Introduction
Acute myocardial infarction (AMI) stem cell
transplantation as a type of treatment is on the increase. A number
of studies such as the REPAIR-AMI, REGENT and BOOST indicated that
stem cell therapy improved the ischemic myocardial remodeling
outcomes. Benefit was also associated with the degree of ischemia
(1–3).
For myocardial regeneration, the bone marrow
mesenchymal stem cells or endothelial progenitor cells (EPCs) need
to be homed to the infarcted area and directional differentiation
into cardiomyocytes, in order to have normal physiological function
and therapeutic effect. Various differentiation inducing factors
such as stromal cell-derived factor-1 (SDF-1), insulin like growth
factor-1 and granulocyte colony-stimulating factor, play a key role
in stem cell directional migration and differentiation (4,5).
However, previous studies focused on the differentiation-inducing
factor promoting the stem cell therapy effect (6,7).
The aim of the present study was to examine whether
SDF-1 affects the apoptosis of AMI, angiogenesis and heart function
changes and the relevant function mechanisms involved.
Materials and methods
Animals
Sixty-four healthy male F344 rats were purchased
from Silaike Experimental Animal Co., Ltd. (Shanghai, China), with
gestational age of 6–8 months and body weight of 250±15 g. The rats
were kept in cages and given unrestricted access to food and water,
with 12-h light/dark cycle, a temperature of 20±3°C, and humidity
of 53±5%.
AMI model
According to the Olivette method, the rats were
fixed in supine position after being anesthetized with ether and
anesthesia was maintained by intraperitoneal injection of ketamine
(100 mg/kg). ALC-V8 ventilator (Alcott Biotechnology Co., Ltd.,
Shanghai, China) was used to support respiration and an ECG
instrument was used for ECG monitoring. The rats were operated with
chest median longitudinal incision, and tissues were separated by
layer separation. Thoracotomy was conducted along the fourth
intercostal space, and the pericardium was separated to expose the
heart. A needle was inserted in the junction below the edge of the
atrioventricular, left atrial appendage and conus and 6-0. Prolene
was used for ligature of the left anterior descending branch. The
left ventricular anterior wall lost its original luster, and had a
pale appearance, with a weak pulse and the ECG showed ST segment
and Q wave changes, indicating that AMI models were successfully
manufactured. The chest was observed continually for 3–5 min until
a stable cycle was obtained. The rats in the sham operation group
had only thread in the corresponding position without ligation.
Experimental grouping
The rats were randomly divided into the sham
operation, model, SDF-1 intervention and SDF-1 antibody groups,
with 16 rats in each group. On day 1 after the model was
successfully established, the rats in the SDF-1 intervention group
were injected with 10 µl recombinant SDF-1 (400 ng/ml; Peprotech,
Inc., Rocky Hill, NJ, USA) in five regions including the myocardial
infarction area and the four surrounding areas. The rats in the
model group were injected with 10 µl normal saline including the
myocardial infarction area and the four surrounding areas, and
those in the SDF-1 antibody group were injected with 1 ml SDF-1
antibody (2 µg/ml; United States Biological, Swampscott, MA, USA).
Four rats were sacrificed (5% isoflurane followed by cervical
dislocation) 1, 3, 7 and 14 days after the intervention, and the
analysis was carried out.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end-labelling
(TUNEL)
TUNEL method was used to count in situ
labeling apoptotic cells. According to the instructions of the kit
procedures, the heart was fixed with 4% polyformaldehyde phosphate
solution, dehydrated and paraffin-embedded, and sectioned (4 µm).
The interval to the long axis was 100 µm perpendicular. After the
conventional xylene dewaxing and gradient ethanol hydration, the
terminal deoxynucleotidyl transfer enzyme-mediated method was used.
TUNEL in situ was used to mark myocardial cell apoptosis.
