Introduction
With the development of invasive cardiology,
contrast-induced nephropathy (CIN) has become an important issue to
tackle in clinical practice. It has become the third leading cause
of hospital-acquired acute renal failure, accounting for 10–25% of
all acquired acute renal damage (1).
The traditional biomarker for diagnosis of CIN is Scr, which is not
sensitive enough to detect early kidney damage, and various factors
affect its accuracy (2), such as
age, gender, muscle mass, diet, medication and hydration status.
Neutrophil gelatinase-associated lipocalin (NGAL), known as NGAL,
lipocalin 2, siderocalin, uterocalin, proteinase-3 and 24p3, is a
mammalian small 25-kDa peptide that belongs to the lipocalin
superfamily, which consists of ~20 small lipoproteins. NGAL was
initially discovered as an antibacterial factor of natural immunity
and an acute-phase protein (3). NGAL
levels are low in normal functioning kidneys; however, when
ischemic injury or nephrotoxicant occurs, NGAL accumulates at high
levels in the renocortical tubule and urine (4). NGAL is readily excreted to serum and
urine (5); therefore, it may be a
novel marker for the early detection of CIN.
N-acetylcysteine (NAC) is an antioxidant agent with
myocardial and renal protective potential (6–8). NAC's
primary use in clinical medicine has been as a mucolytic agent to
treat respiratory diseases. More recently, it has been suggested
that NAC has a vasodilating effect (9). Since CIN may be associated with renal
vasoconstriction and oxidative stress (10–12), we
hypothesize that NGAL may be a novel biomarker that can detect CIN
earlier and more accurately, and that the use of NAC significantly
reduces the incidence of CIN.
Materials and methods
Animals
Adult male Sprague-Dawley rats (n=120), weighing
200±20 g, were obtained from Xuzhou Medical College Laboratory
Animal Center (Xuzhou, China). Rats were housed in standard
stainless steel hanging cages with controlled temperature
(22–25°C), humidity (35–50%), and photocycle (12-h light/dark)
conditions. Experiments were performed in accordance with the Guide
for the Care and Use of Laboratory Animals published in P.R. China
and with the approval of Xuzhou Medical Ethics Committee.
Experimental design and drugs
Rats were randomly divided into four equal groups:
Control (CON; n=30), CIN-treated (CIN; n=30), normal rats treated
with NAC (NAC; n=30), and CIN rats treated with NAC (NAC+CIN;
n=30). A total of 10 mg/kg indomethacin (INDO), 10 mg/kg
Nv-nitro-L-arginine methyl ester (both Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and 2.9 g I/kg iohexol (Jiangsu
Yangtze River Pharmaceutical Group Co., Ltd., Taizhou, China) was
administered intravenously into the tail vein of rats in the CIN
and NAC+CIN groups every 15 min, as described by Yokomaku et
al (13). Rats in the NAC+CIN
group received intraperitoneal injection of 10 mg/kg NAC
(Sigma-Aldrich; Merck Millipore) 12 h prior to INDO treatment. Rats
in the CON group received the same amount of saline injections into
their abdomen and tail vein. Rats in the NAC group were pretreated
with 10 mg/kg NAC by intraperitoneal injection, and the same amount
of saline as delivered to the CIN group was administered 12 h
later.
Measurement of Scr and serum NGAL
Rats were sacrificed at 2, 12, 24, 48 and 72 h by
anesthetic overdose (10% chloral hydrate, 0.5 ml/100 g; Sangon
Biotech Co., Ltd., Shanghai, China) after the procedure. Blood
samples were harvested and centrifuged at 1,500 × g for 15
min at 4°C. Scr values was measured by an automatic biochemical
analyzer (Olympus Corp., Tokyo, Japan), and the concentration of
serum NGAL was evaluated via an enzyme linked immunosorbent assay
(ELISA) with an ELISA kit (Westang Biotech, Shanghai, China).
Hemotoxylin and eosin (HE) staining
and tubular injury score
Kidneys were harvested and separated into two
pieces, one of which was fixed in 10% formalin for 24 h, and the
other was kept at −80°C for subsequent western blot analysis and to
study oxidative status. The fixed piece of kidney was processed and
embedded in paraffin, cut into 4-µm-thick section, embedded in
paraffin sections and stained with HE. Biopsies were examined by
light microscopy in a blinded manner, and scored with a
semi-quantitative scale designed to evaluate the degree of tubular
injury, according to the tubular necrosis, tubular dilatation
and/or atrophy, inflammatory cell infiltration, or cellular edema.
Injury was graded on a scale: 0, normal kidney; 1, minimal damage
(unicellular, patchy isolated damage); 2, mild damage (<25%); 3,
moderate damage (25–50%); and 4, severe damage (>50%) (14). A minimum of 10 fields at ×400
magnification were assessed and graded in each biopsy.
