Introduction
Liver transplantation has been shown to be the only
effective therapy for patients with end-stage liver disease
(1). A small-for-size liver graft is
defined as a graft involving <40% of the standard liver volume
or a graft to recipient weight ratio of 0.8–1.0% (2). In recent years, the demand for partial
liver grafts from either deceased or living donors has been
increasing worldwide, due to the severe shortage of donor organs
(3). However, recent studies
reported that small-for-size liver grafts are associated with
serious complications and graft failure (4,5).
Multiple factors, including transient portal
hypertension, ischemia/reperfusion (I/R) injury and subsequent
severe inflammatory responses in the early phase of reperfusion
after small-for-size liver transplantation may result in graft
failure (6). Among these, I/R injury
severely damages the transplanted liver following temporary
clamping of the hepatoduodenal ligament during liver
transplantation (7). I/R injury
involves the initial tissue damage caused by deprivation of blood
flow and oxygen and additional damage caused by the return of the
blood supply during reperfusion (8).
The mechanisms underlying I/R injury are complex and involve
endothelial cell adhesion, increased neutrophil infiltration,
release of pro-inflammatory cytokines, as well as the generation of
reactive oxygen species (ROS) and reactive nitrogen species
(9). The classical markers used to
assess the severity of hepatic I/R injury include abnormal liver
enzyme levels as well as histological signs of tissue damage
(10). Considering the serious and
unavoidable damage caused by I/R injury to grafts, effective
therapeutic strategies aimed at attenuating I/R injury and reducing
the death of hepatocytes would be of great benefit (11,12).
Nitric oxide (NO) is synthesized from the amino acid
L-arginine by the action of NO synthase (NOS) and plays an
important role in the regulation of renal vascular tone and
hemodynamics (13,14). Three NOS isoforms exist: Endothelial
NOS (eNOS), neuronal NOS and inducible NOS (iNOS) (13).
Excessive levels of iNOS-derived NO production may
be involved in the inflammatory process and promote I/R injury
(15). However, low levels of NO
produced at by eNOS physiologically regulate normal vascular tone
within the sinusoids, prevent leukocyte adhesion and limit ROS
production, thus exerting a beneficial effect on I/R injury
(16).
To date, no study has provided direct evidence
regarding whether delivery of exogenous eNOS via an adenoviral (Ad)
vector is cytoprotective against hepatic I/R injury in the context
of small-for-size liver transplantation. To confirm this, we
evaluated the effects of genetic overexpression of eNOS in the
protection of hepatocytes against I/R injury in a rat model of
small-for-size liver transplantation.
Materials and methods
Ad vector and transfection
The Ad-vector and Ad-eNOS were obtained from the
Second Military Medical University (Shanghai, China). Ad-eNOS was
further amplified in HEK293 cells (Biolef, Shanghai, China). Viral
particles were purified using cesium chloride density gradient
centrifugation. HEK293 cells in serum-free Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Grand
Island, NY, USA) were transfected with Ad-eNOS to identify the
optimal conditions. The Ad-eNOS was applied with a titer of
9.35×109 PFU/ml. L02 cells were transfected with Ad-eNOS
in a humidified atmosphere containing 5% CO2 at 37°C.
Transfection with an empty Ad-vector (Ad-null) served as a control.
The cells were harvested 48 h after transfection for analysis.
In vitro culture
The human normal liver cell line L02 was obtained
from the Shanghai Institute of Biochemistry and Cell Biology
(Shanghai, China). The cells were grown in Roswell Park Memorial
Institute-1640 medium supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and cultured in a
humidified atmosphere containing 5% CO2 at 37°C.
L02 cells were transferred to 24-well plates at a
density of 3.6×104 cells per well. Three groups (six
wells per group) were established: Experimental, control and normal
groups. Ad-eNOS (5×106 PFU) was added to the cells in
the experimental group, whereas Ad-null was added in the control
and normal groups. Then the cells in the experimental and control
groups were placed in a hypoxic environment (95% N2 and
5% CO2; PO2, ≤4 kPa) for 12 h. After
replacement of the medium with fresh culture medium, the cells were
moved to an oxygen enriched atmosphere (85% O2 and 15%
CO2; PO2, ≥13 kPa) for another 12 h. The
cells in the normal group were cultured in a normal atmosphere
containing 5% CO2 at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells or liver
tissues using TRIzol reagent (Gibco; Thermo Fisher Scientific,
Inc.), and DNase was used to treat genomic DNA prior to RT. Total
RNA (500 ng) was reverse transcribed into cDNA using a Prime-Script
RT kit (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's instructions. The sequences of the primers used
(Takara Bio, Inc.) were as follows: eNOS, forward
5′-TCAGTGGCTGGTACATGAGC-3′ and reverse 5′-TATCCAGGTCCATGCAGACA-3′;
GAPDH, forward 5′-ACTGGAACGGTGAAGGTGAC-3′ and reverse
5′-AGAGAAGTGGGGTGGCTTTT-3′. DNA amplification was performed in a
PCR system thermocycler (Biometra GmbH, Göttingen, Germany) using
the following conditions: Initial denaturation step at 95°C for 5
min, followed by 35 cycles of 95°C for 15 sec, 60°C for 30 sec, and
72°C for 30 sec, and a final extension phase at 72°C for 10 min.
The PCR reaction mixture (50 µl) consisted of SYBR Green Mix (32.5
µl), 1.5 µl each primer (20 pmol), 12.5 µl distilled water and 2 µl
template DNA (0.2 µg). A reaction without cDNA was used as a
negative control. Fluorescence quantification (%) was achieved by
calculating the ratio of the integrated optical density value of
eNOS to that of GAPDH, using the 2-ΔΔCq method..
Western blot analysis
The L02 cells or middle right liver tissues of rats
were lysed in lysis buffer (Genechem Co., Ltd., Shanghai, China).
Lysates were centrifuged at 7,500 × g for 10 min at 4°C, and the
supernatant was collected. Total protein levels in supernatant
samples were quantified using a bicinchonic acid assay (Thermo
Fisher Scientific, Inc.). Samples (50 µg protein) underwent 10%
SDS-PAGE. Proteins were then electroblotted onto a polyvinylidene
difluoride membranes. The membrane was blocked with 5% fat-free
milk at 4°C overnight, followed by incubation with rabbit eNOS
primary antibody (1:1,000; cat. no. MAB9028; R&D Systems, Inc.,
Minneapolis, MN, USA) at 37°C for 2 h. Membranes were washed three
times with TBS-T, then incubated at room temperature for 1 h with
horseradish peroxidase-conjugated secondary antibodies (1:2,000;
cat. no. sc-2065; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
The density of the corresponding bands was measured quantitatively
using Image-Pro Plus software, version 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA) and corrected by reference to the value
for GAPDH (1:1,000; cat. no. NB300-221; R&D Systems, Inc.).