Normal myocardial nuclei were blue, while apoptotic-positive
myocardial nuclei were brown. The proportion of the number of
apoptotic-positive cardiac muscle cells to the total number of
nuclei in 5 random high fields of view (×40) were counted under an
optical microscope (Olympus, Tokyo, Japan), and the mean value was
obtained.
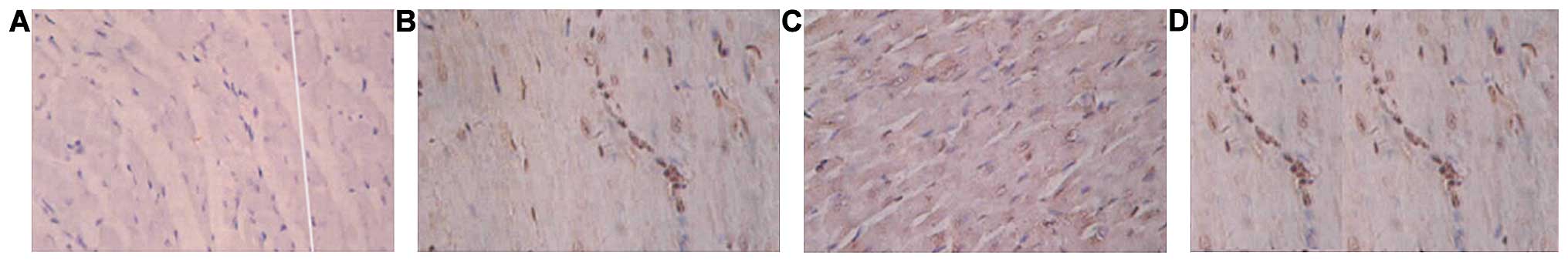
Immunohistochemical staining
CD34 immunohistochemical staining measurement for
microvessel density (MVD) was counted using the Image-Pro Plus 5.0
system (Media Cybernetics, Inc., Rockville, MD, USA) with reference
to the method used by Weidner (8).
First, optical microscope at a magnification of ×20 scanned the
entire section, and five regions of highest vascular density as
‘hot spots’ around the infarction area were selected. The number of
vessels was counted with dyed brown inside the hot spots under a
magnification of ×200 using the optical microscope. The endothelial
cells, the cluster of endothelial cells, and cabled endothelial
cells were counted as one blood vessel without taking the lumen
into account or if there were any red blood cells. The vessel with
a luminal diameter of >20 µm or thick muscle layer was not
included. Each sample was selected for five views (×200) counting
the number of microvessels.
HP SONOS 5500 animal ultrasonic echocardiography
(Hewlett-Packard, Palo Alto, CA, USA) was used to measure the left
ventricular end-diastolic diameter (LVEDd), left ventricular
end-systolic diameter (LVESd), left ventricular fractional
shortening (FS) and ejection fraction (EF) value. The probe was
11.5 MHz and placed in the left parasternal intercostal 4 for 5 of
the rats. According to the American Heart Association ultrasound
recommended guidelines (9), the
measurement was completed within at least three consecutive cardiac
cycles. The maximum and minimum of ventricular volume for the
diastolic and systolic ends were chosen, and the capacity
measurements were performed using the modified Simpson's method.
Ketamine (50 mg/kg) was used for sedation prior to the
examination.
Western blot analysis
The expression level of Toll-like receptor (TLR)-4
and nuclear factor-κB (NF-κB) were selected. WIP tissue cell lysis
was used for cell protein extraction (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China). After measurement of
protein concentration using the BCA protein determination kit
(Beyotime Institute of Biotechnology, Shanghai, China), 30 µg
protein sample was added with the sample buffer and boiled for
denaturation at 95°C for 5 min. Ten percent of SDS-PAGE gel was
used for electrophoresis, and the protein was transferred to a PVDF
membrane (both from Solarbio, Beijing, China). Then, 5% milk was
used to block in a shaker at 25°C for 2 h. Rabbit anti-mouse TLR-4
polyclonal antibody (1:1,000; Affinity Biosciences, Inc.,
Cincinnati, OH, USA; catalog no. AF7017), rabbit anti-mouse
NF-kBp65 polyclonal antibody (1:200; Wuhan Boster Biological
Engineering Co., Ltd., Wuhan, China; catalog no. PB0321) and
negative control rabbit anti-mouse GAPDH IgG antibody (1:1,000;
ZSGB-BIO Technology Co., Ltd., Beijing, China; catalog no.
