
(12.4%) with coronary artery abnormalities. Other lesions include
bicuspid aortic valve malformation, pulmonary stenosis, aortic
stenosis, ventricular septal defect, the main aortic valve prolapse
(26).
iii) Differential diagnosis of LVT: In the 17
misdiagnosed cases reported in the literature, seven cases were
misdiagnosed as aortic valve insufficiency, two cases as Valsalva
sinus aneurysm, one case as coronary artery, left ventricular
fistula, one case as ventricular septal defect with aortic
malformations, one case as four-leaf aortic valve, two cases as
aortic valve prolapse, one case as infective endocarditis, one case
as paravalvular abscess, one case as Marfan syndrome. It is harder
to differ the Valsalva sinus aneurysm and coronary artery, left
ventricular fistula from ALVT. We believe that sinus of Valsalva
aneurysm usually takes the form of a pocket pouch or petals. The
enormous influx of arterial blood into the left ventricle during
the diastole period can increase the sinus volume, and flow into
the left ventricular outflow tract. The two-dimensional image shows
that the sinus wall is interrupted. Remnants of the tumor wall
tissue at the edge of the break are waving like valves. Blood flows
through the break into the left ventricle whose shunt flow signals
originate in the Valsalva sinus below the level of the annulus.
ALVT often originates from above the opening of the coronary artery
(6). The aortic valve is like a
tunnel or an ampulla. The regurgitant beam originates above the
level of annulus. The coronary artery of patients with coronary
artery, left ventricular fistula expands while that of patients
with ALVT no abnormity is observed.
In conclusion, echocardiography is the preferred
method for the non-invasive preoperative diagnosis of ALVT and can
accurately describe the type and the involvement of the cardiac
structure.
References
|
1
|
Edwards JE: An atlas of acquired disease
of the heart and great vessels. 2. 2nd. WB Saunders; Philadelphia:
pp. 11421961
|
|
2
|
Levy MJ, Lillehei CW, Anderson RC, Amplatz
K and Edwards JE: Aortico-left ventricular tunnel. Circulation.
27:841–853. 1963. View Article : Google Scholar
|
|
3
|
Hovaguimian H, Cobanoglu A and Starr A:
Aortico-left ventricular tunnel: a clinical review and new surgical
classification. Ann Thorac Surg. 45:106–112. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooley RN, Harris LC and Rodin AE:
Abnormal communication between the aorta and left ventricle;
aortico-left ventricular tunnel. Circulation. 31:564–571. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terada T, Sakurai H, Nonaka T, Sakurai T,
Sugiura J and Otsuka R: Surgical repair of aortic regurgitation
with left ventricular aneurysm diagnosed preoperatively as
aortico-left ventricular tunnel. World J Pediatr Congenit Heart
Surg. 5:583–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins JD, Sherwood MC, Mayer JE Jr and
Keane JF: Aortico-left ventricular tunnel: 35-year experience. J Am
Coll Cardiol. 44:446–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cook AC, Fagg NL, Ho SY, Groves AM,
Sharland GK, Anderson RH and Allan LD: Echocardiographic-anatomical
correlations in aorto-left ventricular tunnel. Br Heart J.
74:443–448. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hucin B, Horvath P, Skovránek J, Reich O
and Samánek M: Correction of aortico-left ventricular tunnel during
the first day of life. Ann Thorac Surg. 47:254–256. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang XS, Hou YS, Feng B, He Y, Xu Y and
Huang ZG: Transthoracic and transesophageal echocardiography in the
diagnosis of aortico-left ventricular tunnel. Chinese J Ultrasound
Med. 20:304–307. 2004.(In Chinese).
|
|
10
|
Zhang HB, Xu ZW, Su ZZ and Ding WX:
Diagnosis and surgical treatment of aortico-left ventricular
tunnel. Chinese J Thoracic Cardiovas Surg. 20:268–270. 2004.(In
Chinese).
|
|
11
|
Hou CJ, Deng DA, Zhu XY, Han XM, Wang QG,
Sheng XT, Jin Y, Cui CS and Zhang P: A study on imaging
characteristics of color Doppler echocardiography of aortic left
ventricular tunnel. J China Clin Med Imaging. 17:34–36. 2006.(In
Chinese).
|
|
12
|
Horváth P, Balaji S, Skovránek S, Hucin B,
de Leval MR and Stark J: Surgical treatment of aortico-left
ventricular tunnel. Eur J Cardiothorac Surg. 5:113–116; discussion
117. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Qiu LC, Shi HY and Wang LX: One
case of advanced aortico-left ventricular tunnel. Chinese J
Thoracic Cardiovas Surg. 22:3072006.(In Chinese).
|
|
14
|
Grünenfelder J, Zünd G, Prêtre R, Schmidli
J, Vogt PR and Turina MI: Right coronary artery from aorto-left
ventricular tunnel: case report of a new surgical approach. J
Thorac Cardiovasc Surg. 116:363–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu J, Jiang Z, Huang J, Wu S and Mei J:
Aortico-left ventricular tunnel arising from the noncoronary sinus
associated with a ventricular septal defect. Ann Thorac Surg.
98:e135–e137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pockett CR, Chan S and Smallhorn J:
Aortico-left ventricular tunnel: the elusive diagnosis. Pediatr
Cardiol. 34:1743–1745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith BM, Cochran CD and Owens ST:
Aortico-left ventricular tunnel and left ventricular
non-compaction: a case series. Cardiol Young. 26:1–4.
2015.PubMed/NCBI
|
|
18
|
Thomas E, Maskari S and Farqani A:
Percutaneous device closure of aortico-left ventricular tunnel
using Amplatzer vascular plug III. Cardiol Young. 23:755–758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paech C, Pfeil N, Wagner R, Kostelka M and
Weidenbach M: Smart nature. Aortico-left ventricular tunnel
bypassing congenital critical aortic stenosis. Echocardiography.
30:E344–E345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shiraishi S, Takahashi M, Watanabe M and
Tsuchida M: Surgical repair of aortico-left ventricular tunnel:
report of two cases. Asian Cardiovasc Thorac Ann. 21:67–70. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cebeci M, Kalayci S, Demirkan BM, Guray YA
and Tufekcioglu O: Aortico-left ventricular tunnel with uncommon
origin. Eur Heart J Cardiovasc Imaging. 15:9432014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Zhao R, Liu B, Wang W, Yu Y, Shi X
and Xu Y: Noninvasive imaging evaluation of aortico-left
ventricular tunnel: a case report. Zhonghua Xin Xue Guan Bing Za
Zhi. 42:345–346. 2014.(In Chinese). PubMed/NCBI
|
|
23
|
Song L, Jin J and Tao L: Surgical
correction of an aortico-left ventricular tunnel originating from
the left aortic sinus with a left coronary artery anomaly. J Card
Surg. 30:108–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bash SE, Huhta JC, Nihill MR, Vargo TA and
Hallman GL: Aortico-left ventricular tunnel with ventricular septal
defect: two-dimensional/Doppler echocardiographic diagnosis. J Am
Coll Cardiol. 5:757–760. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nezafati MH, Maleki MH, Javan H and Zirak
N: Repair of aorto-left ventricular tunnel arising from the left
sinus of valsalva. J Card Surg. 25:345–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Myers JL and Mehta SM: Congenital Heart
Surgery Nomenclature and Database Project: Aortico-left ventricular
tunnel. Ann Thorac Surg. 69(Suppl 4): S164–S169. 2000. View Article : Google Scholar : PubMed/NCBI
|