Introduction
Inflammatory bowel disease (IBD) is a chronic
non-specific disorder of unknown etiology, and includes ulcerative
colitis (UC), Crohn's disease (CD) and indeterminate colitis.
Recent epidemiological studies indicate a rising incidence of IBD
in adults and children (1,2). However, the majority of published
studies have focused on adults, and delayed diagnosis is common in
children worldwide (3,4). Conventional diagnostic methods may fail
to identify a subset of patients with small bowel involvement.
Double-balloon enteroscopy (DBE), a technique for deep diagnostics
and a therapeutic for small bowel endoscopy, was described in 2001
by Yamamoto et al (5). DBE is
an established modality of investigation in adults, but it has not
been used widely in children and is available in few pediatric
centers in China (6).
The clinical course of CD may include various intra-
and extra-intestinal complications, and its serious and chronic
nature has shown to adversely affect patients' quality of life
(7). Approximately 10% of patients
with UC present with severe clinical symptoms requiring
hospitalization and treatment with intravenous corticosteroids
(8). Infliximab is a chimeric
immunoglobulin G1 monoclonal antibody, which binds to tumor
necrosis factor-α with high affinity and specificity, and has been
proved effective in adults with CD who had an inadequate response
to conventional therapy (9), as well
as in patients with UC (10).
Thalidomide has been intensified in recent years as researchers
have identified and clarified its immunomodulatory and
anti-angiogenic properties (11,12).
Based on the apparent anti-TNF-α properties of thalidomide, a
previous study performed by the authors of the present study used
it successfully to treat a 12-year-old boy with refractory CD and
concomitant tuberculosis (TB) infection in 2006 (13). This experience resulted in the
authors of the current study initiating a thalidomide treatment
trial for refractory cases of CD in 10 children.
Few published studies have reviewed IBD in Chinese
children (14). A steadily
increasing trend of 0–14-year-old childhood IBD in Shanghai, rising
from 0 in 2000 to 6.051 in 2010 per 106 populations for
the year-specific incidence rate, has been found (14). However, few studies have reported the
use of DBE and thalidomide in children with refractory IBD. The
present study is a retrospective, multicenter hospital-based study
that was performed over an 11-year period. The data of 49 pediatric
patients diagnosed with IBD at Fudan University Children's Hospital
(Shanghia, China), and the preliminary experience in the diagnosis
and treatment of IBD in these children, are reviewed.
Materials and methods
Patients and study design
Between July 2001 and May 2012, 49 pediatric
patients (30 males and 19 females) were reviewed at Fudan
University Children's Hospital. The diagnosis of UC or CD was
appropriately evaluated by a physician. All cases met the criteria
established by the Society of Pediatrics of China, 2010 (15). Every child suspected of UC or CD
underwent a complete diagnostic program, consisting of colonoscopy
with ileal intubation, upper gastrointestinal endoscopy and
radiologic contrast imaging of the small bowel. In addition, DBE
was performed in suspected cases with small intestine involvement.
Multiple biopsies from all segments of the gastrointestinal tract
(including pathological and normal tissues) were required for a
complete histologic evaluation. Formalin-fixed, paraffin-embedded
sections (3-µm) were stained with hematoxylin-eosin, then examined
under a BX51 light microscope (Olympus Corporation, Tokyo, Japan).
The data of 49 patients with IBD included general information,
clinical presentation and symptoms, laboratory findings and
therapeutic management.
Physical examinations, laboratory analyses, and
pediatric CD activity index (PCDAI) scoring (16) were performed of the 49 patients at
baseline, weeks 2, 8 and 12, and every 3 months thereafter, until
the patients reached 18 years of age. Endoscopies were repeated at
6 months after thalidomide administration. The patients were
monitored intensively for any side effects during the follow-up
period.
Ethics
The present study was approved by the Institutional
Ethics Committee of the Children's Hospital of Fudan University,
and informed consent was obtained from the patients or guardians of
the patients included in the study.
Results
Patient characteristics
Eight (16.3%) of the 49 patients with partial
enteral nutrition had UC (3 males and 5 females; age, 6–15 years; 2
mild, 4 moderate and 2 severe cases) and 41 (83.7%) had CD (27
males and 14 females; age, 1–17 years; 21 mild and 20
moderate/severe cases). The UC:CD ratio was 1:5.1, and the
male:female ratio was 1:0.63. The patients' age distribution is
presented in Table I. The mean age
at diagnosis was 10.4 years (range, 6.2–14.9 years) in patients
with UC and 10.1 years (range, 1.5–16.9 years) in patients with
CD.
 | Table I.Age distribution of pediatric patients
with inflammatory bowel disease. |
Table I.