Cell cycle analysis
The cultured L02 cells (5×105 cells each
group) or cell suspensions from homogenized middle left liver
tissues were fixed in ice-cold 70% ethanol and centrifuged to
collect a cell pellet that was resuspended in phosphate-buffered
saline (PBS). After washing, cells were incubated with RNAase
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS for 30 min
at 37°C. After further washing, pellets were resuspended in 0.5 ml
propidium iodide staining solution (50 µm/ml) and incubated for 30
min at 4°C. After filtering through a nylon mesh (pore size, 48
µm), apoptosis was detected using a flow cytometer (FACScalibur; BD
Biosciences, San Jose, CA, USA) at an excitation setting of 488
nm.
Immunohistochemical analysis
Paraffin-embedded middle right liver tissues were
cut into 5-µm sections, then deparaffinized in xylene, and
dehydrated in a graded series of ethanol solutions. After antigen
retrieval, the sections were incubated with primary antibody
against CD68 (1:1,000; cat. no. BA3638; Wuhan Boster
Bio-Engineering, Ltd., Co., Wuhan, China) or tumor necrosis factor
(TNF)-α (1:1,000; cat. no. BA0131; Wuhan Boster Bio-Engineering,
Ltd., Co.) at 4°C overnight. After rinsing with PBS, the sections
were incubated with rabbit anti-rat IgG-Biotin secondary antibody
(1:1,000; cat. no. BA1005; Wuhan Boster Bio-Engineering, Ltd., Co.)
for 30 min. The reaction was visualized using 3,3′-diaminobenzidine
(DAB; Sigma-Aldrich; Merck KGaA) staining. The sections were rinsed
with water and counterstained with Mayer's hematoxylin.
Model of small-for-size liver
transplantation
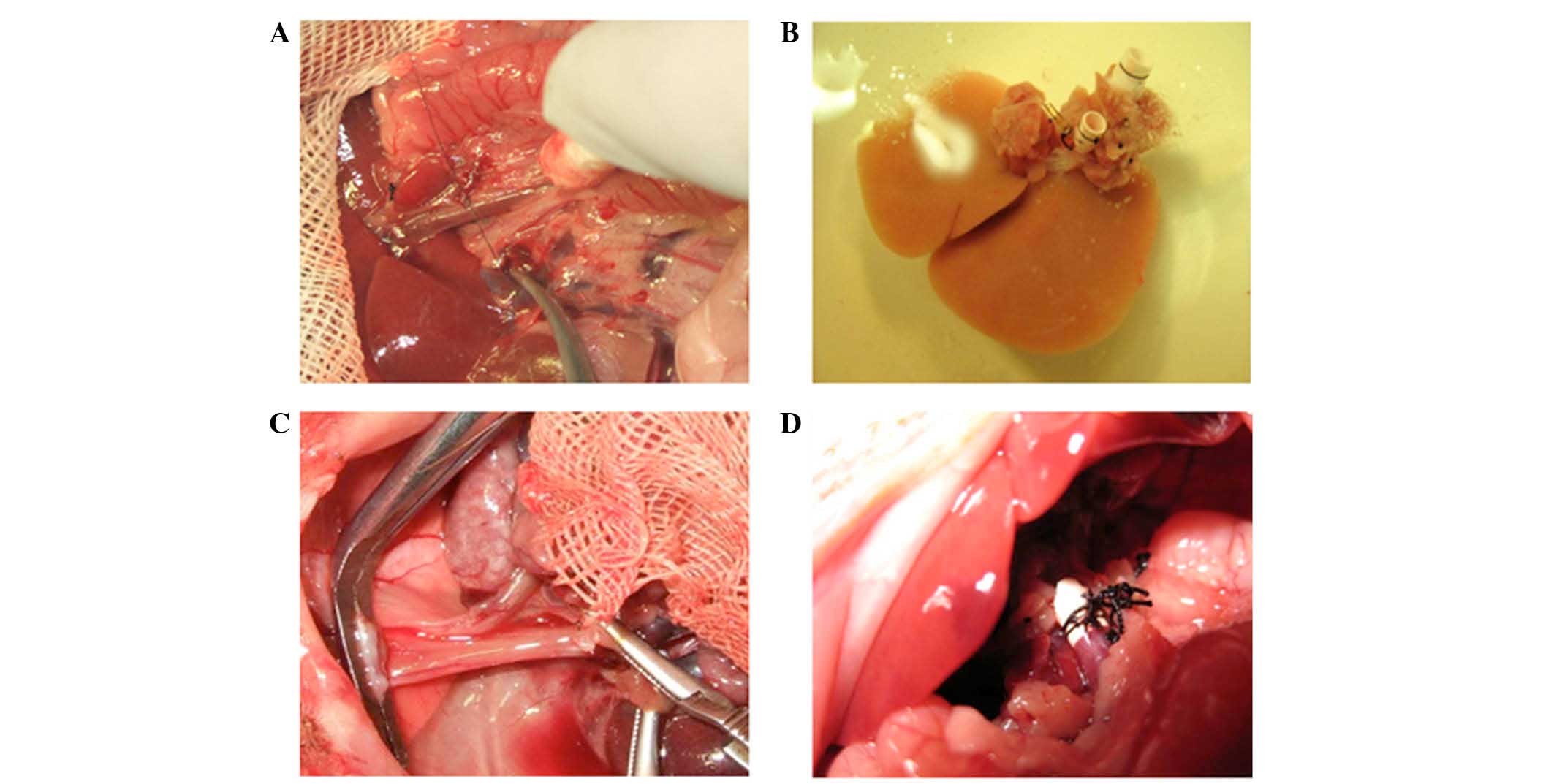
Small-for-size liver transplantation was performed
as described by Kamada and Kalne (17), with minor modifications (Fig. 1). Briefly, the donor rats were
anesthetized by intraperitoneal injection of 100 mg/kg ketamine
(Henrui Medicine, Lianyungang, China). Then, they were sacrificed
by cervical dislocation. The caudate, left lateral, right superior
and right inferior lobes of the rat liver were resected by ligation
with 5–0 or 7-0 silk sutures, with the median lobe spared to obtain
a liver graft of 40% (range, 36–43%) of the original liver size.
