Introduction
Inflammatory bowel disease (IBD) commonly describes
two types of chronic intestinal disorders of the gastrointestinal
tract: Ulcerative colitis (UC) and Crohn's disease (CD) (1). UC is characterized by diffuse
inflammation of the mucosa of the colon and rectum, and has a
relatively high prevalence of 5.3–63.6 per 100,000 individuals in
developing areas and 37.5–238 per 100,000 individuals in developed
areas (2). CD is a disease that can
occur in any part of the gastrointestinal tract, and has a growing
incidence in the Asian countries (3). IBD patients are at high risk of
acquiring cytomegalovirus (CMV) infection, as they are frequently
treated with immunosuppressive drugs (4). These agents have moderate effects on
maintaining corticosteroid-induced remission, while common effects
on relapse (5). Although the
etiology of IBD and the impact of antiviral treatment on the course
of IBD remain poorly understood, various studies have suggested
that viral infections, particularly infection with CMV, are
associated with the onset and aggravation of IBD, thus causing a
poor prognosis (6,7).
CMV belongs to the herpesviridae family of viruses
and contains a double-stranded DNA, with its effect ranging from
asymptomatic infection in immunocompetent hosts to severe end-organ
damage in patients with impaired cellular immunity (8). CMV often results in primary infection
in humans, and later persists in a latent stage for life (9). Accumulating evidence suggested that CMV
disease is more common in patients with severe IBD or
steroid-refractory patients (7). CMV
infection always occurs in immunocompromised hosts, and the
reported prevalence of CMV infection in IBD patients is variable as
the bowel is the largest immunological organ of the body (10). It is estimated that CMV infection in
IBD patients is within the range of 33–36% in patients with
steroid-refractory UC and 21–34% in patients suffering from severe
UC (11). Colonic CMV reactivation
is considered to be an exacerbating factor in patients with UC and
those refractory to immunosuppressive therapies due to the poor
prognosis of UC patients with concomitant CMV infection (12). Previous studies have demonstrated
that local intestinal inflammation and additional immunosuppressive
therapies, such as corticosteroid administration, may induce
colonic CMV reactivation in IBD patients (12,13).
However, another study presented conflicting results on the
association of CMV reactivation with the occurrence or severity of
IBD exacerbation (1). Therefore, the
aim of the present study was to investigate the effect of CMV
infection on the prognosis of IBD using data collected from
previous relevant studies to perform a meta-analysis.
Materials and methods
Literature search
The literature was comprehensively screened and
eligible studies were collected to examine the effects of CMV
infection on the prognosis of IBD. The following computerized
databases were screened: Springerlink (link.springer.com), PubMed (www.ncbi.nlm.nih.gov/pubmed), Web of Science
(wok.mimas.ac.uk), Wiley Online Library
(onlinelibrary.wiley.com), Chinese
Biomedical Database (http://www.sinomed.ac.cn/zh/), China National
Knowledge Infrastructure (www.cnki.net),
Wanfang database (www.wanfangdata.com) and the VIP database (www.cqvip.com). Additional pertinent studies were
obtained by quadratic search. The search included studies published
until September 2014. A combination of keywords and free words was
applied in the process of collecting literature with a highly
efficient and sensitive searching strategy. The search involved a
CMV keyword (‘cytomegalovirus’ or ‘cytomegaloviruses’ or ‘salivary
gland viruses’ or ‘viruses, salivary gland’ or ‘herpesvirus 5,
human’) and an IBM keyword (‘inflammatory bowel diseases’ or
‘inflammatory bowel disease’ or ‘bowel diseases,
inflammatory’).
Inclusion and exclusion criteria
Eligible studies were selected according to the
following inclusion criteria: i) Clinical cohort studies; ii)
subjects in the enrolled studies had a confirmed diagnosis of IBD
based on clinical, radiologic, endoscopic and histologic parameters
(14); iii) the detection method
used was polymerase chain reaction or enzyme-linked immunosorbent
assay; iv) the outcome index included the progress of CMV-positive
and CMV-negative patients with IBD, as well as the effects of
glucocorticoid treatment, colectomy rate, prevalence of severe IBD
and the disease extent; and v) the language of selected studies was
restricted to Chinese and English. The corresponding exclusion
criteria were: i) Studies containing summary and abstracts only;
ii) studies with insufficient information; iii) duplicated
publications; and iv) unpublished studies.
