Introduction
Postoperative pain related to tissue damage during
an operation is the most common acute pain that occurs immediately
after clinical surgery. Postoperative pain may evolve into a
persistent postoperative pain, also known as chronic pain, if
urgent care is not administrated adequately at its initial stage
(1). Postoperative pain may also
affect the prognosis by increasing the risk of postoperative
complications, prolonged hospital stays and additional
hospitalization expenses (2).
Therefore, the effective treatment of postoperative pain is of high
clinical importance. However, administration of single therapies in
the treatment of postoperative pain has shown limitations. In
recent years, studies have indicated that multiple targets are
involved in the formation mechanism of postoperative pain (3), suggesting the potential clinical
efficacy of combinations of multiple analgesics and therapies. One
of the most important tasks at present is to clarify the formation
mechanism of postoperative pain and to propose novel therapies.
The formation mechanism of postoperative pain
involves peripheral sensitization and central sensitization.
Peripheral sensitization decreases the afferent threshold of
noxious stimulation, while central sensitization enhances the
stimulant function and decreases inhibitory function of the pain
transmission of central neurons (4).
Enhanced stimulant function involved in central sensitization was
traditionally considered an important mechanism in the formation of
postoperative pain. However, declined function of the inhibitory
system has gradually attracted attention in recent years. The
descending inhibitory system is an endogenous analgesia system
composed of periaqueductal gray (PAG), nucleus raphe magnus (NRM)
and dorsal horn of the spinal column(5). Its terminal mainly projects at I–II and
IV–V of the spinal cord. Analgesic effects from electroacupuncture
stimulation of the descending inhibitory system have been
identified (6). Thus, the promotion
of postoperative pain relief by enhancing the function of the
descending inhibitory system exogenously is imperative.
5-Hydroxytryptamine (5-HT) is one of the main
neurotransmitters involved in the descending inhibitory system. It
functions by binding with specific 5-HT receptors. In recent years,
the 5-HT2A receptor (5-HT2AR) has gained
attention as a potential new target in pain research. The
expression of 5-HT2AR in the descending pain modulation
pathway of rat brainstem was detected following an increase in
inflammatory pain induced by carrageenan (7). In addition, an agonist of
5-HT2AR may inhibit inflammatory pain (8) and neuropathic pain (9), suggesting that 5-HT2AR is
involved in 5-HT descending pain modulation system. Furthermore,
5-HT2AR is a G protein-coupled receptor which could
couple with Gq and activate phospholipase C (PLC)-β so as to
increase the intracellular Ca2+ concentration and
activate protein kinase C (PKC).
GABA is one of the major inhibitory
neurotransmitters involved in the descending inhibitory system.
GABA can activate the ionic GABAA receptors and cause
Cl− internal flow by transferring extracellular
Cl− intracellularly, hyperpolarizing neurons so as to
inhibit discharging. Internal flow of Cl− depends on the
concentration gradient across the cellular membrane, which depends
on the potassium-chloride cotransporter 2 (KCC2). KCC2, expressed
in neurons specifically, is the primary protein in control of the
inhibitory function of the GABAA receptor. It pumps out
intracellular chlorine ion to maintain the inhibitory function of
GABAA receptors. Reduced KCC2 function on the cell
membrane causes an increased concentration of intracellular
chlorine ion, activating and depolarizing GABAA
receptors, leading to the declined inhibitory function of
GABAA receptors and raised excitability of central
neural network, resulting in the central sensitization. Studies
have reported that reduced function of KCC2 is implicated in the
mechanism of neuropathic pain. It also plays an important role in
the molecular mechanism of pain sensitization (10,11).
The relationship between 5-HT2AR and KCC2
has been demonstrated in previous studies. Activated
5-HT2AR causes the hyperpolarization of inhibitory
postsynaptic potential (IPSP) and increases the expression of KCC2
in the cell membrane through a mechanism mediated by PKC (12). Previous findings demonstrated the
decreased expression of spinal cord KCC2 in a rat incisional pain
model, while the activity and expression level of KCC2 could be
dynamically changed by the phosphorylation of the carboxyl end in
the intracellular structure domain of KCC2 (13). Furthermore, PKC-mediated
phosphorylation may enhance the activity of KCC2 and reduce the
swallowing function (14).
Therefore, 5-HT2AR activity may be mediated by PKC to
increase the expression level of KCC2, resulting in an analgesic
effect.