The time required for graft harvesting was 44.3±3.5 min. The
harvested livers were immediately flushed through the portal vein
and stored in ice-cold Ringer's lactate solution containing 10 U/ml
heparin (Kelun Pharmaceutical, Chengdu, China). After the
suprahepatic vena cava, portal vein and subhepatic vena cava of the
recipient were clamped, the liver was removed and the donor liver
was implanted by connecting the suprahepatic vena cava. The portal
vein and infrahepatic vena cava were anastomosed using the cuff
technique (18). The bile duct was
anastomosed with an intraluminal stent. The time required for graft
implantation was recorded. The transplantation procedure lasted
44.5±4.2 min, during which time the portal vein was clamped for
14.2±4.1 min. All rats survived until the end of the
experiment.
Experimental animals and grouping
A total of 18 male Sprague-Dawley rats (age, 8
weeks) were purchased from the Laboratory Animal Center of Soochow
University (Suzhou, China). All animal care, treatments and
procedures were performed according to the guidelines approved by
the Chinese Association of Animal Care and the standards for animal
use and care set by the Institutional Animal Care and Use
Committee. Rats were randomly divided into three groups:
Experimental, control or sham group (n=6 per group). Rats in the
experimental group were intraperitoneally injected with Ad-eNOS
(4.0×109 PFU), whereas rats in other two groups were
injected with Ad-null (4.0×109 PFU each). After 36 h,
rats in the experimental and control groups underwent
small-for-size liver transplantation. For rats in sham group, the
ligaments around the liver were freed, and the abdomen was closed
40 min later without transplantation. The quantity of bile flow was
evaluated after reperfusion in recipient rats. The quantity of bile
was calculated every minute for a total of ten min. At 6 h after
reperfusion, the rats in each group were sacrificed. Blood samples
were collected from the inferior vena cava, and liver tissues were
dissected and stored at −80°C or in 10% neutral buffered formalin
until further analysis.
Terminal
deoxynucleotidyltransferase-biotin nick end-labeling (TUNEL)
assay
Apoptosis was detected in histological sections
using a commercially available TUNEL kit (Roche Applied Science,
Mannheim, Germany), according to the instructions provided by the
manufacturer. Briefly, after deparaffinization and hydration,
sections were digested with proteinase K (Solarbio Science &
Technology Co., Ltd., Beijing, China) for 10 min. Endogenous
peroxidase activity was quenched with 3% H2O2
for 10 min. The slides were incubated with terminal
deoxynucleotidyl transferase and digoxigenin-dUTP at 37°C for 2 h.
The sections were then incubated with a biotin-conjugated rat
anti-digoxin antibody (1:100; cat. no. AR0147; Wuhan Boster
Bio-Engineering, Ltd., Co.) and streptavidin biotin complex (Wuhan
Boster Bio-Engineering, Ltd., Co.) for 30 min each. After being
rinsed with PBS, the slides were immersed in DAB solution. All
slides were counterstained with Mayer's hematoxylin.
Determination of serum transaminase
levels
Serum alanine transaminase (ALT), aspartate
transaminase (AST), and lactic acid dehydrogenase (LDH) levels were
measured using an automatic biochemical analyzer (AU2700; Olympus
America, Hamburg, Germany).
Quantitative analysis of NO
production
NO production was measured based on the enzymatic
conversion of nitrate to nitrite by nitrate reductase. Aliquots of
culture supernatants (50-µl) or tissue homogenates were collected
and mixed with 50 µl Greiss reagent (1% sulfanilamide and 0.1%
naphthylenediamine dihydrochloride in 2% phosphoric acid). The
mixture was incubated for 10 min with shaking at room temperature,
and the absorbance at 550 nm (A550) was measured using a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Nitrite
concentrations were determined by comparison with a standard
solution of sodium nitrite in water.
Histological examination
Liver tissues of rats in each group were washed with
saline solution, immersed in 10% neutral buffered formalin,
embedded in paraffin, and cut into 10-µm sections. Sections were