Data extraction and quality
assessment
The data from eligible studies were extracted by two
separated investigators. Relevant information was collected as
follows: First author, publication year, study design, country of
study and patient ethnicity, patient gender and age, effects of
glucocorticoid treatment, disease duration, colectomy rate,
severity degree of diseases and disease extent. Subsequently, the
two investigators independently assessed the methodological quality
of the included cohort trials using the Newcastle-Ottawa Scale
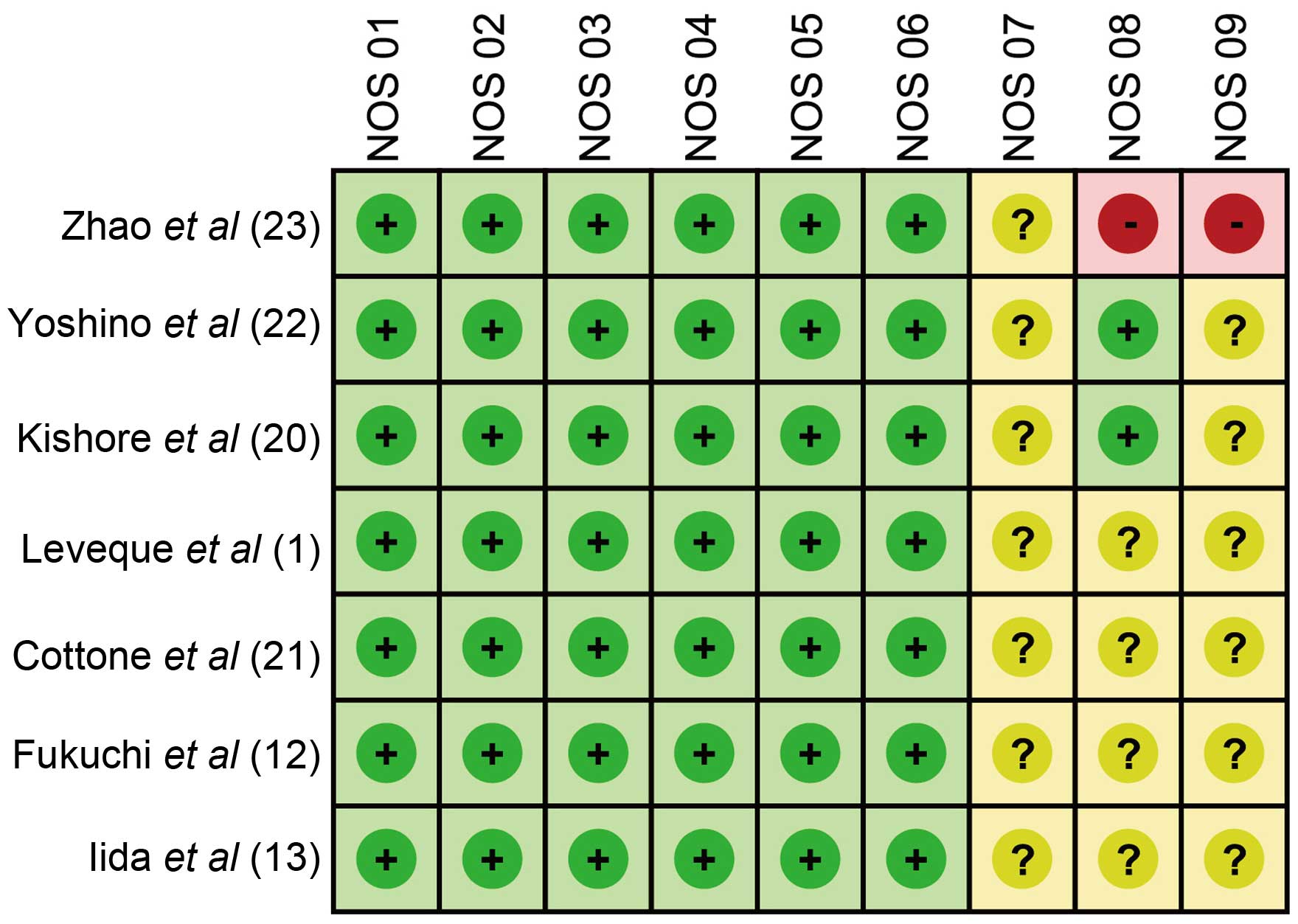
(NOS) criteria (15). The NOS
criteria associated with the selection of the cohort were as
follows: Representativeness of the exposed cohort (NOS1); selection
of the non-exposed cohort (NOS2); ascertainment of exposure (NOS3);
and demonstration that an outcome of interest was not present at
the beginning of the study (NOS4). In addition, NOS criteria
involving the comparability of the cohorts were as follows: The
study was selected and analyzed according to the most important
factor (NOS5); and the study controlled other confounding factors
(NOS6). Finally, the following NOS criteria involved the assessment
of the outcome: Sufficiently long follow-up for outcome to occur
(NOR8); and adequacy of cohort follow-up (NOR9). A third
investigator was prepared to resolve any disagreement on the
inclusion of a single study.