TA309157) were used for incubation at 4°C overnight. TBST buffer
was used for washing, followed by incubation of the membrane with
horseradish peroxidase-labeled goat anti-rabbit IgG (1:5,000;
ZSGB-BIO Technology Co., Ltd.; catalog no. ZDR-5306) at 25°C in the
shaker for 2 h. After washing the membrane with TBST buffer, Super
ECL Plus sensitive luminous liquid was added and placed in the dark
(Applygen Technologies, Inc., Beijing, China). After exposure, the
image was recorded using the SensiAnsys gel imaging system. With
the gray value, the corresponding gray level ratio of target
protein bands with reference protein bands was used to calculate
each protein relative expression level.
Statistical analysis
SPSS software (Chicago, IL, USA) was used for data
analysis and processing. Mean ± standard deviation was used for
quantitative data. Single-factor ANOVA analysis was used for group
comparison, and the number of cases or percentage were determined
for the qualitative data, and χ2 test was used for
comparison between the groups. P<0.05 was considered
statistically different.
Results
Comparison of the number of apoptotic
cells at each time-point
There was no obvious apoptosis in the sham operation
group. The number of apoptotic cells increased with the extension
of time in the model group. The number of apoptotic cells of the
SDF-1 treatment group was significantly lower at each time-point
than the other groups, and the number of apoptotic cells reached
their peak on the 3rd day. The number of apoptotic cells were
reduced further after 7 and 14 days. The number of apoptotic cells
in the SDF-1 antibody group was significantly higher (p<0.05)
(Table I and Fig. 1).
 | Table I.Comparison of the number of apoptotic
cell at each time-point (%). |
Table I.
Comparison of the number of apoptotic
cell at each time-point (%).
| Groups | 1 day | 3 days | 7 days | 14 days |
|---|
| Sham operation | 0.6±0.1 | 0.6±0.1 | 0.6±0.1 | 0.6±0.1 |
| Model | 43.7±6.6 | 68.9±7.4 | 77.5±9.2 | 80.4±10.3 |
| SDF-1
intervention | 26.4±4.2 | 35.5±3.7 | 27.8±3.5 | 23.3±3.2 |
| SDF-1 antibody | 50.6±5.7 | 73.5±7.3 | 82.1±8.8 | 86.7±12.4 |
| F | 6.324 | 6.648 | 7.553 | 7.964 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of MVD at different
time-points
The blood vessel density in the SDF-1 intervention
group was significantly higher than that in the remaining groups.
The vessel density of the SDF-1 antibody group was significantly
smaller at each time-point (p<0.05), (Table II).
 | Table II.Comparison of the microvessel density
at different time-points (/HP). |
Table II.
Comparison of the microvessel density
at different time-points (/HP).