then stained with hematoxylin and eosin (H&E) and observed
under a light microscope.
Statistical analysis
Statistical analysis was performed using the SPSS
software package (version 16.0; SPSS, Inc., Chicago, IL, USA).
Quantitative data are presented as the mean ± standard deviation
values. Data were analyzed using one-way analysis of variance to
evaluate inter-group differences. P<0.05 was considered to
indicate a statistically significant difference.
Results
eNOS expression in L02 liver
cells
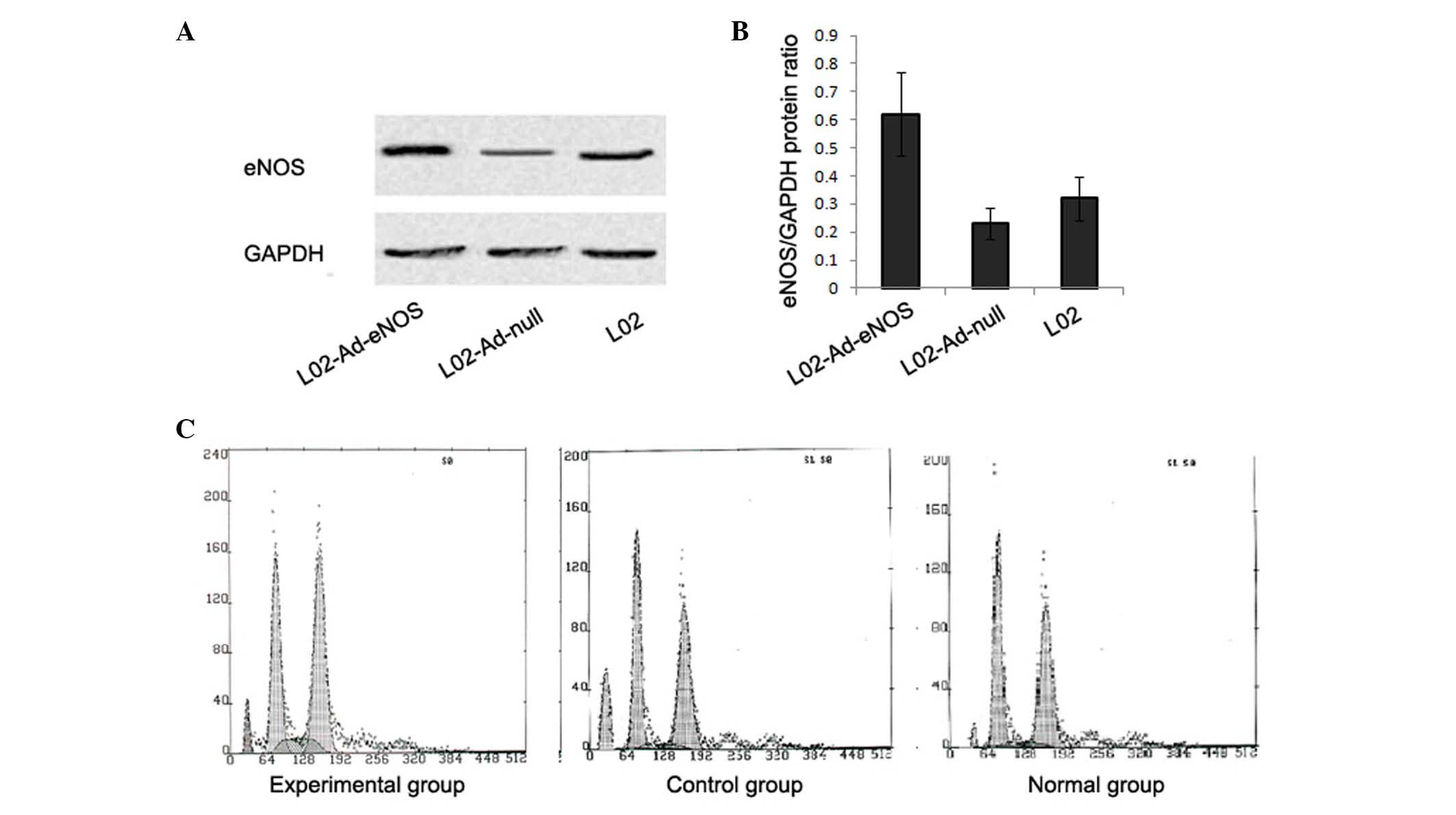
RT-qPCR and western blot analyses were used to
evaluate the mRNA and protein expression levels of eNOS in L02
cells. L02 cells transfected with Ad-eNOS expressed an increased
mRNA level of eNOS (21.92±6.66%), which was significantly higher
than levels in L02 and Ad-null-transfected L02 cells (1.58±2.42 and
2.85±1.52%, respectively; P<0.05). Similar differences in eNOS
expression were observed western blot analysis. The results from
western blot showed greater eNOS protein expression in
Ad-eNOS-transfected L02 cells compared with L02 and
Ad-null-transfected L02 cells (P<0.05; Fig. 2A and B), suggesting successful
transfection of L02 cells with eNOS.
Ad-eNOS transfection increased NO
production and decreased liver cell apoptosis in vitro
The ALT level in the culture supernatants in the
experimental group was significantly lower than that in the control
group (26.26±3.78 vs. 48.42±5.31 U/l; P<0.05), but was
significantly higher than that in the normal group (17.20±2.64 U/l;
P<0.05).
Similar levels of NO products were detected in
culture supernatants from the control and normal groups (6.44±2.11
vs. 8.85±2.40 µmol/l, respectively; P>0.05). However, the NO
concentration was significantly greater in the experimental group
(18.89±3.30 µmol/l) compared to concentrations in the control and
normal groups (P<0.05).
To evaluate whether Ad-eNOS transfection prevented
apoptosis in L02 cells, cells in each group were harvested and
analyzed using flow cytometry. The rates of apoptosis in the
experimental, control and normal groups were 10.10±2.91, 20.53±2.8
and 5.21±1.41%, respectively (P<0.05, experimental vs. control
group), indicating a protective role of Ad-eNOS against apoptosis
in L02 cells (Fig. 2C).
Ad-eNOS transfection improved abnormal
transaminase levels following I/R
Rats in the control group presented reduced bile
secretion and elevated levels of liver enzyme, including ALT, AST
and LDH, after small-for-size liver transplantation (all
P<0.05). However, the quantity of bile secreted in rats in the
experimental group was greater than that in rats in the control
group (P<0.05). Rats in the experimental group exhibited
deceased levels of ALT, AST and LDH (P<0.05), suggesting a
reduction in liver tissue damage with Ad-eNOS pretreatment. A
greater NO level was detected in liver tissue of rats pretreated
with Ad-eNOS compared with that of rats in the control or sham
groups (P<0.05; Table I).
 | Table I.Effects of overexpression of eNOS on
bile secretion, NO concentration and liver enzyme levels in rats
(n=6 per group). |
Table I.
Effects of overexpression of eNOS on
bile secretion, NO concentration and liver enzyme levels in rats
(n=6 per group).
| Parameters | Sham group | Control group | Experimental
group |
|---|
| Bile secretion
(mm3/10 min) | 92.5±12.2 |
29.8±3.8a |
62.3±8.7a,b |
| NO concentration
(µmol/l) | 18.9±6.2 | 23.9±4.1 |
57.9±8.07a,b |
| ALT (U/l) | 20.3±15.0 |
1,947.3±373.0a |
636.4±69.17a,b |
| AST (U/l) | 32.4±13.8 |
2,415.7±259.9a |
1,163.2±252.27a,b |
| LDH (U/l) | 125.3±32.5 |
3,836.3±518.3a |
2,364.6±211.67a,b |
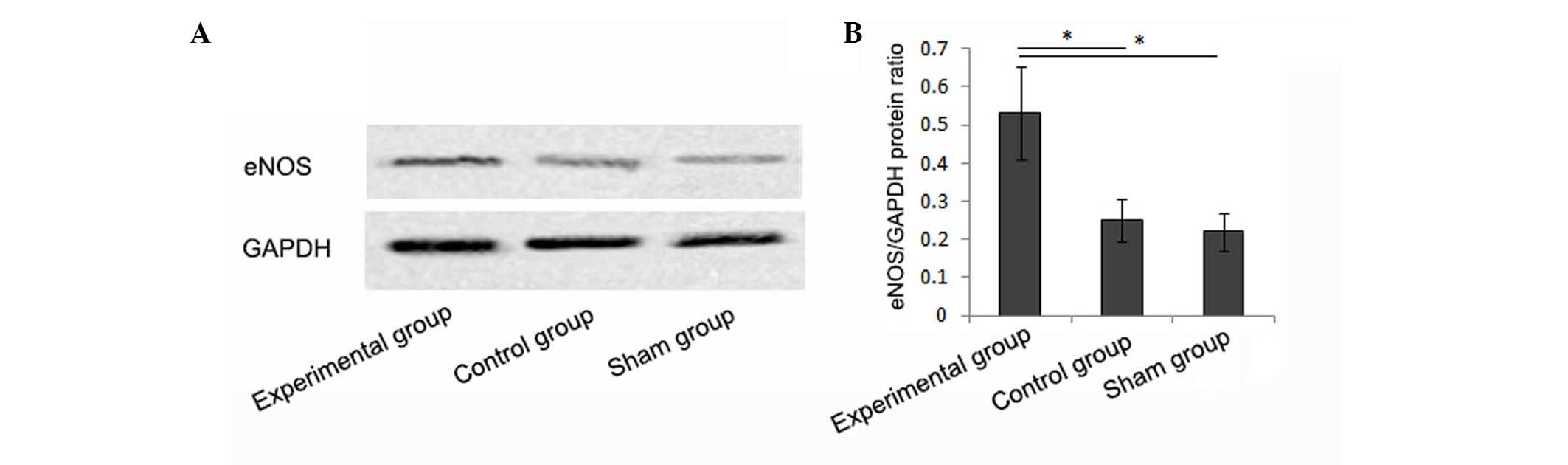
eNOS expression in liver tissues of
rats
Rats pretreated with Ad-eNOS expressed an increased
mRNA level of eNOS in liver tissues (37.8±5.5%), which was
significantly higher than levels in the control and sham groups
(16.8±6.6 and 13.2±6.2%, respectively; P<0.05). Similar
differences were also observed in the western blot analysis, which
revealed greater protein expression of eNOS in the liver tissues of
rats pretreated with Ad-eNOS (Fig. 3A
and B).