Statistical analysis
Stata 12.0 software (Stata Corp., College Station,
TX, USA) was used for statistical analysis. The fixed effects model
or random effects model was adopted for the calculation of the
relative risk (RR) and standardized mean difference (SMD), along
with the 95% confidence interval (95% CI). These statistical models
aimed to evaluate the prognosis of patients with IBD in association
with the effects of corticosteroid therapy, colectomy ratio,
prevalence of severe IBD and disease extent. Z-test was employed to
detect the significance of the pooled effect size (15,16). In
addition, Cochran's Q-test (in which differences with P<0.05
were considered as statistically significant) and I2
tests were employed to quantify heterogeneity among the parameters
of the included trials (17). When
P<0.05 or I2>50% were obtained, which indicated
heterogeneous results, the random effects model was conducted;
otherwise, the fixed effects model was implemented. In order to
evaluate the influence of single study on the overall estimate, a
sensitivity analysis was employed according to the Cochrane
Handbook for Systematic Reviews of Interventions (http://handbook.cochrane.org/). Furthermore, for the
purpose of ensuring the reliability of the results, publication
bias was examined by funnel plots and Egger's linear regression
test (with P<0.05 indicating a statistically significant
difference) (18,19).
Results
Baseline characteristics of eligible
studies
Initially, a total of 195 studies (28 in Chinese and
167 in English) were identified from the literature screening,
including 193 from electronic search and 2 from manual search.
Subsequently, 142 articles were excluded due to not meeting the
inclusion criteria following review of the title and abstract, and
46 full-text articles remained. Ultimately, 7 studies (6 in English
and 1 in Chinese) satisfied the inclusion criteria after
elimination of 36 off-topic studies and 3 studies with insufficient
information. The enrolled studies (1,12,13,20–23)
were published between 2001 and 2013, and provided complete
clinical data for 374 patients with IBD. Among these 7 studies, 5
studies included Asian patients and 2 studies included Caucasian
patients. One was conducted in China, 3 were conducted in Japan, 1
was conducted in India, 1 was conducted in France and 1 was
conducted in Italy. Furthermore, the 7 studies contained 374
patients with IBD; of these, 104 cases were CMV-positive and 270
cases were CMV-negative. The baseline characteristics and NOS