| Groups | 1 day | 3 days | 7 days | 14 days |
|---|
| Sham operation | 1.5±0.3 | 1.4±0.4 | 1.3±0.2 | 1.6±0.5 |
| Model | 0.9±0.2 | 0.8±0.3 | 1.2±0.4 | 1.0±0.3 |
| SDF-1
intervention | 0.8±0.3 | 5.5±1.2 | 8.6±1.5 | 9.3±2.2 |
| SDF-1 antibody | 0.4±0.1 | 0.3±0.1 | 0.5±0.1 | 0.6±0.1 |
| F | 5.436 | 5.647 | 5.968 | 6.302 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
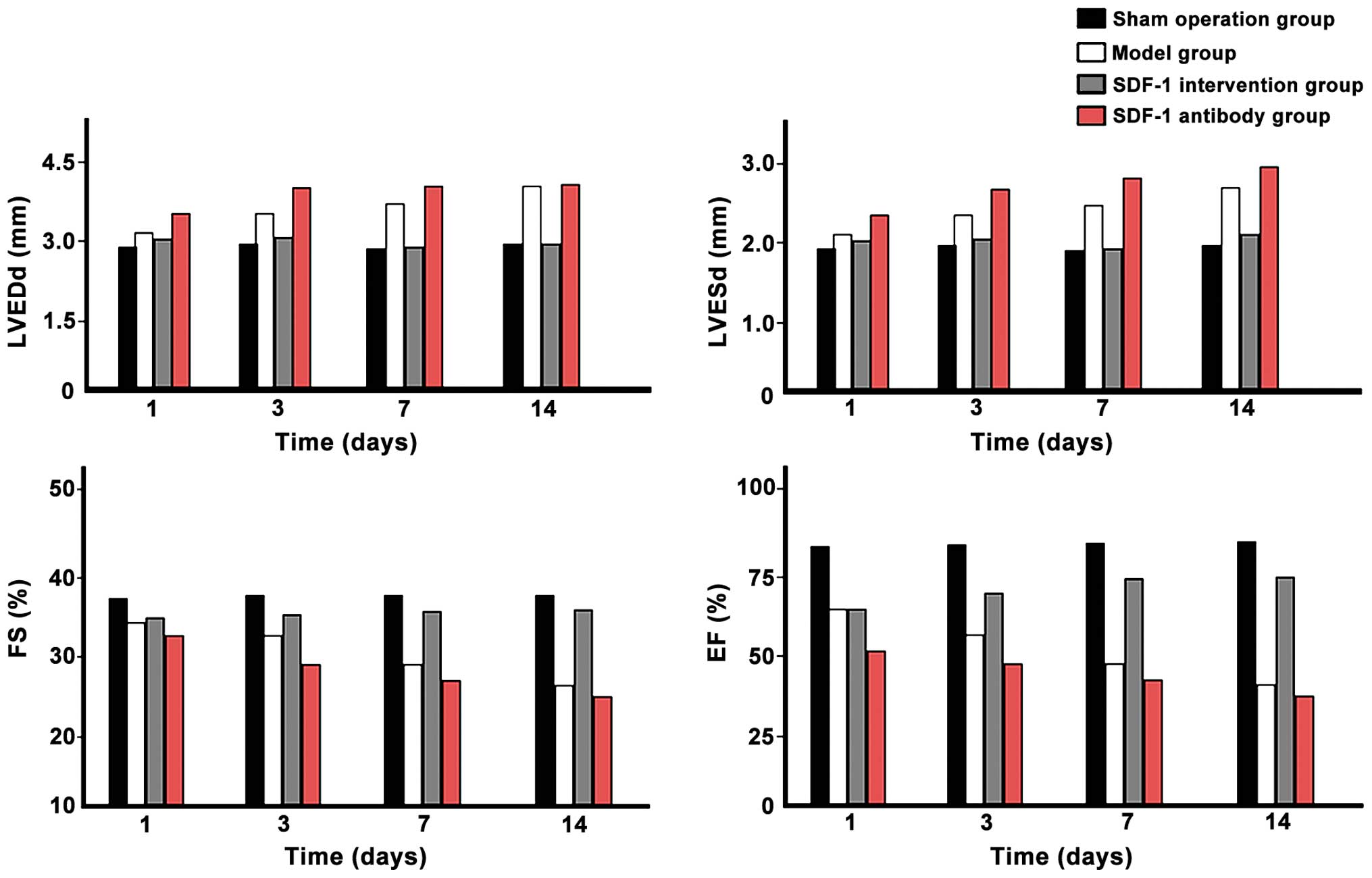
Comparison of cardiac ultrasound
parameters
LVEDd and LVESd values of each time-point in the
model group were significantly higher than those in the sham
operation group, and significantly increased with time. The values
of each time-point in the SDF-1 intervention group were smaller
compared with those of the model group, but larger than those in
the sham operation group and decreased with time. The values in the
SDF-1 antibody group were significantly largest in each time-point
and increased with time (p<0.05). The FS and EF values in model
group were significantly smaller than those in the sham-operated
group and decreased over time. Those in the SDF-1 treatment group
at each time-point were higher than those of the model group, but
smaller than those of the sham operation group and increased over
time. The FS and EF values in the anti-SDF-1 antibody group at each
time-point were significantly minimum and decreased with time
extended (p<0.05) (Fig. 2).