The aim of the study was to investigate the role of
the 5-HT2AR-KCC2 signaling pathway in the formation
mechanism of postoperative pain and the therapeutic effect of
5-HT2AR agonist TCB-2 on postoperative pain. We
evaluated the effects of the 5-HT2AR agonist, TCB-2, and
the 5-HT2AR antagonist, ketanserin, on the mechanical
and thermal hyperalgesia behavioral response in incision pain rats.
PCR and western blotting were used to measure the transcription and
translation levels of KCC2 in the spinal cord. We also investigated
the effects of TCB-2 and ketanserin on the expression levels of
KCC2 and explored the formation mechanism of postoperative pain. We
anticipate that our findings will help provide an experimental
basis for the promotion of clinical treatment options for
postoperative pain.
Materials and methods
Experimental animals and grouping
A total of 21 male Sprague-Dawley rats of clean
class, weighing 200–250 g, were purchased from an Animal Experiment
Center of Ruijin Hospital, Affiliated to the School of Medicine,
Shanghai Jiaotong University (Shanghai, China). The rats were
housed with environmental conditions maintained at 22±1°C and a
12/12-h light-dark cycle. Each rat was treated with intrathecal
catheterization and kept in a single cage with free access to
standard food and water. Approval for the animal experiments was
received from the Animal Ethics Committee of the Second Military
Medical University.
Experimental animals were randomly assigned to the
sham or incision pain group in order to test the incision pain
model. Incision pain rats were then randomly assigned into 3 groups
that were treated with either dimethyl sulfoxide (DMSO) (D4540-500
ml; Sigma-Aldrich, St. Louis, MO, USA), TCB-2 (5-HT2AR
agonist, 2592; Tocris, Bristol, UK) or ketanserin
(5-HT2AR antagonist, S006; Sigma-Aldrich), respectively.
Behavioural experiments were conducted between 9 a.m. and 5
p.m.
Intrathecal catheterization model
The rats were treated with intrathecal
catheterization after being housed for one week and then
anaesthetized by isoflurane inhalation and placed in a stereotactic
frame. Skin was incised by approximately 1.5 cm on the L3-L4 gaps
of the dorsal medialine to expose the superior and inferior
articular process and ligamentum flavum. A no. 7 needle was used to
puncture the ligamentum flavum, endorhachis and arachnoid until a
side swing of the tail or twitch of the hind leg was noted. A
polyurethane (PE-10) catheter prefilled with physiological saline
was inserted in proximity to the lumbar enlargement of the spinal
cord. The exit end of the catheter was taken out through a separate
opening between the ears. The catheter and opening were fixed and
sutured by polyamide sutures (4–0). The rat was then placed in a
single cage with the environment kept warm and quiet. After a 5-day
recovery, 20 µl of 2% lidocaine, followed by 10 µl of physiological
saline were injected by a microsyringe through the catheter. The
rats showed paralysis in the lower extremities and reflection of
clipping tails disappeared within 30 sec, indicating the success of
the intrathecal catheterization model.
Incision pain model
The incision pain model was conducted after the
treatment of intrathecal catheterization, according to Brennan
et al (15). The rats were
given a subcutaneous injection of 80,000 UI of penicillin and
anaesthetized by isoflurane inhalation. Skin was incised by
approximately 1 cm on the plantar aspect of the foot. An ophthalmic
forceps was used to incise lengthwise on the muscle, which was
maintained integrally. Skin incision was sutured by polyamide
sutures (5–0) and then the rats were placed in a warm and quiet
environment.
Behavioural pain testing
To determine the mechanical withdrawal threshold
(MWT), rats with free activities were placed in an overhead plastic
box with a steel net bottom. After adaption for 30 min, von Frey
(mechanical tactile test package, 58011; Stoelting, Wood Dale, IL,
USA) (1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 15.0 and 26.0 g) was used to
stimulate the incision from 4.0 g, according to Coull et al
(16). Mechanical withdrawal was
observed and recorded as O for negative and X for positive. A
higher or lower level of stimulation was applied until mechanical
withdrawal presented as negative. The last level of stimulation was
recorded and the average MWT of each rat was calculated.
Thermal withdrawal latency (TWL)
Ugo Basile (37370; Comerio, Italy) was used to
evaluate the TWL of the left foot. Rats were placed on a special
glass platform in a separate plastic enclosure and adapted for 30
min. The incision was exposed to a thermal stimulus produced by the
infrared ray until the rat withdrew its paw upon feeling pain. The
time between exposure of the incision to the stimulus and its
withdrawal was recorded in seconds by the computer as TWL. The
maximum of TWL was set at 25 sec in case of thermal radiation
damage. The test was performed 5 times for each rat and the average
TWL was calculated without the highest and the lowest values.