scores for the 7 eligible studies are summarized in Table I and Fig.
1, respectively.
 | Table I.Baseline characteristics for the
eligible studies. |
Table I.
Baseline characteristics for the
eligible studies.
|
| Patients | Gender (M/F) | Age (years) |
|
|---|
|
|
|
|
|
|
|---|
| First author | Year | Country | Ethnicity | Study language | N | CMV+ | CMV- | CMV+ | CMV- | CMV+ | CMV- | Ref. |
|---|
| Zhao | 2011 | China | Asian | Chinese | 76 | 21 | 55 | 13/8 | 32/23 | 46.2±19 | 47.6±19.2 | (23) |
| Yoshino | 2007 | Japan | Asian | English | 30 | 17 | 13 | 14/16 | – | 40.8±17.6 | – | (22) |
| Kishore | 2004 | India | Asian | English | 63 | 10 | 53 | 2/8 | 30/23 | 39 | 36 | (20) |
| Leveque | 2010 | France | Caucasian | English | 53 | 7 | 46 | 23/30 | – | 38.5 | – |
(1) |
| Cottone | 2001 | Italy | Caucasian | English | 19 | 7 | 12 | – | – | 48.5 | 35.8 | (21) |
| Fukuchi | 2013 | Japan | Asian | English | 51 | 15 | 36 | 7/8 | 20/16 | 42.9±3.8 | 36.2±2.4 | (12) |
| Iida | 2013 | Japan | Asian | English | 82 | 27 | 55 | 15/12 | 32/23 | 43.2 | 38.9 | (13) |
Association of disease duration with
CMV infection
Of the studies included in the meta-analysis, 3
reported the disease duration of CMV-positive and CMV-negative
patients (12,20,23).
Heterogeneity was observed (I2, 95.8%;
Ph<0.001) and the random effect model was applied to
compare the observations of the studies. According to the
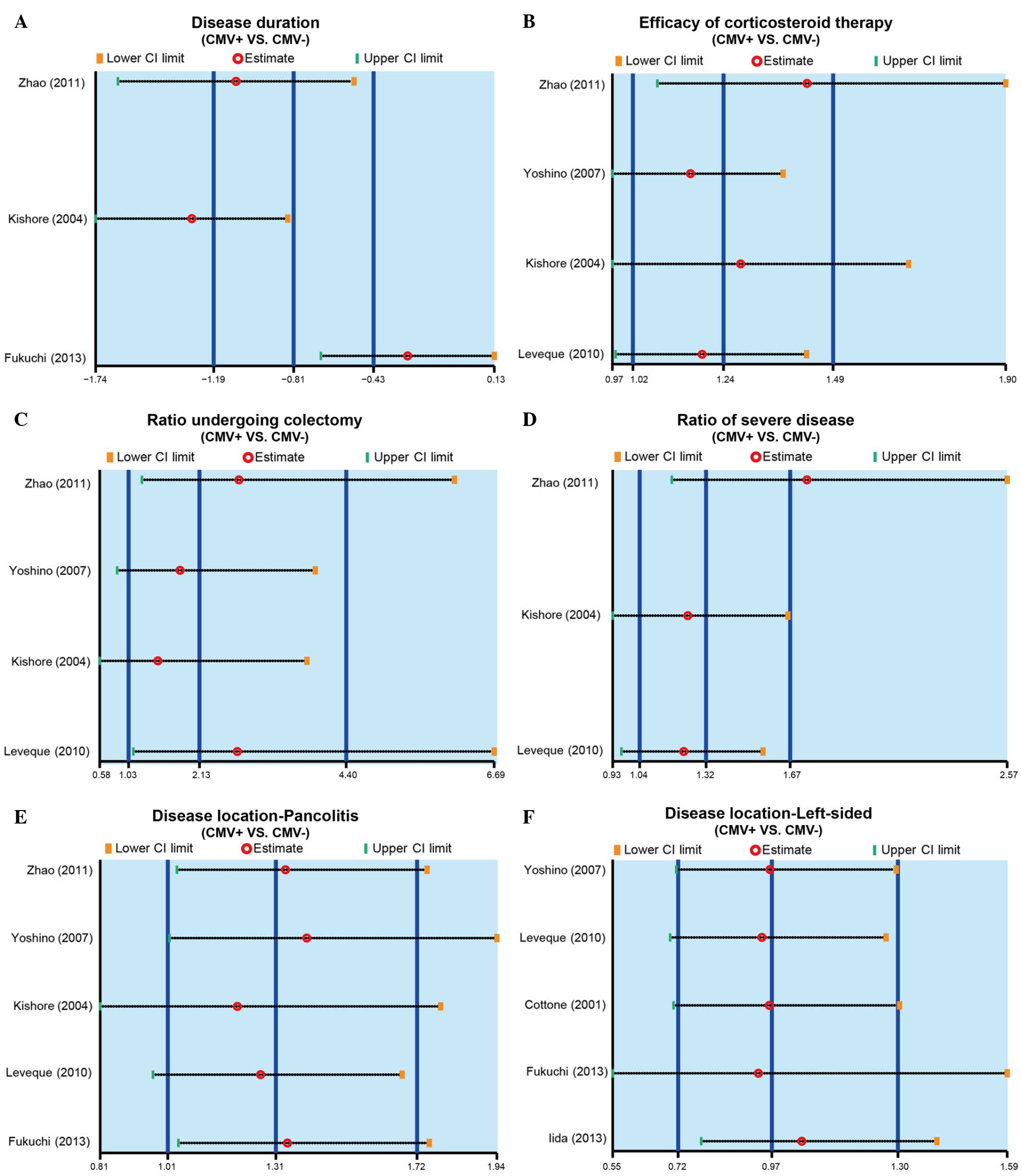
meta-analysis results, the disease duration of CMV-positive
patients was significantly reduced when compared with that of
CMV-negative patients (SMD, −0.81; 95% CI, −1.19 to −0.43;
P<0.001), and the forest plot of this analysis is shown in
Fig. 2A. The results show that CMV
infection may be associated with the disease duration of IBD.
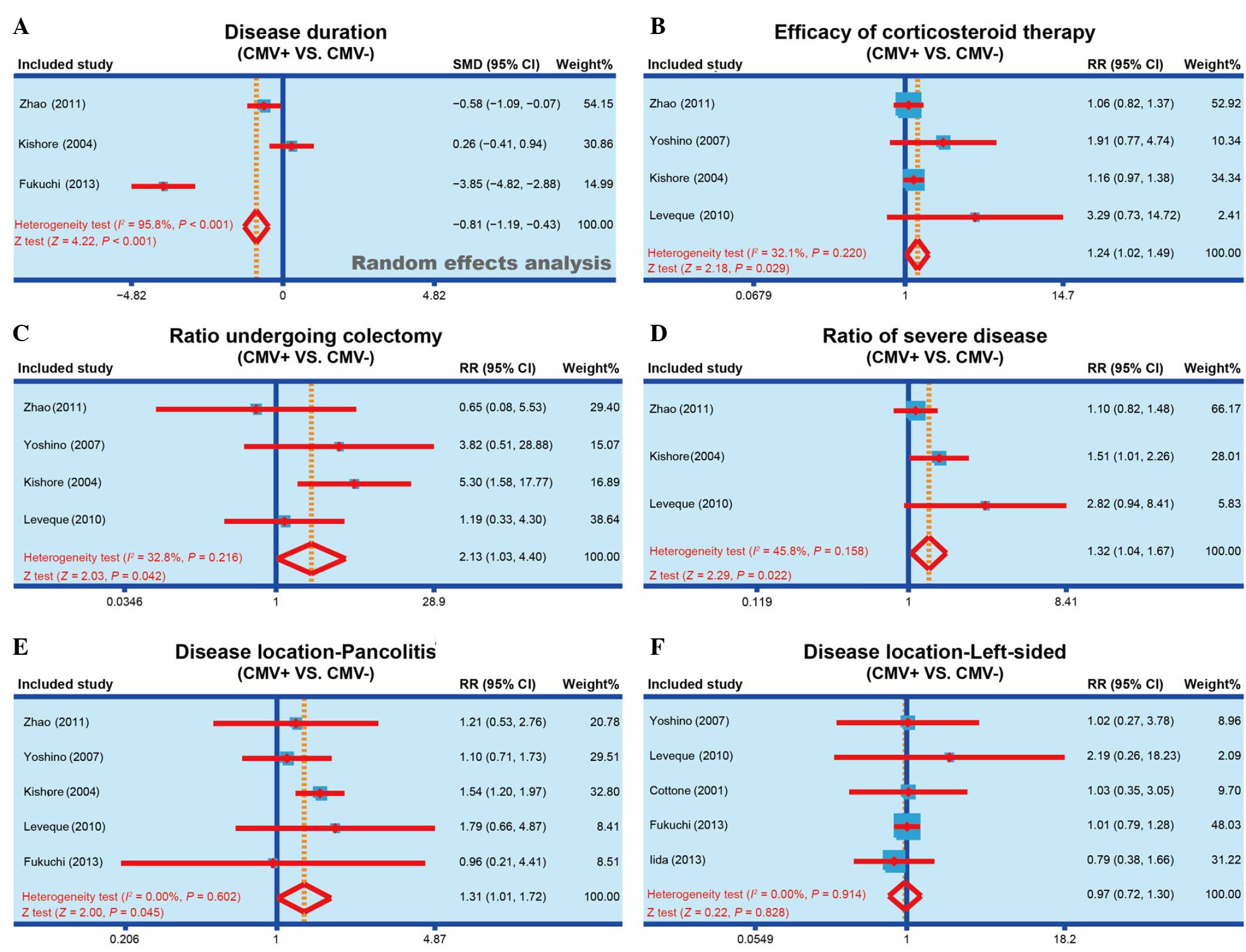 | Figure 2.Forest plots obtained by random
effects model analysis, performed to compare the (A) disease
duration of IBD, (B) efficacy of corticosteroid therapy, (C)
colectomy rate, (D) incidence of severe IBD, (E) incidence of
pancolitis and (F) incidence of life-sided IBD, between the
CMV-positive and CMV-negative patients. IBD, inflammatory bowel
disease; CMV, cytomegalovirus; SMD, standardized mean difference;
RR, relative risk; 95% CI, 95% confidence interval. |
Association of corticosteroid therapy
efficacy with CMV infection
A total of 4 studies reported the efficacy of
corticosteroid therapy in both CMV-positive and CMV-negative
patients (1,20,22,23). No
heterogeneity was observed among the 4 studies (I2,
32.1%; Ph=0.220), and thus the fixed effect model was applied. As