Immunohistochemistry of NGAL
Following deparaffinization, antigen retrieval was
performed in citrate buffer. Non-specific binding was blocked with
0.3% H2O2 for 10 min and, after blocking for
30 min with 10% goat serum at room temperature, anti-NGAL antibody
(1:150 in PBS; PB0641; Boster Biological Technology, Ltd., Wuhan,
China) was applied overnight at 4°C. Samples were washed in buffer
and incubated with the goat anti-rabbit IgG-Biotin secondary
antibody (1:200 in PBS; BA1003; Boster Biological Technology, Ltd.)
for 30 min, followed by the diaminobenzidine-peroxidase reaction
and counterstaining with hematoxylin. Negative control sections
were stained under identical conditions by omitting the primary
antibody instead of PBS. Stained sections were subsequently
visualized using light microscopy at ×200 magnification and the
integrated optical density (IOD) was measured using Image-Pro Plus
6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Western blotting
Total protein extraction was performed by
homogenization and centrifugation at 1,500 × g for 20 min at
4°C, and the concentration of the protein was determined by the
bicinchoninic acid assay (BCA) kit (Biyuntian Biotechnological Co.,
Shanghai, China). Protein samples were separated by 12% SDS-PAGE
and transferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and blocked with 5% skimmed
milk for 2 h. Primary antibodies against rabbit polyclonal
anti-NGAL (1:1,000; ab63929; Abcam, Cambridge, UK) and sheep
anti-mouse anti-GAPDH (1:500; sc-25778; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) were respectively added, and incubated at
4°C overnight. Following washing in TBST for 5 min three times, the
membranes were then incubated with secondary antibody (1:5,000;