Western blotting
Incision pain rats were sacrificed at 12 h, 1, 3 and
5 days after the surgery. Rats were also sacrificed 6 h after the
intrathecal delivery of TCB-2 or ketanserin. Following each
sacrifice, the lumbar enlargement was removed for the measurement
of KCC2 expression. Each 20-mg tissue sample was cut into sections
and mixed with 250 µl histiocytic lysis buffer (RIPA; Solarbio,
Shanghai, China), containing a 0.01% protease inhibitor cocktail
(Sigma-Aldrich, Shanghai, China). After being fully lysed, the
samples were centrifuged at 12,000 RCF for 15 min at 4°C and the
supernatant was collected. A BCA protein quantification kit (BCA;
Thermo Fisher Scientific, Shanghai, China) was used to quantify the
protein contents. The tissue samples were run on a 12% (85 µg/well)
SDS-PAGE gel and transferred to a nitrocellulose filter membrane
(Millipore, Shanghai, China), electrophoretically. Blots were
blocked with 5% skim milk at room temperature for 1 h, followed by
incubation with anti-KCC2 and GAPDH (Cell Signaling Technology,
Danvers, MA, USA) antibodies, and then incubated with goat
anti-mouse or anti-rabbit secondary antibody (Beyotime, Shanghai,
China). Enhanced chemiluminescence (ECL; Thermo Fisher Scientific)
was used to assess the blots visually.
Real-time PCR assay
Total RNA was extracted from tissue samples and
quantified, using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). A
reverse transcription kit (Fermentas, Shanghai, China) was used to
synthesize cDNA through a total volume of 25 µl [12 µl RNA-primer
mix, 5 µl 5X RT reaction buffer, 1 µl 25 mM dNTPs, 1 µl 25 U/µl
RNase inhibitor, 1 µl 200 U/µl M-MLV Rtase, 1 µl
Oligo(dt)18 and 4 µl DNase-free ddH2O]. Then,
cDNA was amplified through a total PCR system of 25 µl (12.5 µl
SYBR-Green mix, 0.5 µl forward primer, 0.5 µl reverse primer, 9.5
µl ddH2O and 2 µl cDNA template) with the PCR conditions
consisting of 10 min at 95°C, and then 15 sec at 95°C and 45 sec at
60°C, for 40 cycles. Amplification kinetic curves were obtained
through 15 sec at 95°C, 1 min at 60°C, 15 sec at 95°C and 15 sec at
60°C.
Statistical analysis
Experimental data were analyzed by SPSS 16.0
statistical software (Chicago, IL, USA), using two-way RM analysis
of variance (ANOVA), and was presented as mean ± SEM. Bonferroni
correction was applied in pairwise comparisons. One-way ANOVA was
used for intra-group comparisons. P<0.05 was considered to
indicate a statistically significant difference. Graphs were
produced by GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
Incision pain model in rats
Changes in MWT in the incision pain
rats
As shown in Fig. 1A,
MWT of rats in the incision group (n=7) decreased on days 1, 2, 3,
4, 5 and 7 after surgery, with statistical significance
(P<0.05), compared with that of the sham group (n=7). Although
there was no statistical significance (P>0.05) detected in the
MWT between the sham and incision groups on day 6, the incision
group showed a much lower MWT in comparison to the sham group
overall.
Changes in TWL in the incision pain
rats
As shown in Fig. 1B,
TWL of rats in the incision group (n=7) decreased from day 1 to 3
after surgery, with statistical significance (P<0.05) compared
with that of the sham group (n=7). However, there was no
statistical significance (P>0.05) detected in the MWT between
the two groups from day 4 to 7.
Behavioural changes of incision pain rats
after intrathecal delivery of TCB-2 and ketanserin
Changes in MWT in incision pain rats
after intrathecal delivery of TCB-2 and ketanserin
Rats were delivered 5-HT2AR agonist,
TCB-2 (100 µg), or antagonist, ketanserin (30 µg), by intrathecal
administration on day 1 after surgery. As shown in Fig. 2A, MWT of the incision + TCB-2 group
(n=10) increased and MWT of the incision + ketanserin group (n=6)
decreased on day 1 after delivery, compared with the incision +
DMSO group (n=7), both with a significant difference
(P<0.05).