shown in Fig. 2B, the results of the
present meta-analysis demonstrated that corticosteroid therapy had
a markedly improved efficacy in CMV-positive patients compared with
their efficacy in CMV-negative patients (RR, 1.24; 95% CI,
1.02–1.49; P=0.029). The results show that CMV infection may be
associated with the corticosteroid therapy efficacy of IBD.
Association of ratio of patients
undergoing colectomy with CMV infection
Out of the 7 eligible studies, 4 studies reported
the colectomy rate in both CMV-positive and CMV-negative patients
(1,20,22,23). No
heterogeneity was observed (I2, 32.8%;
Ph=0.216) and the fixed effect model was applied to
examine the association of colectomy rate with CMV infection. As
shown in Fig. 2C, the findings of
the present study suggested that a significantly higher rate of
CMV-positive patients received colectomy, when compared with
CMV-negative patients (RR, 2.13; 95% CI, 1.03–4.40; P=0.042). The
results show that CMV infection may be associated with the
colectomy rate in IBD patients.
Association of severe IBD incidence
with CMV infection
In total, 3 studies reported the incidence of severe
IBD in both CMV-positive and CMV-negative patients (1,20,23). No
heterogeneity was observed (I2, 45.8%;
Ph=0.158) and the fixed effect model was used to perform
meta-analysis. The results identified that the incidence of severe
IBD in CMV-positive patients was significantly higher compared with
that in CMV-negative patients (RR, 1.32; 95% CI, 1.04–1.67;
P=0.022; Fig. 2D). The results show
that the CMV infection may be associated with the incidence of
severe IBD.
Association of disease location with
CMV infection
The association of the area to which IBD extended
with CMV infection was also investigated. In total 5 of the
eligible studies reported the presence of pancolitis (1,12,20,22,23)
and 5 studies reported left-sided IBD (1,12,13,21,22)
in both CMV-positive and CMV-negative patients. No heterogeneity
was observed (I2<0.01%; Ph=0.602) and the
fixed effect model was applied for meta-analysis. Considering the
IBD onset area, CMV-positive patients were found to present
significantly higher susceptibility to pancolitis compared with the
CMV-negative patients (RR, 1.31; 95% CI, 1.01–1.72; P=0.045;
Fig. 2E). By contrast, no
significant difference was identified in the susceptibility of
CMV-positive or CMV-negative patients to the incidence of
left-sided IBD (RR, 0.97; 95% CI, 0.72–1.30; P=0.828; Fig. 2F). The results show that CMV
infection may be associated with the disease location of IBD.