Age distribution of pediatric patients
with inflammatory bowel disease.
|
| Age (years) |
|---|
|
|
|
|---|
| Disease | <3 | 3–6 | 7–12 | 13–17 |
|---|
| UC (%) | 0 (0) | 0 (0) | 6 (75) | 2 (25) |
| CD (%) | 5 (12.2) | 2 (4.9) | 14 (34.1) | 20 (48.8) |
Clinical manifestations
The mean interval between presentation and diagnosis
was 10.2 months (range, 0.5–24 months) for patients with UC and 9.7
months (range, 0.5 months-5 years) for patients with CD. The
clinical manifestations of the 49 patients with UC or CD are
presented in Table II. In this
study, the common manifestations primarily included abdominal pain,
diarrhea and rectal bleeding, which are typical symptoms of IBD
(17). The percent of patients with
UC with abdominal pain, diarrhea or rectal bleeding were 62.5, 100
and 87.5%, respectively, and the percent of patients with CD were
75.6, 61 and 39%, respectively (Table
II). The most common manifestation was diarrhea in patients
with UC and abdominal pain in patients with CD. Extra-intestinal
manifestations (EIM) were also documented, including being
underweight, and having abdominal masses, oral ulcers, arthritis,
anal fistulas, intestinal perforation and erythra (Table II). The EIM in patients with UC were
being underweight (37.5%), and having oral ulcers (12.5%) and
arthritis (12.5%), and the EIM in patients with CD were being
underweight (34.1%), and having oral ulcers (26.8%), anal fistulas
(22%), arthritis (19.5%), erythra (14.6%) and abdominal masses
(4.9%) (Table II). At least one EIM
was found in 25% of patients with UC and 73.2% of patients with CD.
There were 26.5% (13/49) of cases in the present study who had been
misdiagnosed as dysentery.
 | Table II.Primary manifestations of
inflammatory bowel disease in 49 pediatric patients. |
Table II.
Primary manifestations of
inflammatory bowel disease in 49 pediatric patients.
| Manifestations | UC, n=8 | CD, n=41 |
|---|
| Common
manifestations, n (%) |
|
|
|
Abdominal pain | 5 (62.5) | 31 (75.6) |
|
Diarrhea | 8 (100) | 25 (61) |
| Rectal
bleeding | 7 (87.5) | 16 (39) |
| Extraintestinal
manifestations, n (%) |
|
|
|
Underweight | 3 (37.5) | 14 (34.1) |
|
Abdominal mass | 0 (0) | 2 (4.9) |
| Oral
ulcer | 1 (12.5) | 11 (26.8) |
|
Arthritis | 1 (12.5) | 8 (19.5) |
| Anal
fistula | 0 (0) | 9 (22) |
|
Intestinal perforation | 0 (0) | 0 (0) |
|
Erythra | 0 (0) | 6 (14.6) |
Laboratory findings
Routine blood and other laboratory test results were
obtained. The percent of patients with UC with immunoglobulin G
(>12 g/l), C-reactive protein (>20 mg/l) or erythrocyte
sedimentation rate (>25 mm/h) were 25, 25 and 50%, respectively,
and these percentages in patients with UC were 51.2, 68.3 and
70.7%, respectively (Table III).
In addition, patients with CD had a higher proportion of cases with
increased levels of immunoglobulin G, C-reactive protein and
erythrocyte sedimentation rate compared with patients with UC.
 | Table III.Laboratory findings of pediatric
patients with inflammatory bowel disease. |
Table III.
Laboratory findings of pediatric
patients with inflammatory bowel disease.