P/N926-32231; ODYSSEY; LI-COR Biosciences, Inc., Lincoln, NE, USA)
for 2 h in the dark. Protein bands were analyzed via an ODYSSEY
Laser Imaging system (LI-COR Biosciences, Inc.).
Renal oxidative stress
Renal cortex was homogenized with ice-cold saline
homogenization buffer, and centrifuged at 1,500 × g for 20
min at 4°C. The supernatant was analyzed by commercial
malondialdehyde (MDA) and superoxide dismutase (SOD) kits (cat.
nos. A003-1 and A001-3, respectively; Jiancheng Bioengineering
Institute, China). MDA levels were measured via the thiobarbituric
acid reactive substances method (15). SOD activity was measured by a method
based on the nitro blue tetrazolium (NBT) reduction rate. One unit
for SOD activity was expressed as the enzyme protein amount that
was able to cause a 50% inhibition in NBT reduction rate (15). Sample absorbances were measured at
532 nm and 550 nm respectively. Protein levels of the supernatants
were measured using a BCA assay kit.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). All data were expressed as
mean ± standard deviation. Statistical comparisons were executed by
one-way analysis of variance followed by Student-Newman-Keuls
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Serum Scr and NGAL values
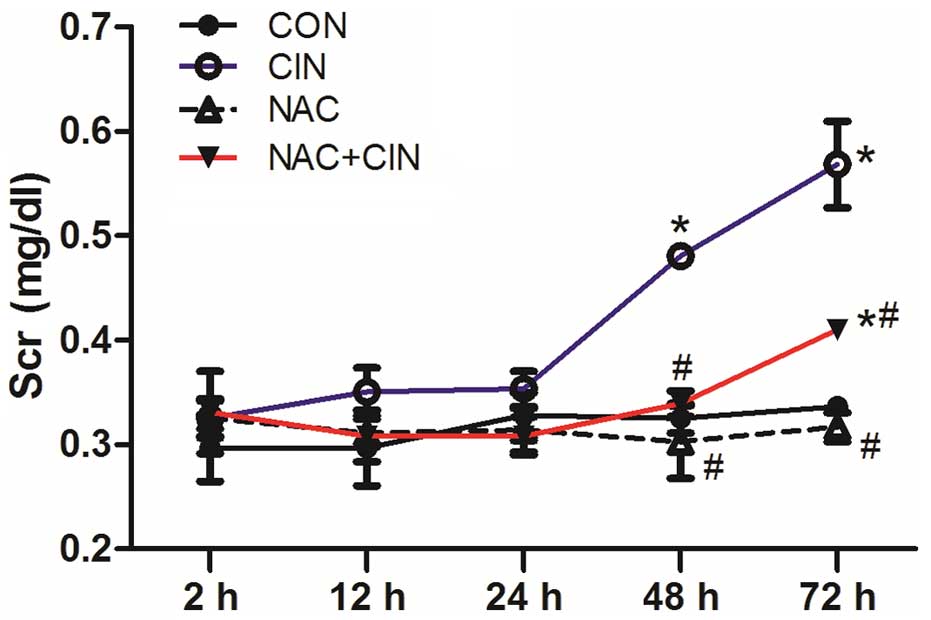
At 2, 12 and 24 h following administration, there
were no significant differences in the Scr values among the four
groups (P>0.05). Scr levels significantly increased in the CIN
group at 48 and 72 h (P<0.05), which was significantly higher
than the NAC+CIN group at the same time point (P<0.05; Fig. 1). However, compared with the CON
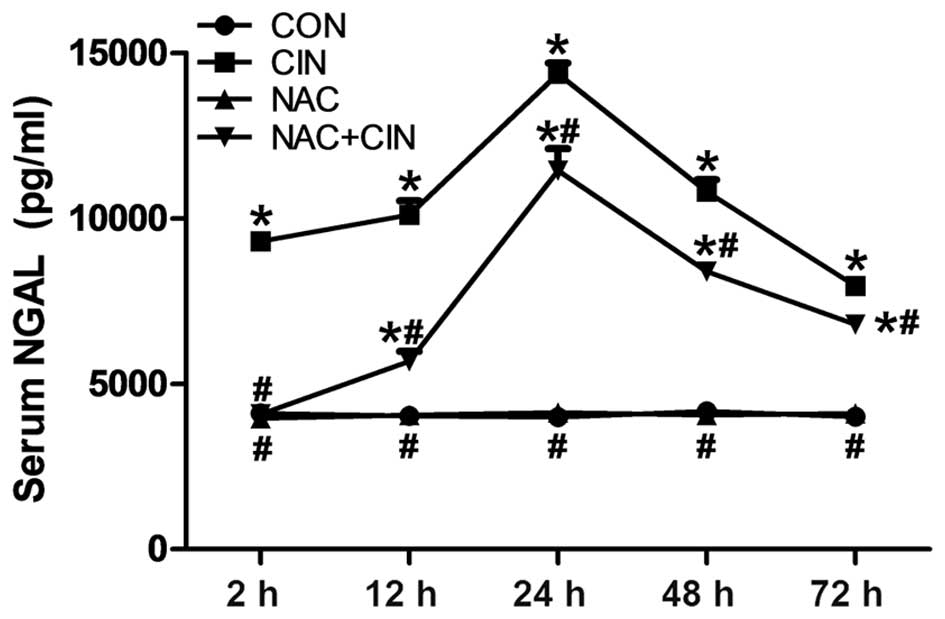
group, the value of serum NGAL in the CIN group had markedly