Sensitivity analysis and publication
bias investigation
The results of sensitivity analysis demonstrated
that no single study was able to impact the overall estimate of the
SMD value of disease duration in the CMV-positive or -negative
patients (Fig. 3A). In addition, the
RR value of efficacy of corticosteroid therapy, colectomy rate, the
incidence of severe IBD and disease location were also not affected
by a single study (Fig. 3B-F).
Furthermore, publication bias was investigated using funnel plots
and Egger's linear regression. As shown in Fig. 4, no evident asymmetry was observed in
the shapes of the funnel plots, which appeared to be symmetric,
while the Egger's regression test suggested the absence of
publication bias. These results suggested that no significant
publication bias was detected in the present meta-analysis
(P>0.05).
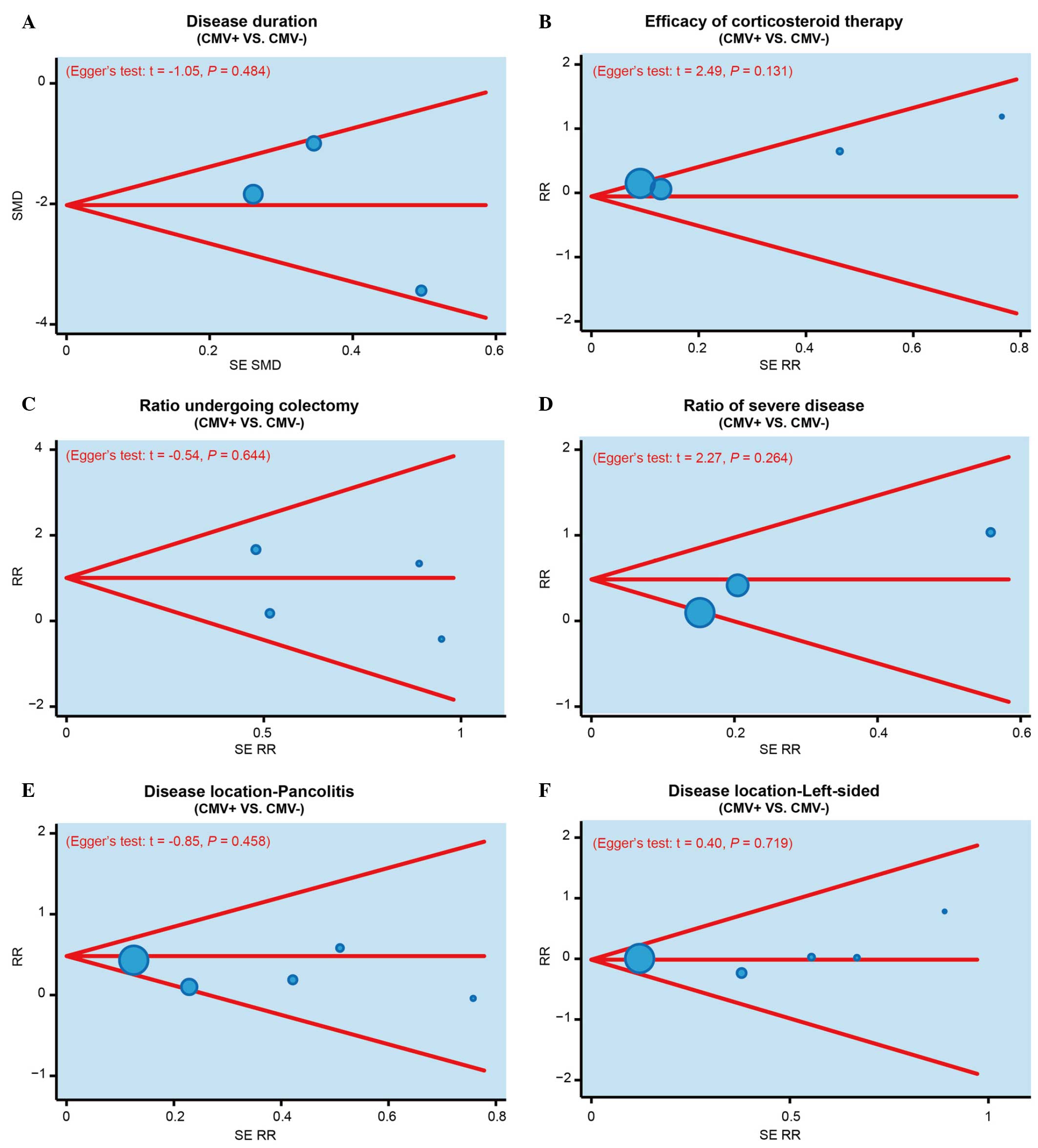 | Figure 4.Funnel plots and Egger's linear
regression tests were performed to investigate the publication bias
in the present meta-analysis, based on the (A) disease duration of
IBD, (B) efficacy of corticosteroid therapy, (C) colectomy rate,
(D) incidence of severe IBD, (E) incidence of pancolitis (disease
location), and (F) the incidence of left-sided IBD (disease
location), in CMV-positive and CMV-negative patients. IBD,
inflammatory bowel disease; CMV, cytomegalovirus; SMD, standardized
mean difference; SE RR, standard error of relative risk. |
Discussion
In the present study, the data of 374 patients
suffering from deterioration of IBD were analyzed retrospectively.