| Parameter | UC n=8 | CD n=41 |
|---|
| White blood cells
>15×109/l, n (%) | 2 (25) | 6 (14.6) |
| Hemoglobin <90
g/l, n (%) | 1 (12.5) | 5 (12.2) |
| Platelets
>400×109/l, n (%) | 1 (12.5) | 18 (43.9) |
| Albumin <35 g/l,
n (%) | 2 (25) | 18 (43.9) |
| Immunoglobulin G
>12 g/l, n (%) | 2 (25) | 21 (51.2) |
| C-reactive protein
>20 mg/l, n (%) | 2 (25) | 28 (68.3) |
| Erythrocyte
sedimentation |
|
| rate >25 mm/h, n
(%) | 4 (50) | 29 (70.7) |
Endoscopic and pathohistological
examination
Colonoscopy was performed in all cases and
gastroscopy was performed in 27 cases. DBE was performed in 13
cases. Six of these patients had both colon and small intestinal
involvement, and one case involved only the small intestine. The
histologic lesions of patients with UC were primarily located in
the sigmoid colon (75%), transverse colon (50%) or pan-colon (50%),
and the histologic lesions in patients with CD were primarily
located in the distal ileum (51.2%), ileocecum (58.5%) or upper
gastrointestinal tract (41.5%) (Table
IV). Analysis of the number and location of lesions revealed
that more than two locations were involved in 70.7% (29/41) of
patients with CD.
 | Table IV.Distribution of histologic lesions in
49 pediatric patients with inflammatory bowel disease. |
Table IV.
Distribution of histologic lesions in
49 pediatric patients with inflammatory bowel disease.
| Lesion
location | UC (n=8) | CD (n=41) |
|---|
| Rectum, n (%) | 2 (25) | 7 (17.1) |
| Sigmoid colon, n
(%) | 6 (75) | 9 (22.0) |
| Descending colon, n
(%) | 3 (37.5) | 8 (19.5) |
| Transverse colon, n
(%) | 4 (50) | 14 (34.1) |
| Ascending colon, n
(%) | 1 (12.5) | 11 (26.8) |
| Pan-colon, n
(%) | 4 (50) | 10 (24.4) |
| Ileocecum, n
(%) | 3 (37.5) | 24 (58.5) |
| Distal ileum, n
(%) | 0 (0) | 21 (51.2) |
| Ileum, n (%) | 0 (0) | 6 (14.6) |
| Upper
gastrointestinal tract, n (%) | 0 (0) | 17 (41.5) |
In patients with UC, histological findings
identified inflammatory cell infiltration and lymph follicle
formation. A crypt abscess was identified in one patient with UC.
Lymphocyte and plasmocyte aggregation were observed in all patients
with CD. The characteristic histological finding of non-caseating
granuloma was seen in 29.2% (12/41) cases of CD.
Treatment
The goals of IBD treatment were to induce and
maintain the remission of mucosal inflammation and other clinical
symptoms, and to reduce the rate of growth retardation. The
standard treatment for IBD was administered to all patients,
including corticosteroids such as prednisone [5 mg per tablet;
Actavis (Foshan) Pharmaceutical Co., Ltd., Foshan, Guangdong,
China] at an initial dose of 2 mg/kg per day and adjusted according
to patients condition, 5-aminosalicylate (500 mg per bag; Shanghai
Ethypharm Pharmaceutical Co., Ltd., Shanghai, China) at a dose of
50–80 mg/kg per day, immunosuppressants such as 6-mercaptopurine
(50 mg per tablet; Zhejiang Hisun Pharmaceutical Co., Ltd.,
Taizhou, China) and azathioprine (AZA; 50 mg per tablet; Beijing
Jialin Pharmaceutical Co., Ltd., Beijing, China) according to the
patients condition, as well as nutritional support. The majority of
the patients who received the standard treatment for IBD responded
well.
Two patients (one with UC, one with CD) with
moderate-severe active IBD were administered infliximab (100 mg per
branch; Parkedale Pharmaceuticals, Inc., Rochester, Minnesota, USA)
a dose of 5 mg/kg per day in weeks 0, 2 and 6 and once in week 8
because oral glucocorticoids and immunosuppressants failed. In
addition, one patient with CD and arthritis received infliximab
treatment. All patients showed improvement in symptoms after the
first infliximab infusion (5 mg/kg). No side effects following
treatment were observed during the follow-up period.