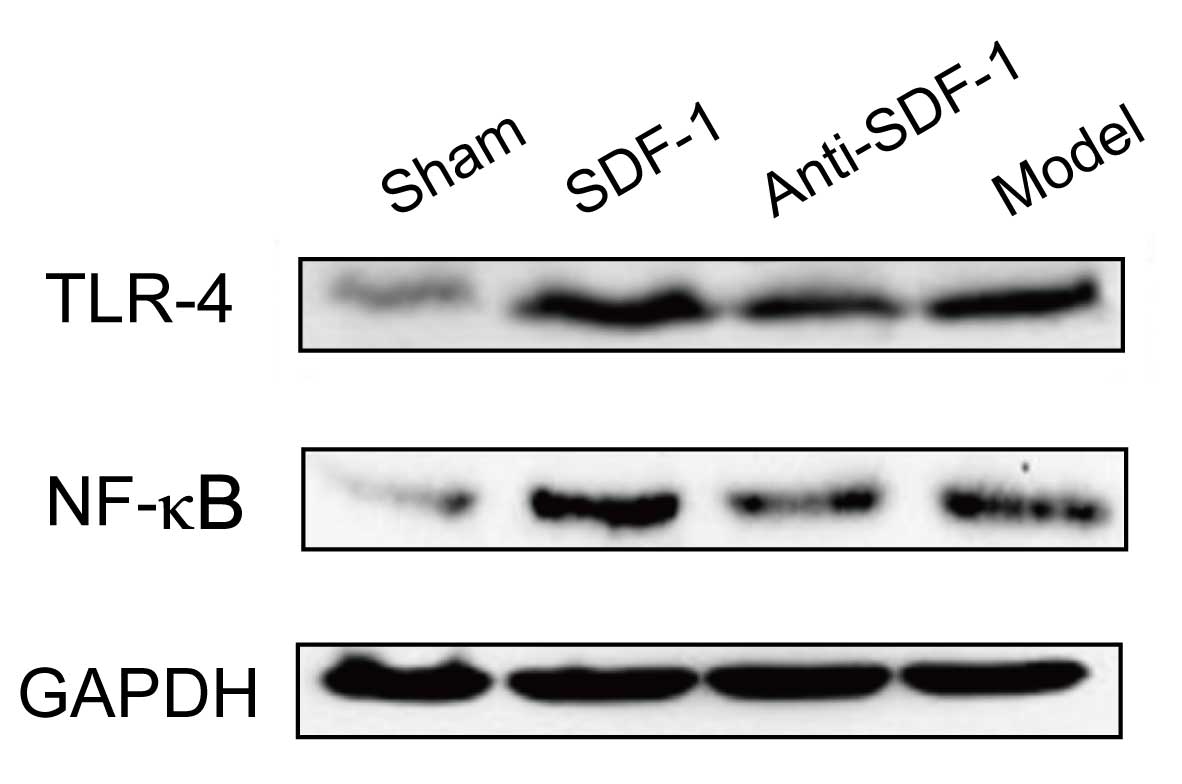
Comparison of the expression levels of
TLR-4 and NF-κB
The expression levels of TLR-4 and NF-κB in the
SDF-1 intervention group were significantly higher than those in
the other groups at each time-point and increased with time
(p<0.05). In other groups, the differences were not
statistically significant (p>0.05), as shown in Table III and Fig. 3.
 | Table III.Comparison of TLR-4 and NF-κB
expression levels (%). |
Table III.