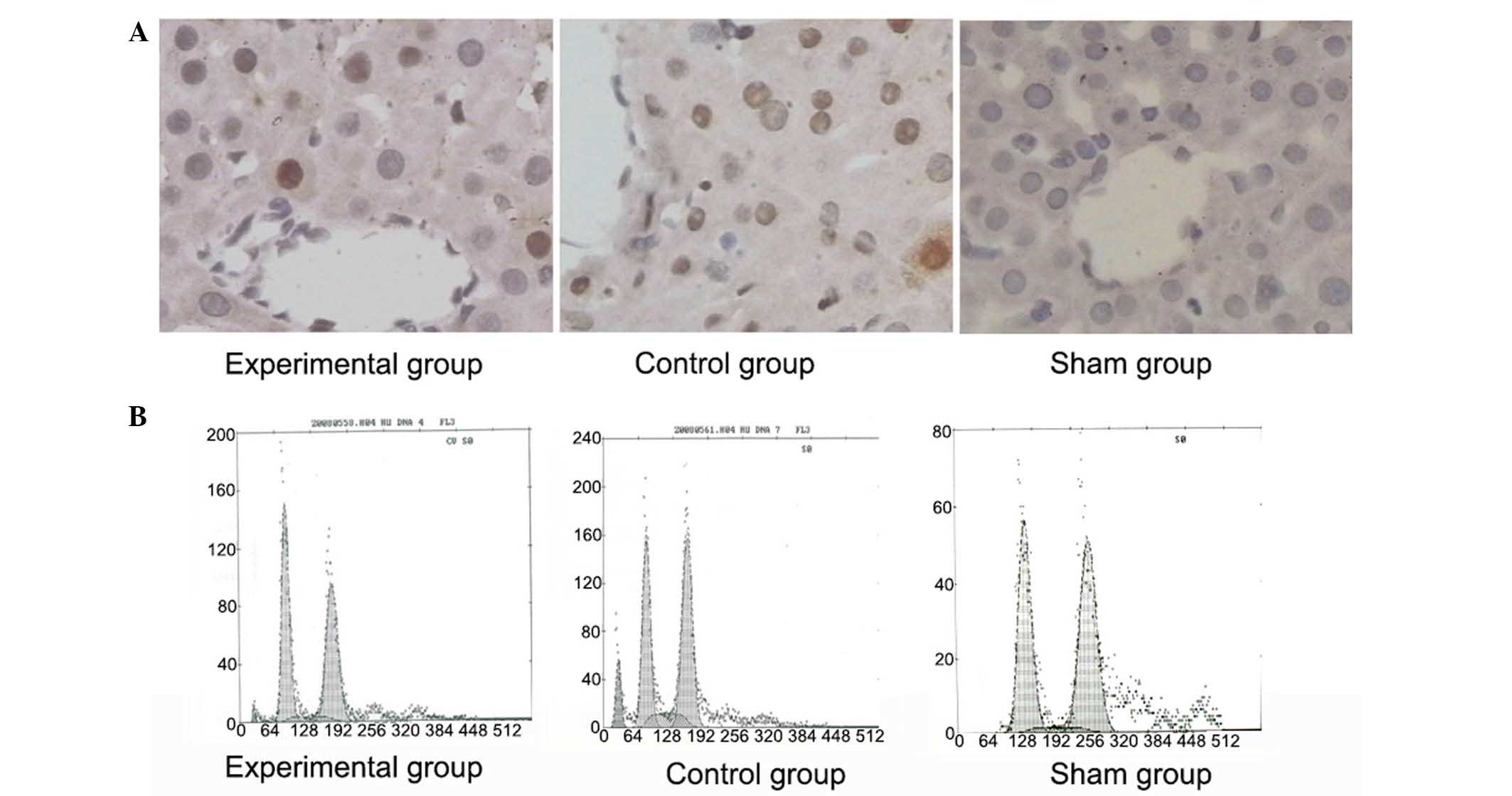
Ad-eNOS treatment inhibited apoptosis
in liver tissues after small-for-size liver transplantation
Rats in the control group displayed significantly
more TUNEL-positive apoptotic cells in liver tissues compared with
sham-operated rats (Fig. 4A).
However, pretreatment with eNOS decreased the number of
TUNEL-positive apoptotic cells in liver tissues. These results are
consistent with the results of the flow cytometry analysis, which
revealed a reduced percentage of apoptotic cells in liver tissues
of rats pretreated with eNOS (3.9±0.9 vs. 11.8±1.3% in the control
group; P<0.05), although this value remained higher than that in
the sham group (1.2±0.4%, P<0.05; Fig. 4B).