Ten patients received thalidomide treatment (25 mg
per tablet; Changzhou Pharmaceutical Co., Ltd., Changzhou, China)
at an initial dose of 2 mg/kg per day and adjusted according to
patient condition. Among these patients, seven with refractory CD,
which was defined as standard induction therapy with high-dose
intravenous steroids, failed to induce remission either at
diagnosis or during subsequent relapse; one developed a
simultaneous TB infection and another two developed a suspected TB
infection). Thalidomide was administered at an initial dose of 2.0
mg/kg/day, then increased to 2.5–3.0 mg/kg/day or decreased to 1.5
mg/kg/day, depending on each patient's response to drug. Six of the
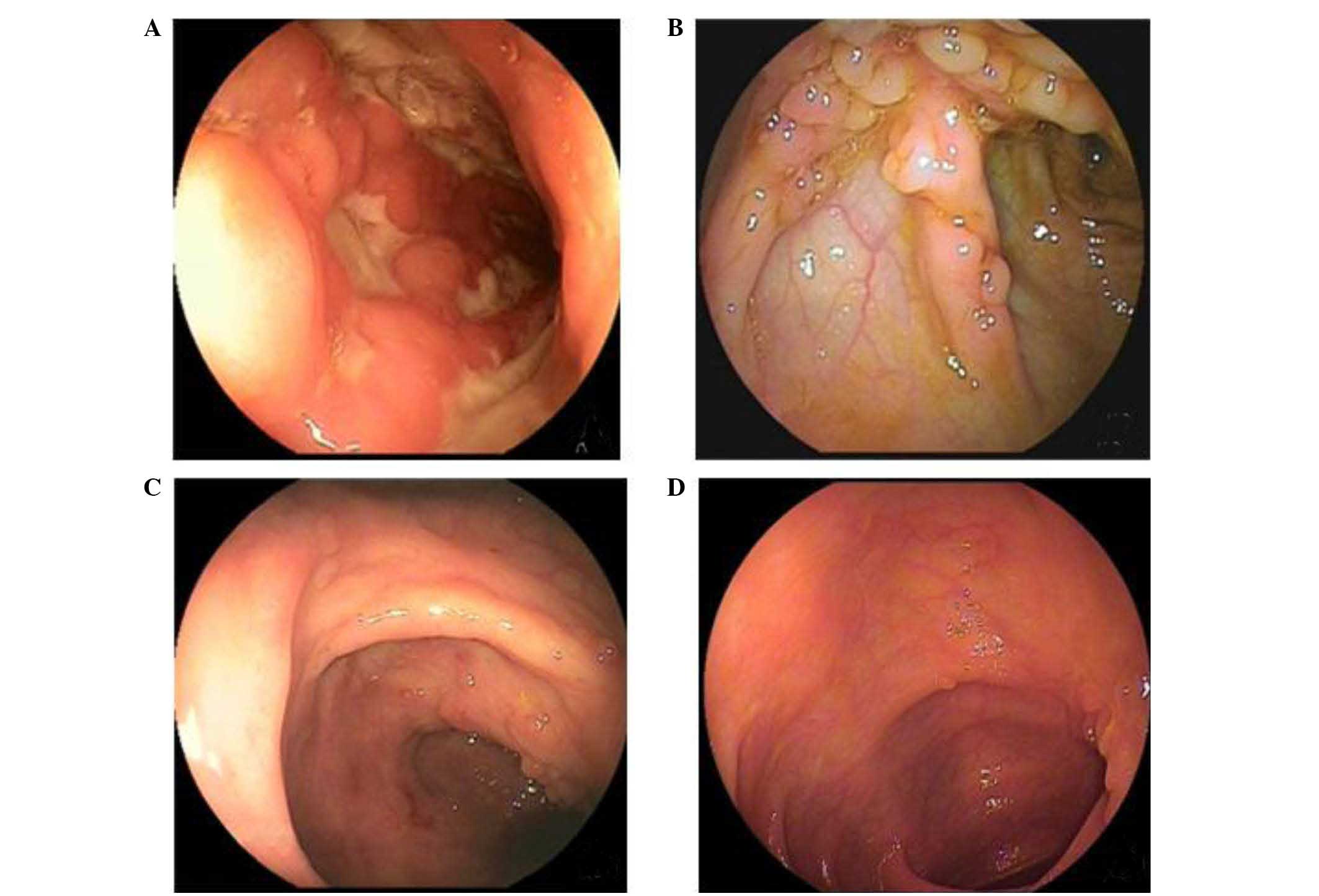
ten patients completed all follow-up assessments (13). After 3 months of thalidomide
treatment, all patients achieved complete clinical remission with
significant improvement of laboratory data, showed good tolerance
of the drug, and then stopped steroid use. The endoscopic findings
revealed marked improvement (Fig.
1). The remaining four patients who received thalidomide were
still being followed-up at the time of manuscript submission, but
their clinical symptoms and endoscopic characteristics improved in
response to the treatment.