 | Figure 2.Changes in MWT and TWL in incision
pain rats were recorded after intrathecal delivery of the
5-HT2AR agonist, TCB-2, and the 5-HT2AR
antagonist, ketanserin, at baseline and 1–7 days after. (A) MWT
increased in the incision+TCB-2 group, while it decreased in the
incision+ketanserin group after 1 day (*P<0.05, **P<0.01),
compared with the incision+DMSO group. (B) Changes in TWL were
detected in incision pain rats after intrathecal delivery of the
5-HT2AR agonist, TCB-2, and the 5-HT2AR
antagonist, ketanserin (*P<0.05), compared with the
incision+DMSO group. MWT, mechanical withdrawal threshold; TWL,
thermal withdrawal latency; 5-HT2AR, 5-hydroxytryptamine
2A receptor; DMSO, dimethyl sulfoxide. |
Changes in TWL in the incision pain
rats after intrathecal delivery of TCB-2 and ketanserin
As shown in Fig. 2B,
TWL of the incision + TCB-2 group (n=10) showed no significant
difference in comparison to the incision + DMSO group (P>0.05,
n=7). The rats in the incision + ketanserin group (n=6) showed a
significantly decreased TWL on day 2 after surgery compared with
the incision + DMSO group (P<0.05, n=7).
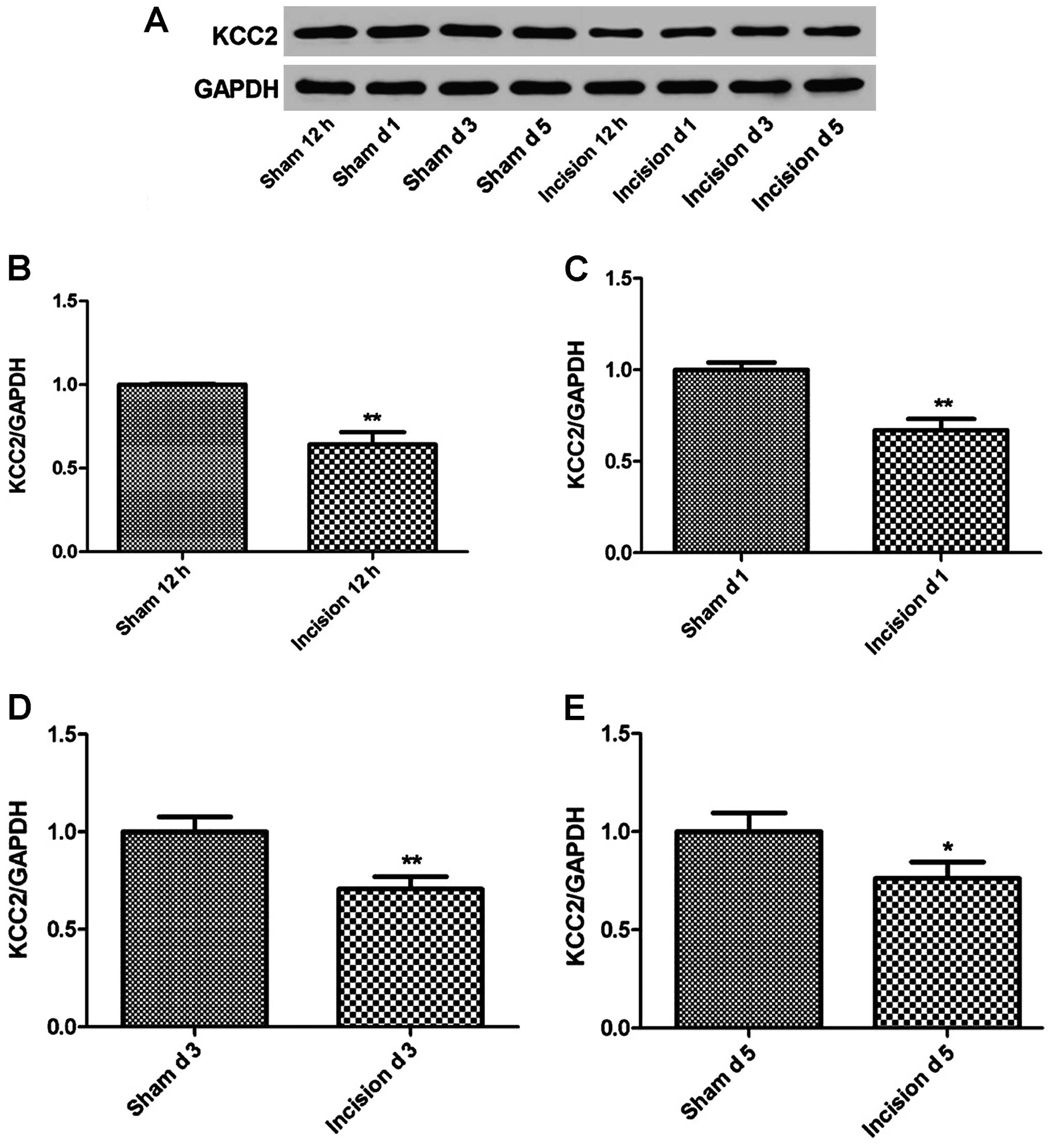
Changes in KCC2 expression in the
incision pain rats
The KCC2 expression in the incision pain rats (n=7)
was measured using western blot analysis at 12 h, 1, 3 and 5 days
after the surgery. As shown in Fig.