increased, with a significant increase at 2 h after administration
(P<0.05; Fig. 2). NGAL values in
the NAC+CIN group were significantly decreased, as compared with
the CIN group (P<0.05; Fig.
2).
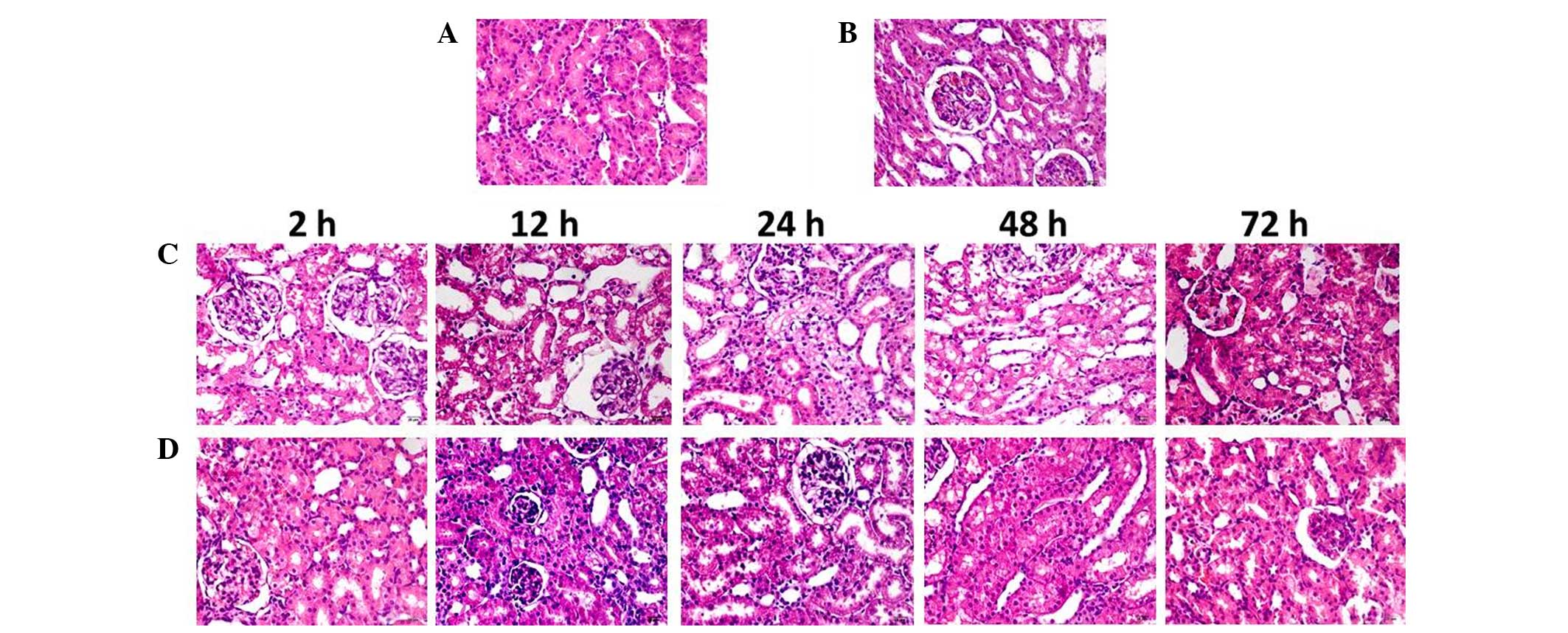
HE staining and renal injury
score
In contrast-injected rats, vacuolar degeneration of
tubular epithelial cells, tubular dilation, protein cast (protein
deposition in the tubular lumen), tubular brush border loss,
increased epithelial cell shedding, and necrosis of partial tubular
epithelial cells were observed (Fig.
3). Semi-quantitative analysis demonstrated no difference
between the renal injury scores of the NAC and CON groups. Renal
injury scores in the NAC+CIN group were higher than that of the CON
group, but significantly lower than that of the CIN group
(P<0.05; Table I).
 | Table I.Renal injury score of each group
(n=30) at the indicated time points. |
Table I.
Renal injury score of each group
(n=30) at the indicated time points.
| Groups | CON | CIN | NAC | NAC+CIN |
|---|
| 2 h | 0.19±0.03 | 0.28±0.08 | 0.21±0.07 | 0.21±0.07 |
| 12 h | 0.21±0.04 |
1.94±0.21a |
0.17±0.06b |
1.40±0.16a,b |
| 24 h | 0.18±0.07 |
2.42±0.66a |
0.21±0.08b |
1.43±0.08a,b |
| 48 h | 0.26±0.06 |
2.60±0.65a |
0.20±0.02b |
1.79±0.24a,b |
| 72 h | 0.26±0.04 |
2.02±0.16a |
0.25±0.06b |
1.25±0.15a,b |
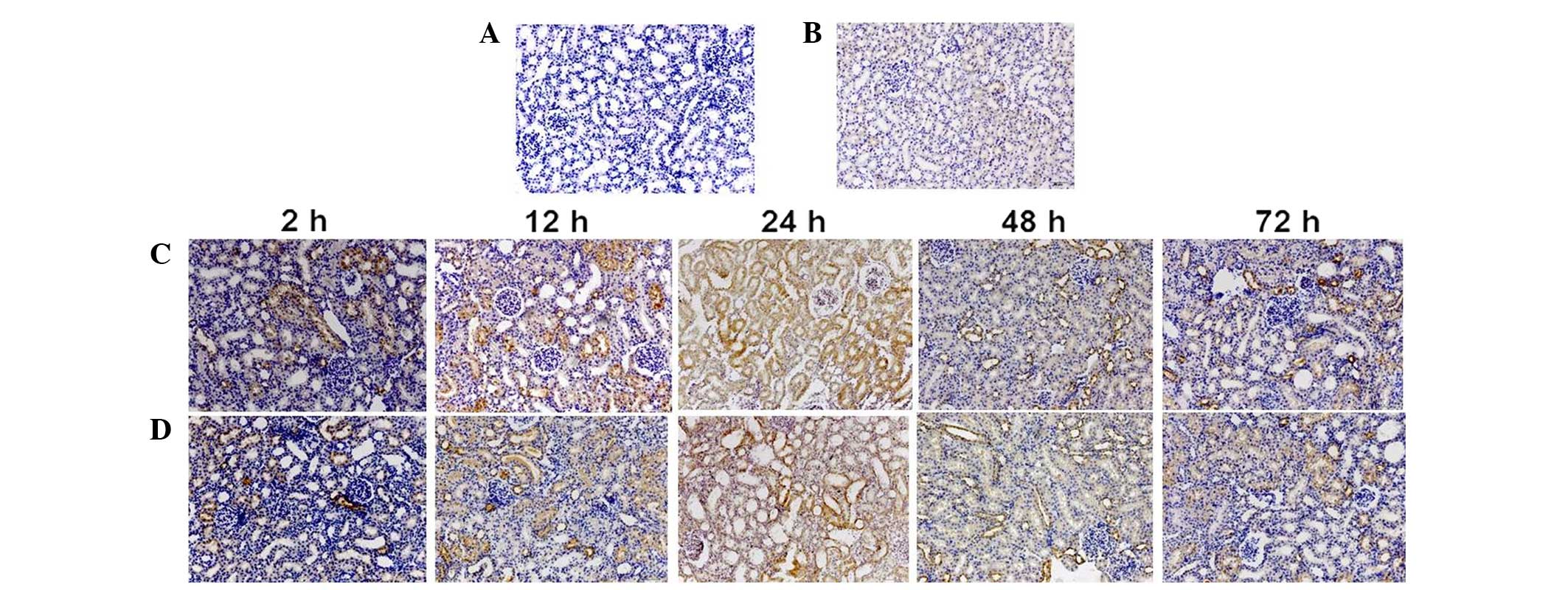
Immunohistochemistry
Immunohistochemistry findings demonstrated that NGAL
was predominantly expressed in the cytoplasm of proximal tubular
cells (Fig. 4). Compared with the
CON group, the positive staining IOD value of NGAL significantly
increased 2 h after administration in the CIN group (P<0.05;
Table II), and was significantly
higher than that of the NAC+CIN group at same time point
(P<0.05; Table II).