Discussion
Epidemiological studies have identified that the
incidence of IBD has markedly increased during the past several
decades (18). The onset of IBD
within the first year of life has been documented in 1% of
pediatric patients (19). In the
patient sample in the present study, CD was more common compared
with UC and showed a male preponderance; these findings are in
agreement with previously published results (5). In a retrospective Chinese study, 82
patients with CD and 71 patients with UC were reported with a male
predominance (14). One larger study
from the Japanese nationwide registry reported that between 2003
and 2006, newly registered patients, who were aged ≤16 years,
included 311 cases of CD (10.6%) and 880 cases of UC (5.9%)
(20). The authors of the present
study purposed that the variances between studies were due to
subjects being obtained from different regions and sample size.
In a study of children in France, the mean interval
between presentation and diagnosis 2 months for UC and 4 months for
CD (21). In the present study
group, the mean intervals were longer; 10.2 months for UC and 9.7
months for CD, and 13 cases (13/49, 26.5%) were initially
misdiagnosed as dysentery, which manifested diarrhea, mucous bloody
stool, positive occult blood test and effective antibacterial
treatment. As a result, these cases experienced delayed final
diagnosis. Children with diarrhea, abdominal pain and blood in
stool can often be mistakenly diagnosed with infectious diarrhea,
which may lead to a delay in the true diagnosis. In pediatric IBD,
growth failure may present in affected children before digestive
symptoms develop (22). Two of the
patients in the present study exhibited growth retardation; they
visited their physicians because of microplasia without digestive
symptoms, which did not develop until 1–2 years later.
In the current study, the most common manifestation
was diarrhea in patients with UC and abdominal pain in patients
with CD. The EIM for patients with UC and CD were being
underweight, and having oral ulcers and arthritis. Diarrhea,
abdominal pain, being underweight, oral ulcers and arthritis are
symptoms of IBD (23–25). Patients with CD presented a higher
proportion of immunoglobulin G, C-reactive protein and erythrocyte
sedimentation rate compared with patients with UC. The results were
consisted with the work of Vermerire et al (17), who determined that C-reactive protein
was a marker to differentiate IBD (17). In addition, it has been determined
that immunoglobulin G and erythrocyte sedimentation rates are
closely associated with the development of IBD (26,27).
CD is a chronic transmural IBD that may involve any
part of the alimentary tract. The incidence rate of CD involving
the esophagus, stomach and duodenum was identified as 63% (17/27)
in the present study, which is similar to the results in the study
by Castellaneta et al (28).
Castellaneta et al (28)
suggested that multiple biopsies should be performed in children
with suspected IBD who cannot be diagnosed enteroscopically, even
in the absence of upper gastrointestinal symptoms. The majority of
patients with CD in the present study had more than two lesions,
and the lesions were more extensive compared with those in adults
(29). Conventional diagnostic
studies may fail to identify a subset of patients with small bowel
involvement. In the current study, 13 patients underwent DBE with
an anal approach. Six of these patients had colonic and small
intestinal involvement, and one case involved only the small
intestine. DBE has been proved to be a useful diagnostic tool for
the evaluation of small bowel lesions in patients with CD (30), and can enable the appropriate
adjustment of treatment to achieve clinical improvement. The
significance of this finding is emphasized by the significant and
sustained clinical improvement in the majority of patients
following the adjustment of therapy (30–32).
The present study identified one crypt abscess among
the patients with UC. Similar to other studies, pathohistological
examination revealed markedly less obvious evidence of disease in
children compared with adults (33,34).
Studies in adults have reported pathological changes in CD,
including crack ulcers and epithelioid granuloma in submucosa and
subserous layer, in 70–75% of adults (35). The current study observed
non-caseating granuloma in only 12 patients in the study sample;
the low incidence rate may be due to the restricted dimensions and
depth of the biopsies performed.
Infliximab, a TNF-α antibody, was administered to
three patients, whose symptoms were improved after treatment. A
number of studies have confirmed that infliximab is safe and
effective in the treatment of IBD in children (36,37). In
China, infliximab is mostly used in patients with refractory IBD.
Further follow-up studies should be conducted to evaluate the
long-term efficacy and safety of infliximab therapy. In addition,
infliximab is useful for relieving the EIM of IBD, such as pyoderma
gangrenosum, angiitis, arthritis, nodular erythema and uveitis.
However, infliximab should not be administered to patients with
sepsis, TB infection and/or intestinal tract stenosis. In
comparison with conventional ‘step-up’ therapy, ‘top-down’ or early
aggressive treatment (infliximab + AZA) show superiority in mucosal
healing, rapid remission and remission rates (38).