Ad-eNOS treatment restored
histological changes in liver tissues after small-for-size liver
transplantation
To assess the protective effects of eNOS on liver
tissues, histological analysis by H&E staining was performed at
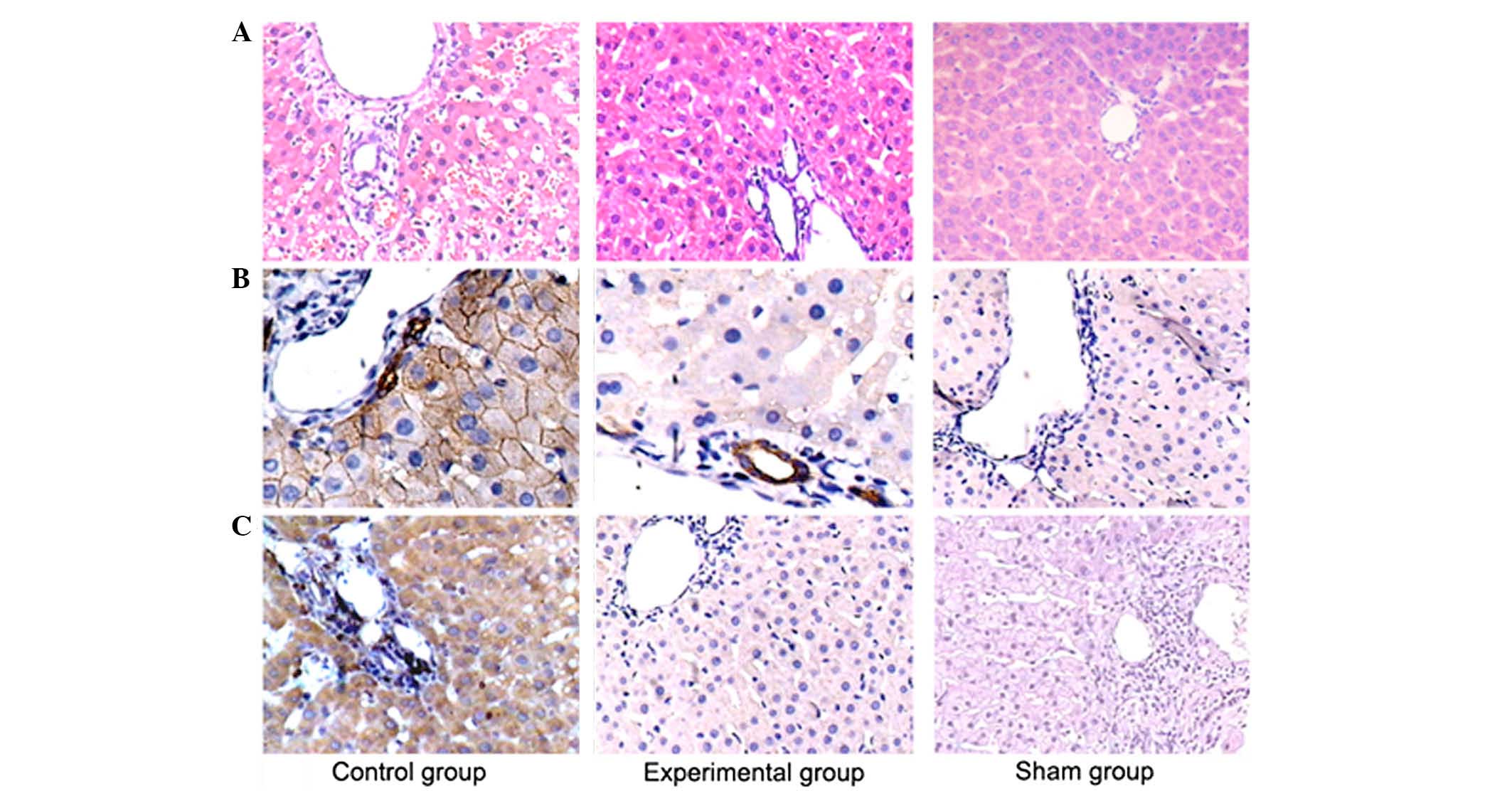
6 h after reperfusion. Control liver sections showed swelling and
redness of liver tissues, with severe endothelial cell damage and
massive inflammatory cell infiltration surrounding the periportal
region (Fig. 5A). However, there was
a significant improvement in the liver histological abnormalities
in Ad-eNOS-pretreated rats, which showed normal hepatocyte
morphology, complete vascular walls, and reduced inflammatory cell
infiltration in the periportal region.
The number of TNF-α-positive cells in liver tissues
was reduced in Ad-eNOS-pretreated rats compared with the
Ad-null-pretreated rats (control) after small-for-size liver
transplantation (Fig. 5B).
Similarly, the rats pretreated with Ad-eNOS showed significantly
fewer CD68-positive immunoreactive macrophages in vascular walls at
6 h after reperfusion than compared with the control group
(Fig. 5C).
Discussion
I/R injury, caused by cold ischemia during organ
storage and subsequent reperfusion, may inevitably occur during
small-for-size liver transplantation, resulting in delayed graft
function and decreased long-term graft survival (19). In the present study, in vivo
experimental studies showed that Ad-eNOS pretreatment significantly
increased bile production, improved abnormal transaminase levels,
diminished apoptotic liver cells and decreased hepatocellular
damage. These findings suggested that eNOS-mediated NO production
plays a crucial role in the protection of hepatocytes against I/R
injury following small-for-size liver transplantation. The
eNOS-mediated renal protective effects may associate with
downregulation of TNF-α and a reduction in macrophage activation
during the early stage of reperfusion in small-for-size liver
allografts.
Previous experiments suggests that NO functions as a
protective factor during I/R injury (20). It has been reported that endogenous
NO produced by eNOS or activation of NOS may protect liver cells
from I/R injury (21). Furthermore,
the delivery of exogenous NO during an ischemic insult has been
shown to limit the extent of reperfusion damage, suggesting a
beneficial role of NO against I/R injury following organ
transplantation (22,23). Moreover, administration of L-arginine
or FK409 (potent spontaneous NO releasers) into the hepatic
vasculature results in improved hepatic tissue blood flow, serum
liver enzyme levels, and well-preserved endothelial cells, thereby
enhancing graft survival following I/R (24–26). In
the present study, we detected an increased NO concentration in
culture supernatants of L02 cells pretreated with Ad-eNOS. In
addition, the NO level was greater in the liver tissue of rats
pretreated with Ad-eNOS as compared with the level in control rats
(P<0.05). Histological findings revealed severe endothelial cell
damage and massive inflammatory cell infiltration surrounding the
periportal region in Ad-null-pretreated rats following I/R. By
contrast, in the Ad-eNOS-pretreated rats there was a significant
improvement in histological abnormalities in the liver, with normal
hepatocyte morphology, complete vascular walls and reduced
inflammatory cell infiltration in the periportal region. Thus, we
propose that Ad-eNOS-mediated NO may be responsible for the renal
protective effect against I/R injury following small-for-size liver
transplantation. The exact mechanism by which eNOS exerts its
protective effects via NO requires further investigation.