 | Table II.Integrated optical density value of
each group (n=30) at the indicated time points. |
Table II.
Integrated optical density value of
each group (n=30) at the indicated time points.
| Groups | CON | CIN | NAC | NAC+CIN |
|---|
| 2 h | 2.81±0.27 |
15.54±3.31a |
2.67±0.19b |
10.90±1.42a,b |
| 12 h | 2.34±0.17 |
32.30±3.31a |
2.43±0.25b |
20.39±2.2a,b |
| 24 h | 2.79±0.32 |
55.74±3.62a |
2.58±0.34b |
32.94±0.31a,b |
| 48 h | 2.42±0.39 |
27.38±2.15a |
2.32±0.14b |
16.55±2.46a,b |
| 72 h | 2.47±0.29 |
13.41±0.10a |
2.42±0.20b |
8.57±0.55a,b |
Western blotting
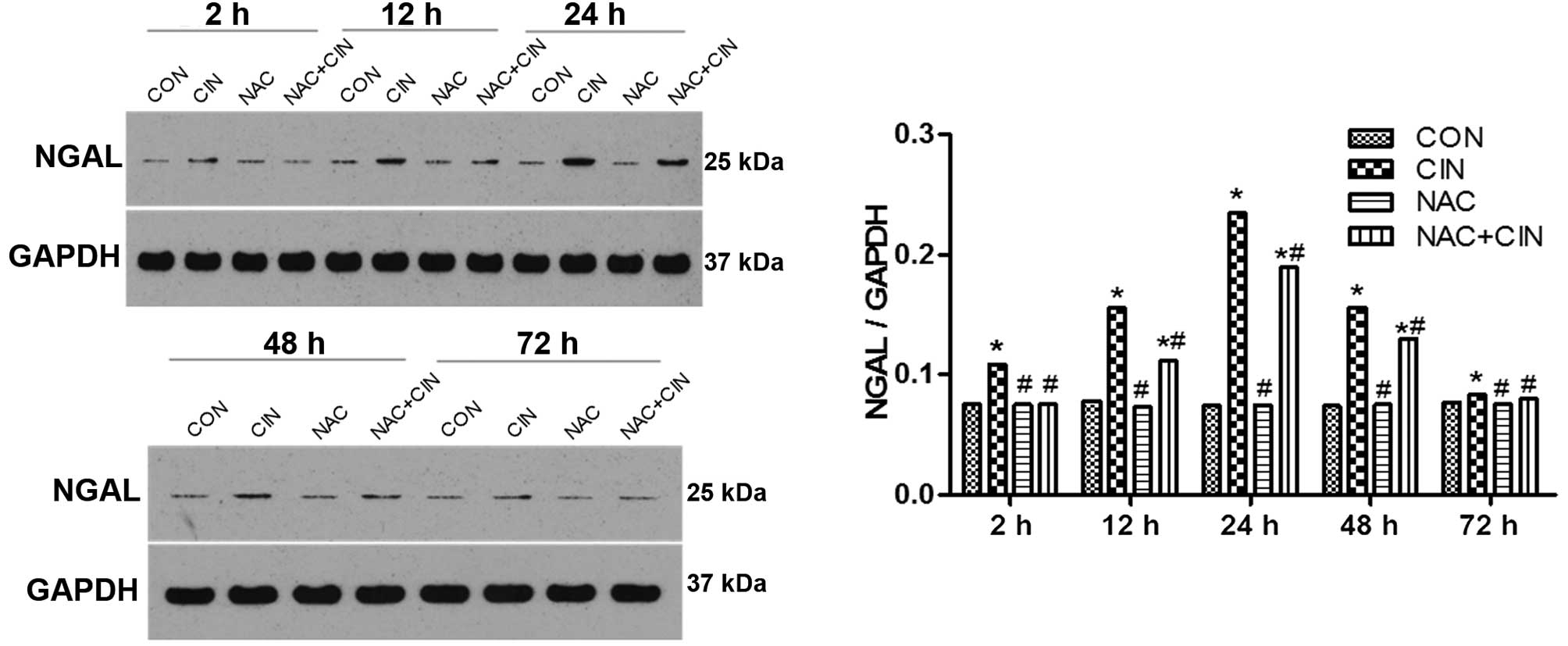
According to the western blotting results, the
expression levels of NGAL in the kidney tissue of the CIN group
began to significantly increase 2 h after the procedure
(P<0.05), and were significantly decreased in the NAC+CIN group
at the same time point (P<0.05). No significant differences were
detected between the NAC and CIN groups (P>0.05; Fig. 5).