Biological treatment is expensive and cannot be used
in patients with TB infection, which remains common in China. The
treatment of patients with simultaneous CD and TB infection is
challenging because the administration of steroids or
immunosuppressants may result in the reactivation of latent TB or
exacerbate the infection. In China, few patients can afford the
high cost of infliximab. The development of novel treatment
modalities to help this group of patients is urgently needed.
In agreement with the results of other studies
(39,40), thalidomide (13) treatment improved the symptoms of 10
patients with refractory CD in the present study. Studies have
demonstrated that thalidomide therapy can enhance the response to
anti-TB drugs (41,42). In the current study, it was observed
that thalidomide was a good therapeutic option for patients with
IBD and concomitant or suspected TB. In addition, positive
responses, including weight gain, improved endoscopic findings and
improved PCDAI scores, were observed at 2–3 weeks after the
initiation of thalidomide treatment in the present study, which is
an improvement compared with patients who received steroids only.
The side effects of thalidomide, including peripheral neuropathy,
drowsiness, fatigue, constipation, xerosis cutis and
granulocytopenia, were tolerable for patients during the follow-up
period in the current study, and no severe neurotoxicity was
observed. Due to the irreversible neurotoxicity with a dose- and
time-dependent manner, the clinical application of thalidomide
treatment is limited and nerve conduction velocity tests should be
performed during follow-up examinations (43).
The present study was limited by a number of
factors. Firstly, the sample size of patients with UC was too small
to compare the difference between patients with UC and CD. Hence, a
multicenter study with a large sample size is required. Secondly, a
small number of patients in the present study received thalidomide.
The long-term efficacy and safety of thalidomide should be further
evaluated in a large-scale randomized controlled trial.
In conclusion, in the patient sample in the current
study, CD was more common than UC and showed a male preponderance.
The mean age at diagnosis was 10.4 years in patients with UC and
10.1 years in patients with CD. The most common manifestation was
diarrhea in patients with UC and abdominal pain in patients with
CD. The EIM for patients with UC and CD were being underweight, and
oral ulcers and arthritis. Patients with CD presented a higher
proportion of immunoglobulin G, C-reactive protein and erythrocyte
sedimentation rate compared with patients with UC. In addition, the
administration of infliximab and thalidomide achieved remission in
refractory cases and the tolerance was acceptable.
Acknowledgements
The authors thank Professor Huang's team members
(Department of Gastroenterology, Children's Hospital of Fudan
University, Shanghai, China) for their helpful discussion and
critical reading of the manuscript.
References
|
1
|
Shin DH, Sinn DH, Kim YH, Kim JY, Chang
DK, Kim EJ, Ryu HY, Song HU, Kim IY, Kim do H, et al: Increasing
incidence of inflammatory bowel disease among young men in Korea
between 2003 and 2008. Dig Dis Sci. 56:1154–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cucchiara S and Stronati L: Incidence in
pediatric IBD is rising: Help from health administrative data.
Inflamm Bowel Dis. 17:1048–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rufo PA and Bousvaros A: Challenges and
progress in pediatric inflammatory bowel disease. Curr Opin
Gastroenterol. 23:406–412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hugot JP and Bellaiche M: Inflammatory
bowel diseases: The paediatric gastroenterologist's perspective.
Pediatr Radiol. 37:1065–1070. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto H, Sekine Y, Sato Y, Higashizawa
T, Miyata T, Iino S, Ido K and Sugano K: Total enteroscopy with a
nonsurgical steerable double-balloon method. Gastrointest Endosc.
53:216–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Nardo G, de Ridder L, Oliva S, Casciani
E, Escher JC and Cucchiara S: Enteroscopy in paediatric Crohn's
disease. Dig Liver Dis. 45:351–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine JS and Burakoff R: Extraintestinal
manifestations of inflammatory bowel disease. Gastroenterol Hepatol
(N Y). 7:235–241. 2011.PubMed/NCBI
|
|
8
|
Sands BE, Tremaine WJ, Sandborn WJ,
Rutgeerts PJ, Hanauer SB, Mayer L, Targan SR and Podolsky DK:
Infliximab in the treatment of severe, steroid-refractory
ulcerative colitis: A pilot study. Inflamm Bowel Dis. 7:83–88.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danese S, Colombel JF, Reinisch W and
Rutgeerts P: Review article: Infliximab for Crohn's disease
treatment-shifting therapeutic strategies after 10 years of
clinical experience. Aliment Pharmacol Ther. 33:857–869. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Järnerot G, Hertervig E, Friis-Liby I,
Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Danielsson A,
Verbaan H, et al: Infliximab as rescue therapy in severe to
moderately severe ulcerative colitis: A randomized,
placebo-controlled study. Gastroenterology. 128:1805–1811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dredge K, Marriott JB, Macdonald CD, Man
HW, Chen R, Muller GW, Stirling D and Dalgleish AG: Novel
thalidomide analogues display anti-angiogenic activity
independently of immunomodulatory effects. Br J Cancer.