Comparison of TLR-4 and NF-κB
expression levels (%).
| Groups | 1 day | 3 days | 7 days | 14 days |
|---|
|
|
|
|
|
|
|---|
|
| TLR-4 | NF-κB | TLR-4 | NF-κB | TLR-4 | NF-κB | TLR-4 | NF-κB |
|---|
| Sham operation | 1.2±0.2 | 0.9±0.2 | 1.3±0.3 | 0.8±0.2 | 1.1±0.3 | 1.0±0.3 | 1.0±0.3 | 1.1±0.3 |
| Sham operation | 1.3±0.3 | 1.0±0.3 | 1.2±0.3 | 0.9±0.3 | 1.1±0.3 | 1.0±0.3 | 1.2±0.3 | 0.9±0.3 |
| SDF-1
intervention | 13.2±3.2 | 10.5±3.3 | 21.4±5.4 | 17.8±5.6 | 38.7±6.6 | 26.5±6.7 | 40.2±8.7 | 32.3±8.9 |
| SDF-1 antibody | 0.8±0.2 | 0.9±0.2 | 0.7±0.2 | 0.8±0.2 | 0.9±0.2 | 0.8±0.2 | 0.7±0.2 | 0.7±0.2 |
| F | 10.532 | 12.354 | 13.624 | 14.857 | 16.359 | 18.427 | 20.312 | 23.635 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
SDF-1 is a type of CXC chemokine, which is the only
receptor for CXCR4 and is increased significantly in the early
stage of myocardial infarction. It can be sustained for 56 days
after infarction and is expressed in the hematopoietic stem cell
surface (10). Reconstruction of
SDF-1 expression in the cell around the infarction area can promote
CD117+ and CD34+ stem cells gradually homing
to the myocardial infarction area (11). Nevertheless, only a small fraction of
bone marrow mesenchymal stem cells can survive after
transplantation and apoptosis is considered an important factor
that affects the curative effect (12). SDF-1 has directed chemotaxis and
anti-apoptotic effects.
The present study showed that with the extension of
time in the model group, the number of apoptotic cells increased
accordingly. Myocardial infarction is a process of cardiomyocyte
apoptosis. Cardiac remodeling after revascularization may be
associated with blocking of cardiomyocytes apoptosis (13). The number of apoptotic cells in the
SDF-1 intervention group was significantly less than that in the
other groups at all time-points and the number of apoptotic cells
on day 3 was the greatest in number. By contrast, the number of
apoptotic cells on days 7 and 14 were decreased. The number of
apoptotic cells in the SDF-1 antibody group was the greatest,
proving that SDF-1 obviously inhibited the process of apoptosis of
cardiac muscle cells (14). The new
blood vessel density in the SDF-1 intervention group was
significantly higher than that in the other groups. The SDF-1
antibody group had the lowest vascular density at all time-points,
suggesting that SDF-1 has the potential of cell proliferation and
neovascularization. In animal models, SDF-1 and vascular
endothelial growth factor (VEGF) have similar effects, which can
mobilize hematopoietic stem cells and circulating EPCs (15). The mechanism of SDF-1 chemotaxis
attracting circulating EPCs to ischemia or injury site may be
similar to its ability of raising tissue-committed stem cells
(16). Neointimal thickening was
attributed to smooth muscle-like cells, and the origin of the
smooth muscle-like cells is considered to be induced by the trend
of SDF-1 in endothelial cells selectively up-regulating in
recruiting CXCR4+ stem cells (17).