3, the expression level of KCC2 demonstrated a significant
decrease in the incision group compared to the sham group after
surgery (P<0.05 or P<0.01).
Effect of TCB-2 and ketanserin on the
expression of KCC2 after intrathecal delivery
The transcription and translation levels of KCC2
were measured using quantitative PCR and western blotting,
respectively. As shown in Figs. 4
and 5, the transcription and
translation levels were decreased in the incision + ketanserin
group (P<0.05 or P<0.01), indicating the inhibitory effect of
ketanserin on the expression level of KCC2. On the other hand,
significant increases in the transcription and translation levels
were detected in the incision + TCB-2 group (P<0.05 or
P<0.01).
Discussion
In this study, we applied the classic rat incision
pain model and demonstrated significant decrease in MWT and TWL.
Decreased MWT and TWL lasted for 3 and 7 days, respectively,
indicating a successful simulation of postoperative pain in this
model. Given the unclear formation mechanism of postoperative pain,
it remains one of the most challenging problems in clinical pain
therapeutics. We explored the mechanism of pain sensitization by
using a rat incision pain model in order to provide an experimental
basis for the promotion of clinical treatment of postoperative
pain.
We found that the MWT of the incision pain rats
increased on day 1 after the delivery of 5-HT2AR
agonist, TCB-2, while the TWL showed no significant change. On the
other hand, the MWT and TWL of rats decreased on day 1 and 2 after
the delivery of 5-HT2AR antagonist, ketanserin,
respectively, indicating that the activation of 5-HT2AR
alleviated mechanical hyperalgesia caused by incision pain, while
the antagonism of 5-HT2AR aggravated mechanical and
thermal hyperalgesia. The mechanism of TCB-2 in pain sensitization
may involve the activation of 5-HT2AR, leading to the
increased expression of downstream signaling molecules, which may
be downregulated by ketanserin. 5-HT2AR is a G
protein-coupled receptor which mediates a series of functions by
the activation of PKC after coupling with Gq. The activation of
5-HT2AR was reported to induce the expression of KCC2 on
the cell membrane through the mediation of PKC, resulting in the
recovery of endogenous inhibitory function in a spinal cord injury
(SCI) model (12). In the present
study, alleviated pain sensitization was detected after the
intrathecal delivery of 5-HT2AR agonist TCB-2. By
contrast, aggravated mechanical and thermal hyperalgesia were
observed after the delivery of 5-HT2AR antagonist,
ketanserin. Thus, we suggest that the 5-HT2AR-KCC2
pathway may be involved in the formation of incision pain.
The study of 5-HT2AR in pain research
has, thus far, mainly focused on neuropathic pain and inflammatory
pain models. 5-HT2AR belongs to the group of 5-HT
receptors involved in the descending inhibitory system. It was
reported that a significant increase in the expression of
5-HT2AR in the descending pain modulation pathway of rat
brainstem was detected during inflammatory pain induced by
carrageenan (7). An agonist of
5-HT2AR may inhibit inflammatory pain (8) or neuropathic pain (9), suggesting that 5-HT2AR is
involved in the 5-HT descending pain modulation system. KCC2,
another member of the inhibitory function system, is also a hotspot
in pain research (16,17). KCC2 is directly involved in
neuropathic pain pathways and plays a crucial role in the molecular
mechanism of pain sensitization. Previous studies detected a
decreased expression of spinal cord KCC2 in a rat incisional pain
model, indicating the participation of 5-HT2AR-KCC2
signaling pathway in the formation of SCI. However, the mechanism
of the 5-HT2AR-KCC2 pathway involvement in the rat
incision pain model has not been clearly elucidated. We found that
the mechanical hyperalgesia caused by incision pain may be
alleviated by TCB-2, while aggravated mechanical and thermal
hyperalgesia were induced by ketanserin, suggesting that the
expression of KCC2 may be regulated by the 5-HT2AR-KCC2
pathway to participate in the formation of incision pain. We
explored the mechanism of 5-HT2AR-KCC2 pathway in the
formation of incision pain through animal behaviour. Further study
should be conducted to characterize and define the relationship
between KCC2 expression and formation of incision pain.