87:1166–1172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lentzsch S, LeBlanc R, Podar K, Davies F,
Lin B, Hideshima T, Catley L, Stirling DI and Anderson KC:
Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan
cells and angiogenesis in vivo. Leukemia. 17:41–44. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng CF, Xu JH, Huang Y and Leung YK:
Treatment of pediatric refractory Crohn's disease with thalidomide.
World J Gastroenterol. 17:1286–1291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XQ, Zhang Y, Xu CD, Jiang LR, Huang
Y, Du HM and Wang XJ: Inflammatory bowel disease in Chinese
children: A multicenter analysis over a decade from Shanghai.
Inflamm Bowel Dis. 19:423–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Society of Pediatrics of China,
Digestology group, . Consensus on diagnostic criteria of pediatric
inflammatory bowel disease. Chinese J Practical Pediatr.
25:263–265. 2010.(In Chinese).
|
|
16
|
Hyams JS, Ferry GD, Mandel FS, Gryboski
JD, Kibort PM, Kirschner BS, Griffiths AM, Katz AJ, Grand RJ, Boyle
JT, et al: Development and validation of a pediatric Crohns disease
activity index. J Pediatr Gastroenterol Nutr. 12:439–447. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vermeire S, Van Assche G and Rutgeerts P:
Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut.
55:426–431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pozler O, Maly J, Bonova O, Dedek P,
Frühauf P, Havlickova A, Janatova T, Jimramovsky F, Klimova L,
Klusacek D, et al: Incidence of Crohn disease in the Czech Republic
in the years 1990 to 2001 and assessment of pediatric population
with inflammatory bowel disease. J Pediatr Gastroenterol Nutr.
42:186–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heyman MB, Kirschner BS, Gold BD, Ferry G,
Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T and
El-Serag HB: Children with early-onset inflammatory bowel disease
(IBD): Analysis of a pediatric IBD consortium registry. J Pediatr.
146:35–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prideaux L, Kamm MA, De Cruz PP, Chan FK
and Ng SC: Inflammatory bowel disease in Asia: A systematic review.
J Gastroenterol Hepatol. 27:1266–1280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Auvin S, Molinié F, Gower-Rousseau C,
Brazier F, Merle V, Grandbastien B, Marti R, Lerebours E, Dupas JL,
Colombel JF, et al: Incidence, clinical presentation and location
at diagnosis of pediatric inflammatory bowel disease: A prospective
population-based study in northern France (1988–1999). J Pediatr
Gastroenterol Nutr. 41:49–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
IBD Working Group of the European Society
for Paediatric Gastroenterology, Hepatology and Nutrition, .
Inflammatory bowel disease in children and adolescents:
Recommendations for diagnosis-the Porto criteria. J Pediatr
Gastroenterol Nutr. 41:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mutalib M, Blackstock S, Evans V, Huggett
B, Chadokufa S, Kiparissi F and Elawad M: Eosinophilic
gastrointestinal disease and inflammatory bowel disease in
children: Is it a disease continuum? Eur J Gastroenterol Hepatol.
27:20–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dodell GB, Albu JB, Attia L, McGinty J,
Pi-Sunyer FX and Laferrère B: The bariatric surgery patient: Lost
to follow-up; From morbid obesity to severe malnutrition. Endocr
Pract. 18:e21–e25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yüksel I, Ataseven H, Başar O, Köklü S,
Ertuğrul I, Ulker A, Dağlı U and Saşmaz N: Peripheral arthritis in
the course of inflammatory bowel diseases. Dig Dis Sci. 56:183–187.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai C, Shen J, Zhao D, Qiao Y, Xu A, Jin
S, Ran Z and Zheng Q: Serological investigation of food specific
immunoglobulin G antibodies in patients with inflammatory bowel
diseases. PLoS One. 9:e1121542014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Ren J, Xia Q, Wu X, Han G, Ren H,
Yan D, Wang G, Gu G and Li J: Preliminary case-control study to
evaluate diagnostic values of C-reactive protein and erythrocyte
sedimentation rate in differentiating active Crohn's disease from
intestinal lymphoma, intestinal tuberculosis and Behcet's syndrome.