Oxidative stress indicators
As shown in Tables
III and IV, a significant
decline of kidney SOD was observed in CIN rats compared with the
CON group (P<0.05), and there was also a significant decline
between the CIN and NAC+CIN groups (P<0.05). This was
accompanied by an increase of MDA in the CIN group, which was
significantly higher than the other three groups (P<0.05).
 | Table III.The level of SOD in renal tissue
(U/mgprot). |
Table III.
The level of SOD in renal tissue
(U/mgprot).
| Groups | CON (n=30) | CIN (n=30) | NAC (n=30) | NAC+CIN (n=30) |
|---|
| 2 h | 144.39±1.786 |
113.46±2.114a |
150.32±4.968b |
130.03±0.791a,b |
| 12 h | 160.64±0.807 |
97.32±0.833a |
167.15±1.576b |
105.08±0.356a,b |
| 24 h | 124.88±4.521 |
67.20±0.464a |
128.78±2.474b |
97.32±0.833a,b |
| 48 h | 159.04±1.187 |
90.78±0.672a |
163.63±1.018b |
100.36±0.858a,b |
| 72 h | 137.78±1.383 |
107.15±1.568a |
139.45±1.929b |
125.00±1.609a,b |
 | Table IV.The level of MDA in renal tissue
(nmol/mgprot). |
Table IV.
The level of MDA in renal tissue
(nmol/mgprot).
| Groups | CON (n=30) | CIN (n=30) | NAC (n=30) | NAC+CIN (n=30) |
|---|
| 2 h | 2.42±0.101 |
3.01±0.133a |
2.33±0.188b |
2.52±0.215b |
| 12 h | 2.76±0.215 |
3.19±0.175a |
2.72±0.136b |
3.25±0.303a,b |
| 24 h | 2.26±0.122 |
5.17±0.257a |
2.13±0.131b |
4.16±0.258a,b |
| 48 h | 3.00±0.293 |
4.51±0.309a |
2.86±0.252b |
3.96±0.199a,b |
| 72 h | 2.36±0.152 |
3.33±0.154a |
2.22±0.028b |
3.14±0.088a,b |
Discussion
CIN has become the third leading cause of
hospital-acquired acute renal failure, accounting for 10–25% of all
acquired acute renal damage (16).
CIN is defined as an increase in serum creatinine by 0.3 mg/dl
within 48 h (16). However, serum
creatinine is an unreliable indicator of acute changes in renal
function (17). Therefore, a
biomarker that can accurately reflect the kidney injury of early
stage is urgently required, to improve the prognosis of
patients.
NGAL is a protein with a small molecular weight (25
kDa), which is a member of lipocalin family, that can be covalently
combined with neutrophil gelatinase (18). NGAL is present in various organs;
under normal conditions, NGAL remains at a low expression level in
the kidneys, trachea and epithelial cells of the gastrointestinal
tract. However, when ischemia or ischemic reperfusion injury
occurs, the secretion of NGAL in the thick ascending limb segment
of renal tubular increases rapidly. In the present study, a rat
model of CIN was established and treated with NAC. The expression
of NGAL was subsequently detected in the kidney and serum and the
presence of kidney damage was assessed, in order to explore whether
NGAL may be used as an early diagnostic marker of CIN, and whether
NAC has a protective effect on the kidneys. The present data showed
that significant kidney damage occurred in the rat model 12 h after
the injection of contrast medium; however, Scr values did not
increase until 48 h after injection. Meanwhile,
immunohistochemistry and western blot analysis indicated that NGAL
expression levels in the kidney had increased by 2 h
post-injection, and peaked at 24 h. ELISA analysis demonstrated
that the serum level of NGAL increased 2 h after the injection,
demonstrating the same variation trends as NGAL in the kidney. When
Mishra et al (19) conducted
a study of 71 children undergoing cardiac surgery, the NGAL level
in serum and urine significantly increased 2 h after acute kidney
injury; however, but Scr levels did not change until 24 h later.