According to the results, the current study suggests that CMV
infection may be an important biomarker in determining the
prognosis of IBD. The human response to CMV infection is complex
and encompasses multiple aspects of the human immune system
(24). Although the role of CMV in
active IBD has been controversial, early studies have highlighted
the association of CMV infection with severe active IBD and high
doses of immunosuppressive medication (10,25).
The findings of the present study demonstrated that
CMV infection is potentially associated with the disease duration,
efficacy of corticosteroid therapy, colectomy rate, the incidence
of severe IBD and the disease location in patients with IBD. CMV
may present in immunosuppressed patients due to reactivation of a
latent infection, and it has a detrimental effect on the disease
severity and outcome of IBD patients (26). Increased CMV reactivity has been
demonstrated to be associated with active IBD disease and longer
disease duration (11). Due to the
association of CMV with severe steroid-refractory IBD, CMV can be
involved in flares of IBD by causing CMV colitis directly or by
exacerbating underlying IBD (10).
It has been observed that the prevalence of CMV infection in active
UC patients is lower than that previously reported in patients with
acute severe colitis and steroid-refractory colitis, suggesting
that the CMV infection is correlated with the incidence of severe
IBD (27). It is suggested that
patients with steroid-refractory or steroid-dependent IBD should be
screened for CMV infection prior to increasing the dose and number
of immunosuppressive drugs administrated (27,28).
The findings of Leveque et al (1) also demonstrates a significant
association between the treatment with high-dose systemic
corticosteroids and the detection of CMV in IBD patients.
Furthermore, CMV is present in its latent form in the majority of
healthy subjects, so it is important to know the disease location
for better treatment of IBD patients. In the present meta-analysis,
disease location data demonstrated that the prevalence of
pancolitis may be affected by the presence of CMV infection in the
IBD patient. In general, CMV infection results to severe IBD or
recovery of IBD, and thus it may be a useful biomarker in the
determination of prognosis in IBD patients.
However, certain limitations of the present
meta-analysis should be noted. Firstly, due to the limited number
of IBD patients in the enrolled studies, the sample size in each
subgroup analysis was rather small, which may decrease the accuracy
of this meta-analysis. In addition, all included studies were
published in English or Chinese in the various electronic databases
searched, which may bias the results, since other languages or
unpublished studies were not considered.
In conclusion, the present meta-analysis revealed
that CMV infection is strongly correlated with IBD prognosis. The
findings demonstrated that CMV infection may be associated with the
disease duration of IBD, efficacy of corticosteroid therapy,
colectomy rate, the incidence of severe IBD and disease location.
Therefore, CMV infection is suggested to be a biomarker of IBD
prognosis, and determining the presence of CMV infection in IBD
patients may assist in the selection of an efficient treatment
strategy.
Acknowledgements
The authors would like to thank their colleagues for
their valuable advice and comments on the present study.
References
|
1
|
Leveque N, Brixi-Benmansour H, Reig T,
Renois F, Talmud D, Brodard V, Coste JF, De Champs C, Andréoletti L
and Diebold MD: Low frequency of cytomegalovirus infection during
exacerbations of inflammatory bowel diseases. J Med Virol.
82:1694–1700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Da Silva BC, Lyra AC, Rocha R and Santana
GO: Epidemiology, demographic characteristics and prognostic
predictors of ulcerative colitis. World J Gastroenterol.
20:9458–9467. 2014.PubMed/NCBI
|
|
3
|
Lee KM and Lee JM: Crohn's disease in
Korea: Past, present, and future. Korean J Intern Med. 29:558–570.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D'Ovidio V, Vernia P, Gentile G,
Capobianchi A, Marcheggiano A, Viscido A, Martino P and Caprilli R:
Cytomegalovirus infection in inflammatory bowel disease patients
undergoing anti-TNFalpha therapy. J Clin Virol. 43:180–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lichtenstein GR, Rutgeerts P, Sandborn WJ,
Sands BE, Diamond RH, Blank M, Montello J, Tang L, Cornillie F and
Colombel JF: A pooled analysis of infections, malignancy, and
mortality in infliximab- and immunomodulator-treated adult patients
with inflammatory bowel disease. Am J Gastroenterol. 107:1051–1063.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delvincourt M, Lopez A, Pillet S, Bourrier
A, Seksik P, Cosnes J, Carrat F, Gozlan J, Beaugerie L, Roblin X,
et al: The impact of cytomegalovirus reactivation and its treatment
on the course of inflammatory bowel disease. Aliment Pharmacol
Ther. 39:712–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones A, McCurdy JD, Loftus EV Jr,
Bruining DH, Enders FT, Killian JM and Smyrk TC: Effects of
antiviral therapy for patients with inflammatory bowel disease and
a positive intestinal biopsy for cytomegalovirus. Clin
Gastroenterol Hepatol. 13:949–955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCurdy JD, Jones A, Enders FT, et al: A
model for identifying cytomegalovirus in patients with inflammatory
bowel disease. Clin Gastroenterol Hepatol. 13:131–137; quiz e7.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garrido E, Carrera E, Manzano R and
Lopez-Sanroman A: Clinical significance of cytomegalovirus
infection in patients with inflammatory bowel disease. World J
Gastroenterol. 19:17–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JJ, Simpson N, Klipfel N, Debose R,
Barr N and Laine L: Cytomegalovirus infection in patients with
active inflammatory bowel disease. Dig Dis Sci. 55:1059–1065. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nowacki TM, Bettenworth D, Ross M,
Heidemann J, Lehmann PV and Lügering A: Cytomegalovirus
(CMV)-specific perforin and granzyme B ELISPOT assays detect
reactivation of CMV infection in inflammatory bowel disease. Cells.