Am J Med Sci. 346:467–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castellaneta SP, Afzal NA, Greenberg M,
Deere H, Davies S, Murch SH, Walker-Smith JA, Thomson M and
Srivistrava A: Diagnostic role of upper gastrointestinal endoscopy
in pediatric inflammatory bowel disease. J Pediatr Gastroenterol
Nutr. 39:257–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mamula P, Telega GW, Markowitz JE, Brown
KA, Russo PA, Piccoli DA and Baldassano RN: Inflammatory bowel
disease in children 5 years of age and younger. Am J Gastroenterol.
97:2005–2010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin TK and Erdman SH: Double-balloon
enteroscopy: Pediatric experience. J Pediatr Gastroenterol Nutr.
51:429–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mensink PB, Groenen MJ, Van Buuren HR,
Kuipers EJ and Van der Woude CJ: Double-balloon enteroscopy in
Crohn's disease patients suspected of small bowel activity:
Findings and clinical impact. J Gastroenterol. 44:271–276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Y, Shao CH and Leung YK: Application
of double balloon enteroscopy in pediatric patients. Zhonghua Er Ke
Za Zhi. 48:599–602. 2010.(In Chinese). PubMed/NCBI
|
|
33
|
Robert ME, Tang L, Hao LM and Reyes-Mugica
M: Patterns of inflammation in mucosal biopsies of ulcerative
colitis: Perceived differences in pediatric populations are limited
to children younger than 10 years. Am J Surg Pathol. 28:183–189.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Washington K, Greenson JK, Montgomery E,
Shyr Y, Crissinger KD, Polk DB, Barnard J and Lauwers GY:
Histopathology of ulcerative colitis in initial rectal biopsy in
children. Am J Surg Pathol. 26:1441–1449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pierik M, De Hertogh G, Vermeire S, Van
Assche G, Van Eyken P, Joossens S, Claessens G, Vlietinck R,
Rutgeerts P and Geboes K: Epithelioid granulomas, pattern
recognition receptors, and phenotypes of Crohn's disease. Gut.
54:223–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tiemi J, Komati S and Sdepanian VL:
Effectiveness of infliximab in Brazilian children and adolescents
with Crohn disease and ulcerative colitis according to clinical
manifestations, activity indices of inflammatory bowel disease, and
corticosteroid use. J Pediatr Gastroenterol Nutr. 50:628–633. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hyams JS, Lerer T, Griffiths A,
Pfefferkorn M, Stephens M, Evans J, Otley A, Carvalho R, Mack D,
Bousvaros A, et al: Pediatric Inflammatory Bowel Disease
Collaborative Research Group: Outcome following infliximab therapy
in children with ulcerative colitis. Am J Gastroenterol.
105:1430–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oldenburg B and Hommes D: Biological
therapies in inflammatory bowel disease: Top-down or bottom-up?
Curr Opin Gastroenterol. 23:395–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Facchini S, Candusso M, Martelossi S,
Liubich M, Panfili E and Ventura A: Efficacy of long-term treatment
with thalidomide in children and young adults with Crohn disease:
Preliminary results. J Pediatr Gastroenterol Nutr. 32:178–181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lazzerini M, Martelossi S, Marchetti F,
Scabar A, Bradaschia F, Ronfani L and Ventura A: Efficacy and
safety of thalidomide in children and young adults with intractable
inflammatory bowel disease: Long-term results. Aliment Pharmacol
Ther. 25:419–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stefan DC, Andronikou S, Freeman N and
Schoeman J: Recovery of vision after adjuvant thalidomide in a
child with tuberculous meningitis and acute lymphoblastic leukemia.
J Child Neurol. 24:166–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schoeman JF, Fieggen G, Seller N,
Mendelson M and Hartzenberg B: Intractable intracranial tuberculous
infection responsive to thalidomide: Report of four cases. J Child
Neurol. 21:301–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Priolo T, Lamba LD, Giribaldi G, De Negri
E, Grosso P, De Grandis E, Veneselli E, Buoncompagni A, Viola S,
Alpigiani MG, et al: Childhood thalidomide neuropathy: A clinical
and neurophysiologic study. Pediatr Neurol. 38:196–199. 2008.
View Article : Google Scholar : PubMed/NCBI
|