The protective effect of eNOS against I/R injury
following organ transplantation has been investigated by directly
altering eNOS expression in several studies (26,27). It
has been reported that eNOS overexpression can lead to reduced
infarct size after cardiac I/R injury (27,28). A
study performed by Duranski et al showed that transgenic
mice with eNOS overexpression exhibit less severe I/R injury than
wild-type mice, and this hepatoprotective effect was probably
mediated through the soluble guanylyl cyclase-cGMP pathway
independent of heme oxygenase-1 (29). By contrast, liver injury is more
severe in eNOS-deficient mice subjected to liver I/R injury than in
wild-type counterparts (30). In a
murine liver transplant model from eNOS-deficient donor to
wild-type mice, eNOS-deficient grafts resulted in greater I/R
injury, increased microcirculatory disturbances and increased
macrophage infiltration (31).
However, the dual role for eNOS in hepatic I/R injury remains
controversial. An animal study by Palanisamy et al showed
that adenovirus-mediated eNOS overexpression is detrimental to the
mouse liver during I/R, leading to elevated AST and ALT levels and
significantly increased apoptosis at 24 h after reperfusion
(32). In the present study, eNOS
overexpression significantly attenuated hepatic I/R injury in
Ad-eNOS-infected mouse livers, as evidenced by the correction of
increased transaminase levels and improvement in histological signs
of liver damage following small-for-size liver transplantation. The
present results are consistent with the findings of Duranski et
al, which suggested that genetic overexpression of eNOS
protected against hepatic I/R injury in vivo following 5 h
of reperfusion (29).
Apoptosis has been identified as a key mechanism
underlying hepatic I/R injury. Reperfusion of livers after cold
ischemia during organ storage results in endothelial cell adhesion,
microcirculatory disturbances, activation of Kupffer cells, and
concomitantly, the release of ROS and proinflammatory cytokines
such as TNF-α, thereby promoting hepatocellular apoptosis after
liver transplantation (33). A
previous study found increased numbers of apoptotic and necrotic
cells in small-for-size grafts vs. whole grafts after liver
transplantation in rats (34).
Therefore, inhibition of apoptosis may provide protection for liver
grafts against I/R injury, particularly for small-for-size grafts.
In the present study, rats pretreated with Ad-eNOS displayed
significantly fewer TUNEL-positive apoptotic cells in liver tissues
in comparison with rats pretreated with Ad-null, and this result is
consistent with the findings from the flow cytometric analysis.
Thus, we speculate that overexpression of eNOS using an adenoviral
vector resulted in sustained NO production and protection of
hepatocytes from apoptosis in both in vitro and in
vivo experiments.
TNF-α is a cytokine produced by numerous cell types
in response to inflammatory stimuli (35). TNF-α serves a crucial function in
hepatocyte injury during I/R, and the mechanisms are complex, with
interactions involving ROS, NO, adhesion molecules and various
cytokines and chemokines (36). It
has been reported that proinflammatory cytokines, such as TNF-α,
interleukin (IL)-1 and IL-6, are observed in acute lung injury
following orthotopic liver transplantation at 4 h after reperfusion
(37). In the present study, a
significant reduction in the number of TNF-α-positive cells at 6 h
after reperfusion was observed in liver tissues of
Ad-eNOS-pretreated rats in comparison with control counterparts.
These results were consistent with those of a previous study
showing that ischemic preconditioning is responsible for a
protective effect on liver morphology and associated with a
decrease in the serum TNF-α level (38). In addition, early activation of
macrophages also accelerates an early immune process following
graft I/R injury in small-for-size allografts (39). In the present study, rats pretreated
with Ad-eNOS showed significantly fewer CD68-positive
immunoreactive macrophages in vascular walls, suggesting Ad-eNOS
may inhibit the inflammatory process by decreasing early activation
of macrophages in small-for-size allografts. These effects may
contribute to the eNOS-mediated hepatic protection following
I/R.
In conclusion, the present results indicate that
eNOS-derived NO production significantly attenuates hepatic I/R
injury. Thus, eNOS overexpression may constitute a promising
therapeutic approach to prevent liver I/R injury following
small-for-size liver transplantation.
Acknowledgements
The present study was supported by the Science and
Technology Development Plan of Soochow (grant no. SYSD2014012).
References
|
1
|
Li KK and Neuberger J: The management of
patients awaiting liver transplantation. Nat Rev Gastroenterol
Hepatol. 6:648–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma T, Liu H, Chen W, Xia X, Bai X, Liang
L, Zhang Y and Liang T: Implanted adipose-derived stem cells
attenuate small-for-size liver graft injury by secretion of VEGF in
rats. Am J Transplant. 12:620–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park GC, Song GW, Moon DB and Lee SG: A
review of current status of living donor liver transplantation.
Hepatobiliary Surg Nutr. 5:107–117. 2016.PubMed/NCBI
|
|
4
|
Wang HS, Ohkohchi N, Enomoto Y, Usuda M,
Miyagi S, Asakura T, Masuoka H, Aiso T, Fukushima K, Narita T, et
al: Excessive portal flow causes graft failure in extremely
small-for-size liver transplantation in pigs. World J
Gastroenterol. 11:6954–6959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamada N, Sanada Y, Hirata Y, Okada N,
Ihara Y, Sasanuma H, Urahashi T, Sakuma Y, Yasuda Y and Mizuta K:
The outcomes of pediatric living donor liver transplantation using
small-for-size grafts: Experience of a single institute. Pediatr
Surg Int. 32:363–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Du Z, Yan J, Ma D, Shi M, Zhang M,
Peng C and Li H: Mesenchymal stem cells promote liver regeneration
and prolong survival in small-for-size liver grafts: Involvement of
C-Jun N-terminal kinase, cyclin D1 and NF-κB. PLoS One.
9:e1125322014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carini R and Albano E: Recent insights on
the mechanisms of liver preconditioning. Gastroenterology.
125:1480–1491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weigand K, Brost S, Steinebrunner N,
Büchler M, Schemmer P and Müller M: Ischemia/reperfusion injury in
liver surgery and transplantation: pathophysiology. HPB Surg.
2012:1767232012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choukér A, Martignoni A, Schauer RJ, Dugas
M, Schachtner T, Kaufmann I, Setzer F, Rau HG, Löhe F, Jauch KW, et
al: Alpha-gluthathione S-transferase as an early marker of hepatic
ischemia/reperfusion injury after liver resection. World J Surg.
29:528–534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Selzner N, Rudiger H, Graf R and Clavien
PA: Protective strategies against ischemic injury of the liver.
Gastroenterology. 125:917–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iñiguez M, Dotor J, Feijoo E, Goñi S,
Prieto J, Berasain C and Avila MA: Novel pharmacologic strategies
to protect the liver from ischemia-reperfusion injury. Recent Pat
Cardiovasc Drug Discov. 3:9–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han KH, Jung JY, Chung KY, Kim H and Kim
J: Nitric oxide synthesis in the adult and developing kidney.