Bennett et al (20) drew the
same conclusion, and the present findings were consistent.
Therefore, NGAL is a superior indicator for the kidney injury of
early stage than Scr.
Due to the high incidence and mortality rates of
CIN, it is important that a strategy is adopted to prevent the
occurrence of CIN in high-risk patients. Hydration therapy has been
demonstrated to be effective (21,22);
however, individuals who do not have good cardiac function, cannot
receive a large liquid infusion, thus hydration therapy is not
viable. A previous study has demonstrated the harm of hydration
therapy (23). Studies have shown
that oxidative stress has an important role in CIN (24–26). In
oxidative stress, radicals reacts with lipids to form MDA. MDA is
cytotoxic, and can cause the polymerization of protein and nucleic
acid. There are two types of antioxidant systems in the human body,
antioxidant enzyme systems and non-enzymatic antioxidant system.
SOD is an important member of the antioxidant enzyme family. These
enzymes constitute one of the primary defense mechanisms of cells
against oxidative stress and serve a role in the pathogenesis of
certain invasive bacteria.
NAC has been used as a type of mucolytics to treat
respiratory diseases in clinical settings for ≥30 years. It is a
precursor of intracellular reduced glutathione, with various
biological activities, including interfering radical generation,
clearing radicals, regulating intracellular metabolic activities
and preventing DNA damage (8,27). In
the present study, NAC was investigated for its potential to reduce
the kidney damage caused by contrast agents. Scr values in the CIN
group has significantly increased 48 h after the injection of
contrast medium; however, at the same time point, the difference
between the NAC+CIN and CON groups was not statistically
significant. Scr values in the NAC+CIN group did not significantly
increase until 72 h later, and remained lower than that of the CIN
group at the same time point. Conversely, HE staining of the kidney
tissue demonstrated that the NAC+CIN group exhibited markedly
reduced pathological manifestations of renal injury compared with
the CIN group, such as brush border disappearance, vacuolization,
tubular necrosis and protein deposition. Meanwhile, the renal
injury scores of the NAC+CIN group were significantly lower than
those of the CIN group at each time point. These findings suggest
that NAC treatment may be a useful method to protect the kidneys
from the injury caused by contrast agents.
According to the present data, a decline in kidney
SOD levels was observed in the CIN group compared with the CON or
NAC+CIN groups, accompanied by an increase of kidney MDA, which was
significantly higher than the other three groups. This indicates
that NAC altered the influence of contrast agent on renal oxidative
stress. No significant differences were detected between all the
indicators of the NAC and CON groups. Therefore, these findings
indicated that NAC did not have a significant impact on normal
kidneys, but may inhibit the contrast agent-induced oxidative
stress damage in the kidney.
In conclusion, the present study demonstrated that
NGAL may be a superior indicator of early stage kidney injury than
the conventional diagnostic indicator, Scr. Prevention of NAC was
able to reduce the kidney damage caused by contrast agents, and
this may be associated with the inhibition of oxidative stress. The
present research demonstrated the feasibility of a novel indicator
for the prevention of kidney injury, and provides a new choice for
the prevention of contrast-induced nephropathy.
References
|
1
|
Nash K, Hafeez A and Hou S:
Hospital-acquired renal insuffciency. Am J Kidney Dis. 39:930–936.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonventre JV, Vaidya VS, Schmouder R, Feig
P and Dieterle F: Next-generation biomarkers for detecting kidney
toxicity. Nature Biotechnol. 28:436–440. 2010. View Article : Google Scholar
|
|
3
|
Patsaoura A, Tatsi E, Margeli A, Kanavaki
I, Delaporta P, Kyriakopoulou D, Kouraklis-Symeonidis A, Kattamis A
and Papassotiriou I: Plasma neutrophil gelatinase-associated
lipocalin levels are markedly increased in patients with
non-transfusion-dependent thalassemia: Lack of association with
markers of erythropoiesis, iron metabolism and renal function. Clin
Biochem. 47:1060–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finn WF: The clinical and renal
consequences of contrast-induced nephropathy. Nephrol Dial
Transplant. 21:i2–i10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tepel M, Aspelin P and Lameire N:
Contrast-induced nephropathy: A clinical and evidence-based
approach. Circulation. 113:1799–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mehran R and Nikolsky E: Contrast-induced
nephropathy: Definition, epidemiology, and patients at risk. Kidney
Int Suppl. S11–S15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldenberg I and Matetzky S: Nephropathy
induced by contrast media: Pathogenesis, risk factors and
preventive strategies. CMAJ. 172:1461–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Briguori C, Tavano D and Colombo A:
Contrast agent-associated nephrotoxicity. Prog Cardiovasc Dis.