The LVEDd and LVESd values of each time-point in the
SDF-1 intervention group were smaller than those of the model
group, but larger than those of the sham operation group, decrease
with time. Those in the SDF-1 antibody group at each time-point
were the largest and increased with time. The FS and EF values of
each time-point in the the SDF-1 intervention group were higher
than those in the model group, but less than those in the sham
operation group, increasing with time. Those in the SDF-1 antibody
group at each time-point were the least and decreased with the
time. It was suggested that SDF-1 can further improve the cardiac
function, which may be related to its ability to inhibit myocardial
apoptosis, and increase angiogenesis and chemotaxis of endogenous
or exogenous pluripotent stem cell differentiation. In some
studies, the autologous bone marrow stem cell of AMI patients was
mobilized by subcutaneous injection of SDF-1 (18). Subsequently, peripheral blood stem
cell suspension was separated. The collected stem cell suspension
was injected to the infarct-related artery by over-the-wire balloon
catheter in the center cavity. After 6 months, in comparison to the
conventional treatment group (drugs and interventional therapy),
the end systolic volume (ESV) of heart decreased significantly
(p=0.01). The left ventricular EF increased significantly
(p<0.001), while the left ventricular segmental wall motion
score index decreased significantly (p<0.001), whereas the end
diastolic volume (EDV) had no significant change (p=0.07),
indicating that SDF-1 improved the percutaneous transluminal
coronary artery transplantation of autologous peripheral blood stem
cells effect in the treatment of AMI for reducing myocardial
infarction area, reducing left ventricular remodeling and improving
cardiac function (18).
TLR-4 and NF-κB protein expression levels of the
SDF-1 treatment group were significantly higher than those of other
groups and increased with time. The difference was not
statistically significant compared with those of the other groups
at each time-point, suggesting that SDF-1 be involved in biological
activity through the TLR-4/NF-κB signaling pathway (19). The TLR-4/NF-κB signaling pathway
plays an important role in the inflammatory response mediated by
inflammatory mediators such as neutrophils, inflammatory cytokines
including IL-2, IL-10, γ-IFN and TNF-α, and inflammatory mediators,
which are involved in cell injury and repair. Previous findings
have shown that the SDF-1/CXCR4 axis played a role through the
phosphoinositide 3-kinase/protein kinase C/NF-κB and p42/44
mitogen-activated protein kinase pathways (20,21).
In conclusion, SDF-1 is capable of reducing the
apoptosis of myocardial cells in AMI, promoting blood vessel
regeneration and improving cardiac function, which may be
associated with activation of the TLR-4/NF-κB signaling
pathway.
References
|
1
|
Assmus B, Leistner DM, Schächinger V, Erbs
S, Elsässer A, Haberbosch W, Hambrecht R, Sedding D, Yu J, Corti R,
et al: REPAIR-AMI Study Group: Long-term clinical outcome after
intracoronary application of bone marrow-derived mononuclear cells
for acute myocardial infarction: migratory capacity of administered
cells determines event-free survival. Eur Heart J. 35:1275–1283.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ripa RS and Kastrup J: Stem cells: REGENT
trial-the end of cell therapy for MI? Nat Rev Cardiol. 6:567–568.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyer GP, Wollert KC, Lotz J, Pirr J,
Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L,
Ganser A and Drexler H: Intracoronary bone marrow cell transfer
after myocardial infarction: 5-Year follow-up from the
randomized-controlled BOOST trial. Eur Heart J. 30:2978–2984. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zgraggen S, Huggenberger R, Kerl K and
Detmar M: An important role of the SDF-1/CXCR4 axis in chronic skin
inflammation. PLoS One. 9:e936652014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Youssef A and Han VK: Low oxygen tension
modulates the insulin-like growth factor-1 or −2 signaling via both
insulin-like growth factor-1 receptor and insulin receptor to
maintain stem cell identity in placental mesenchymal stem cells.