1:35–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukuchi T, Nakase H, Matsuura M, Yoshino
T, Toyonaga T, Ohmori K, Ubukata S, Ueda A, Eguchi T, Yamashita H,
et al: Effect of intensive granulocyte and monocyte adsorptive
apheresis in patients with ulcerative colitis positive for
cytomegalovirus. J Crohns Colitis. 7:803–811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iida T, Ikeya K, Watanabe F, Abe J,
Maruyama Y, Ohata A, Teruyuki S, Sugimoto K and Hanai H: Looking
for endoscopic features of cytomegalovirus colitis: A study of 187
patients with active ulcerative colitis, positive and negative for
cytomegalovirus. Inflamm Bowel Dis. 19:1156–1163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dubinsky M and Braun J: Diagnostic and
prognostic microbial biomarkers in inflammatory bowel diseases.
Gastroenterology. 149:1265.e3–1274.e3. 2015. View Article : Google Scholar
|
|
15
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H, Manning AK and Dupuis J: A method
of moments estimator for random effect multivariate meta-analysis.
Biometrics. 68:1278–1284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zintzaras E and Ioannidis JP: HEGESMA:
Genome search meta-analysis and heterogeneity testing.
Bioinformatics. 21:3672–3673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song F and Gilbody S: Bias in
meta-analysis detected by a simple, graphical test. Increase in
studies of publication bias coincided with increasing use of
meta-analysis. BMJ. 316:4711998.PubMed/NCBI
|
|
19
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kishore J, Ghoshal U, Ghoshal UC,
Krishnani N, Kumar S, Singh M and Ayyagari A: Infection with
cytomegalovirus in patients with inflammatory bowel disease:
Prevalence, clinical significance and outcome. J Med Microbiol.
53:1155–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cottone M, Pietrosi G, Martorana G, Casà
A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A and Pagliaro
L: Prevalence of cytomegalovirus infection in severe refractory
ulcerative and Crohn's colitis. Am J Gastroenterol. 96:773–775.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshino T, Nakase H, Ueno S, Uza N, Inoue
S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S, et al:
Usefulness of quantitative real-time PCR assay for early detection
of cytomegalovirus infection in patients with ulcerative colitis
refractory to immunosuppressive therapies. Inflamm Bowel Dis.
13:1516–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao K, Li ZM, Zuo YN, Xie ZX, Li LK,
Zhang B and Peng D: Correlation of cytomegalovirus infection in the
intestine of patients with inflammatory bowel disease. Zhong Guo Yi
Yao Dao Kan. 13:1863–1865. 2011.(In Chinese).
|
|
24
|
Miller-Kittrell M and Sparer TE: Feeling
manipulated: Cytomegalovirus immune manipulation. Virol J. 6:42009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pillet S, Pozzetto B, Jarlot C, Paul S and
Roblin X: Management of cytomegalovirus infection in inflammatory
bowel diseases. Dig Liver Dis. 44:541–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maconi G, Lombardini M, Furfaro F, Bezzio
C, Zerbi P and Ardizzone S: Long-term outcome of inflammatory bowel
diseases with cytomegalovirus colitis: Effect of antiviral
treatment. Eur J Gastroenterol Hepatol. 26:1146–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osaki R, Andoh A, Tsujikawa T, Ogawa A,
Koizumi Y, Nakahara T, Hata K, Sasaki M, Saito Y and Fujiyama Y:
Acute cytomegalovirus infection superimposed on
corticosteroid-naïve ulcerative colitis. Intern Med. 47:1341–1344.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Domenech E, Vega R, Ojanguren I, Hernández
A, Garcia-Planella E, Bernal I, Rosinach M, Boix J, Cabré E and
Gassull MA: Cytomegalovirus infection in ulcerative colitis: A
prospective, comparative study on prevalence and diagnostic
strategy. Inflamm Bowel Dis. 14:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|