Electrolyte Blood Press. 4:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lundberg JO and Weitzberg E: NO-synthase
independent NO generation in mammals. Biochem Biophys Res Commun.
396:39–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukhopadhyay P, Rajesh M, Horváth B,
Bátkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L,
et al: Cannabidiol protects against hepatic ischemia/reperfusion
injury by attenuating inflammatory signaling and response,
oxidative/nitrative stress, and cell death. Free Radic Biol Med.
50:1368–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siriussawakul A, Zaky A and Lang JD: Role
of nitric oxide in hepatic ischemia-reperfusion injury. World J
Gastroenterol. 16:6079–6086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamada N and Calne RY: A surgical
experience with five hundred thirty liver transplants in the rat.
Surgery. 93:64–69. 1983.PubMed/NCBI
|
|
18
|
Tan F, Chen Z, Zhao Y, Liang T, Li J and
Wei J: Novel technique for suprahepatic vena cava reconstruction in
rat orthotopic liver transplantation. Microsurgery. 25:556–560.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gueler F, Gwinner W, Schwarz A and Haller
H: Long-term effects of acute ischemia and reperfusion injury.
Kidney Int. 66:523–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimamura T, Zhu Y, Zhang S, Jin MB,
Ishizaki N, Urakami A, Totsuka E, Kishida A, Lee R, Subbotin V, et
al: Protective role of nitric oxide in ischemia and reperfusion
injury of the liver. J Am Coll Surg. 188:43–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grossini E, Pollesello P, Bellofatto K,
Sigaudo L, Farruggio S, Origlia V, Mombello C, Mary DA, Valente G
and Vacca G: Protective effects elicited by levosimendan against
liver ischemia/reperfusion injury in anesthetized rats. Liver
Transpl. 20:361–375. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phillips L, Toledo AH, Lopez-Neblina F,
Anaya-Prado R and Toledo-Pereyra LH: Nitric oxide mechanism of
protection in ischemia and reperfusion injury. J Invest Surg.
22:46–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lang JD Jr, Teng X, Chumley P, Crawford
JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, et
al: Inhaled NO accelerates restoration of liver function in adults
following orthotopic liver transplantation. J Clin Invest.
117:2583–2591. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aiba M, Takeyoshi I, Ohwada S, Kawashima
Y, Iwanami K, Sunose Y, Yamada T, Tsutsumi H, Matsumoto K and
Morishita Y: Novel nitric oxide donor (FK409) ameliorates liver
damage during extended liver resection with warm ischemia in dogs.
J Am Coll Surg. 193:264–271. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li SQ and Liang LJ: Protective mechanism
of L-arginine against liver ischemic-reperfusion injury in rats.
Hepatobiliary Pancreat Dis Int. 2:549–552. 2003.PubMed/NCBI
|
|
26
|
Trocha M, Merwid-Lad A, Szuba A, Chlebda
E, Pieśniewska M, Sozański T and Szelag A: Effect of simvastatin on
nitric oxide synthases (eNOS, iNOS) and arginine and its
derivatives (ADMA, SDMA) in ischemia/reperfusion injury in rat
liver. Pharmacol Rep. 62:343–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones SP, Greer JJ, Kakkar AK, Ware PD,
Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S,
Yokoyama M and Lefer DJ: Endothelial nitric oxide synthase
overexpression attenuates myocardial reperfusion injury. Am J
Physiol Heart Circ Physiol. 286:H276–H282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghavami S, Hashemi M, Kadkhoda K, Alavian
SM, Bay GH and Los M: Apoptosis in liver diseases - detection and
therapeutic applications. Med Sci Monit. 11:RA337–RA345.
2005.PubMed/NCBI
|
|
29
|
Duranski MR, Elrod JW, Calvert JW, Bryan
NS, Feelisch M and Lefer DJ: Genetic overexpression of eNOS
attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart
Circ Physiol. 291:H2980–H2986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abu-Amara M, Yang SY, Quaglia A, Rowley P,
Fuller B, Seifalian A and Davidson B: Role of endothelial nitric
oxide synthase in remote ischemic preconditioning of the mouse
liver. Liver Transpl. 17:610–619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Theruvath TP, Zhong Z, Currin RT, Ramshesh
VK and Lemasters JJ: Endothelial nitric oxide synthase protects
transplanted mouse livers against storage/reperfusion injury: Role
of vasodilatory and innate immunity pathways. Transplant Proc.
38:3351–3357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palanisamy AP, Cheng G, Sutter AG, Liu J,
Lewin DN, Chao J and Chavin K: Adenovirus-mediated eNOS expression
augments liver injury after ischemia/reperfusion in mice. PLoS One.
9:e933042014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mendes-Braz M, Elias-Miró M,
Jimenez-Castro MB, Casillas-Ramírez A, Ramalho FS and Peralta C:
The current state of knowledge of hepatic ischemia-reperfusion
injury based on its study in experimental models. J Biomed
Biotechnol. 2012:2986572012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang TB, Man K, Kin-Wah Lee T, Hong-Teng
Tsui S, Lo CM, Xu X, Zheng SS, Fan ST and Wong J: Distinct
intragraft response pattern in relation to graft size in liver
transplantation. Transplantation. 75:673–678. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perry BC, Soltys D, Toledo AH and
Toledo-Pereyra LH: Tumor necrosis factor-α in liver
ischemia/reperfusion injury. J Invest Surg. 24:178–188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perry BC, Soltys D, Toledo AH and
Toledo-Pereyra LH: Tumor necrosis factor-α in liver
ischemia/reperfusion injury. J Invest Surg. 24:178–188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang A, Chi X, Luo G, Hei Z, Xia H, Luo
C, Wang Y, Mao X and Xia Z: Mast cell stabilization alleviates
acute lung injury after orthotopic autologous liver transplantation
in rats by downregulating inflammation. PLoS One. 8:e752622013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Czigány Z, Turóczi Z, Ónody P, Harsányi L,
Lotz G, Hegedüs V and Szijártó A: Remote ischemic perconditioning
protects the liver from ischemia-reperfusion injury. J Surg Res.
185:605–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang ZF, Ho DW, Chu AC, Wang YQ and Fan
ST: Linking inflammation to acute rejection in small-for-size liver
allografts: The potential role of early macrophage activation. Am J
Transplant. 4:196–209. 2004. View Article : Google Scholar : PubMed/NCBI
|