45:493–503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toprak O and Cirit M: Risk factors for
contrast-induced nephropathy. Kidney Blood Press Res. 29:84–93.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katzberg RW: Urography into the 21st
century: New contrast media, renal handling, imaging
characteristics, and nephrotoxicity. Radiology. 204:297–312. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tumlin J, Stacul F, Adam A, Becker CR,
Davidson C, Lameire N and McCullough PA: CIN Consensus Working
Panel: Pathophysiology of contrast-induced nephropathy. Am J
Cardiol. 98:14K–20K. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perrin T, Descombes E and Cook S:
Contrast-induced nephropathy in invasive cardiology. Swiss Med
Wkly. 142:w136082012.PubMed/NCBI
|
|
13
|
Yokomaku Y, Sugimoto T, Kume S, Araki S,
Isshiki K, Chin-Kanasaki M, Sakaguchi M, Nitta N, Haneda M, Koya D,
et al: Asialoerythropoietin prevents contrast-induced nephropathy.
J Am Soc Nephrol. 19:321–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
James MT, Ghali WA, Tonelli M, Faris P,
Knudtson ML, Pannu N, Klarenbach SW, Manns BJ and Hemmelgarn BR:
Acute kidney injury following coronary angiography is associated
with a long-term decline in kidney function. Kidney Int.
78:803–809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
James MT, Ghali WA, Knudtson ML, Ravani P,
Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn
BR, et al: Associations between acute kidney injury and
cardiovascular and renal outcomes after coronary angiography.
Circulation. 123:409–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice
guideline for acute kidney injury. Kidney Int Suppl. 2:1–138.
2012.
|
|
17
|
Bellomo R, Kellum JA and Ronco C: Defining
acute renal failure: Physiological principles. Intensive Care Med.
30:33–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cowland JB and Borregaard N: Molecular
characterization and pattern of tissue expression of the gene for
NGAL from humans. Genomics. 45:17–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishra J, Mor IK, Ma Q, Kelly C, Yang J,
Mitsnefes M, Barasch J and Devarajan P: Amelioration of ischemic
acute renal injury by neutrophil gelatinase-associated lipocalin. J
Am Soc Nephrol. 15:3073–3082. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennett M, Dent CL, Ma Q, Dastrala S,
Grenier F, Workman R, Syed H, Ali S, Barasch J and Devarajan P:
Urine NGAL predicts severity of acute kidney injury after cardiac
surgery: A prospective study. Clin J Am Soc Nephrol. 3:665–673.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Navaneethan SD, Singh S, Appasamy S, Wing
RE and Sehgal AR: Sodium bicarbonate therapy for prevention of
contrast-induced nephropathy: A systematic review and
meta-analysis. Am J Kidney Dis. 53:617–627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Recio-Mayoral A, Chaparro M, Prado B,
Cózar R, Méndez I, Banerjee D, Kaski JC, Cubero J and Cruz JM: The
reno-protective effect of hydration with sodium bicarbonate plus
N-acetylcysteine in patients undergoing emergency percutaneous
coronary intervention: The RENO study. J Am Coll Cardiol.
49:1283–1288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reinecke H, Fobker M, Wellmann J, Becke B,
Fleiter J, Heitmeyer C, Breithardt G, Hense HW and Schaefer RM: A
randomized controlled trial comparing hydration therapy to
additional hemodialysis or N-acetylcysteine for the prevention of
contrast medium-induced nephropathy: The Dialysis-versus-Diuresis
(DVD) Trial. Clin Res Cardiol. 96:130–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toprak O, Cirit M, Tanrisev M, Yazici C,
Canoz O, Sipahioglu M, Uzum A, Ersoy R and Sozmen EY: Preventive
effect of nebivolol on contrast-induced nephropathy in rats.
Nephrol Dial Transplant. 23:853–859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodman A, Olszanecki R, Yang LM, Quan S,
Li M, Omura S, Stec DE and Abraham NG: Heme oxygenase-1 protects
against radiocontrast-induced acute kidney injury by regulating
anti-apoptotic proteins. Kidney Int. 72:945–953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katholi RE, Woods WT Jr, Taylor GJ,
Deitrick CL, Womack KA, Katholi CR and McCann WP: Oxygen free
radicals and contrast nephropathy. Am J Kidney Dis. 32:64–671.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heyman SN, Rosen S, Khamaisi M, Idée JM
and Rosenberger C: Reactive oxygen species and the pathogenesis of
radiocontrast-induced nephropathy. Invest Radiol. 45:188–195. 2010.
View Article : Google Scholar : PubMed/NCBI
|