Endocrinology. 157:1163–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalonneau F, Liu XQ, Sadir R, Almodovar J,
Mertani HC, Bruckert F, Albiges-Rizo C, Weidenhaupt M, Lortat-Jacob
H and Picart C: The effect of delivering the chemokine SDF-1α in a
matrix-bound manner on myogenesis. Biomaterials. 35:4525–4535.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mierzejewska K, Klyachkin YM, Ratajczak J,
Abdel-Latif A, Kucia M and Ratajczak MZ:
Sphingosine-1-phosphate-mediated mobilization of hematopoietic
stem/progenitor cells during intravascular hemolysis requires
attenuation of SDF-1-CXCR4 retention signaling in bone marrow.
BioMed Res Int. 2013:8145492013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
9
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American Society of
Echocardiography endorsed by the European Association of
Echocardiography, a registered branch of the European Society of
Cardiology, and the Canadian Society of Echocardiography. J Am Soc
Echocardiogr. 23:685–713, 786–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong J, Meng HB, Hua J, Song ZS, He ZG,
Zhou B and Qian MP: The SDF-1/CXCR4 axis regulates migration of
transplanted bone marrow mesenchymal stem cells towards the
pancreas in rats with acute pancreatitis. Mol Med Rep. 9:1575–1582.
2014.PubMed/NCBI
|
|
11
|
Roy LD, Sahraei M, Schettini JL, Gruber
HE, Besmer DM and Mukherjee P: Systemic neutralization of IL-17A
significantly reduces breast cancer associated metastasis in
arthritic mice by reducing CXCL12/SDF-1 expression in the
metastatic niches. BMC Cancer. 14:225–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Belle JI, Petrov JC, Langlais D, Robert F,
Cencic R, Shen S, Pelletier J, Gros P and Nijnik A: Repression of
p53-target gene Bbc3/PUMA by MYSM1 is essential for the survival of
hematopoietic multipotent progenitors and contributes to stem cell
maintenance. Cell Death Differ. 23:759–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang J, Zhang G and Zhang L, Hou YP, Liu
XL and Zhang L: High admission glucose levels increase Fas
apoptosis and mortality in patients with acute ST-elevation
myocardial infarction: a prospective cohort study. Cardiovasc
Diabetol. 12:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan Y, Yang F, Cao X, Chen C, Zhang X,
Zhang X, Lin W, Wang X and Liang C: Gab1 regulates SDF-1-induced
progression via inhibition of apoptosis pathway induced by
PI3K/AKT/Bcl-2/BAX pathway in human chondrosarcoma. Tumour Biol.
16:12–14. 2015.
|
|
15
|
Moore MA, Hattori K, Heissig B, Shieh JH,
Dias S, Crystal RG and Rafii S: Mobilization of endothelial and
hematopoietic stem and progenitor cells by adenovector-mediated
elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N
Y Acad Sci. 938:36–45; discussion 45–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ratajczak MZ, Majka M, Kucia M, Drukala J,
Pietrzkowski Z, Peiper S and Janowska-Wieczorek A: Expression of
functional CXCR4 by muscle satellite cells and secretion of SDF-1
by muscle-derived fibroblasts is associated with the presence of
both muscle progenitors in bone marrow and hematopoietic
stem/progenitor cells in muscles. Stem Cells. 21:363–371. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao C and Li Y: GaoC and Li Y: SDF-1 plays
a key role in the repairing and remodeling process on rat
allo-orthotopic abdominal aorta grafts. Transplant Proc.
39:268–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing Q, de Vos P, Faas MM, Ye Q and Ren Y:
LPS promotes pre-osteoclast activity by up-regulating CXCR4 via
TLR-4. J Dent Res. 90:157–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Džumhur A, Zibar L, Wagner J, Simundić T,
Dembić Z and Barbić J: Association studies of gene polymorphisms in
toll-like receptors 2 and 4 in Croatian patients with acute
myocardial infarction. Scand J Immunol. 75:517–523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roland J, Murphy BJ, Ahr B, Robert-Hebmann
V, Delauzun V, Nye KE, Devaux C and Biard-Piechaczyk M: Role of the
intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood.
101:399–406. 2003. View Article : Google Scholar : PubMed/NCBI
|