In conclusion, we succeeded in simulating the
descending inhibitory system in a rat incision pain model by
demonstrating decreased MWT and TWL. The 5-HT2AR
agonist, TCB-2, showed mitigative effects on postoperative pain
which could be exacerbated by the 5-HT2AR antagonist,
ketanserin, indicating the relationship between 5-HT2AR
and the formation of incision pain.
References
|
1
|
Macrae WA: Chronic post-surgical pain: 10
years on. Br J Anaesth. 101:77–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavlin DJ, Chen C, Penaloza DA, Polissar
NL and Buckley FP: Pain as a factor complicating recovery and
discharge after ambulatory surgery. Anesth Analg. 95:627–634. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chou R, Gordon DB, de Leon-Casasola OA,
Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL,
Chittenden EH, Degenhardt E, et al: Management of postoperative
pain: a clinical practice guideline from the American Pain Society,
the American Society of Regional Anesthesia and Pain Medicine, and
the American Society of Anesthesiologists Committee on Regional
Anesthesia, Executive Committee, and Administrative Council. J
Pain. 17:131–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dirks J, Møiniche S, Hilsted KL and Dahl
JB: Mechanisms of postoperative pain: Clinical indications for a
contribution of central neuronal sensitization. Anesthesiology.
97:1591–1596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dostrovsky JO, Shah Y and Gray BG:
Descending inhibitory influences from periaqueductal gray, nucleus
raphe magnus, and adjacent reticular formation. II. Effects on
medullary dorsal horn nociceptive and nonnociceptive neurons. J
Neurophysiol. 49:948–960. 1983.PubMed/NCBI
|
|
6
|
Hosobuchi Y, Adams JE and Linchitz R: Pain
relief by electrical stimulation of the central gray matter in
humans and its reversal by naloxone. Science. 197:183–186. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie H, Ma F, Zhang YQ, Gao X and Wu GC:
Expression of 5-HT2A receptor mRNA in some nuclei of
brain stem enhanced in monoarthritic rats. Brain Res. 954:94–99.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okamoto K, Imbe H, Kimura A, Donishi T,
Tamai Y and Senba E: Activation of central 5HT2A
receptors reduces the craniofacial nociception of rats.
Neuroscience. 147:1090–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Obata H, Saito S, Sasaki M and Goto F:
Interactions of 5-HT2 receptor agonists with
acetylcholine in spinal analgesic mechanisms in rats with
neuropathic pain. Brain Res. 965:114–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lavertu G, Côté SL and De Koninck Y:
Enhancing K-Cl co-transport restores normal spinothalamic sensory
coding in a neuropathic pain model. Brain. 137:724–738. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cramer SW, Baggott C, Cain J, Tilghman J,
Allcock B, Miranpuri G, Rajpal S, Sun D and Resnick D: The role of
cation-dependent chloride transporters in neuropathic pain
following spinal cord injury. Mol Pain. 4:362008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bos R, Sadlaoud K, Boulenguez P, Buttigieg
D, Liabeuf S, Brocard C, Haase G, Bras H and Vinay L: Activation of
5-HT2A receptors upregulates the function of the
neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci USA.
110:348–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaesse P, Airaksinen MS, Rivera C and
Kaila K: Cation-chloride cotransporters and neuronal function.
Neuron. 61:820–838. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HH, Walker JA, Williams JR, Goodier
RJ, Payne JA and Moss SJ: Direct protein kinase C-dependent
phosphorylation regulates the cell surface stability and activity
of the potassium chloride cotransporter KCC2. J Biol Chem.
282:29777–29784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brennan TJ, Van der Meulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coull JA, Boudreau D, Bachand K, Prescott
SA, Nault F, Sík A, De Koninck P and De Koninck Y: Trans-synaptic
shift in anion gradient in spinal lamina I neurons as a mechanism
of neuropathic pain. Nature. 424:938–942. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Price TJ, Cervero F and de Koninck Y: Role
of cation-chloride-cotransporters (CCC) in pain and hyperalgesia.
Curr Top Med Chem. 5:547–555. 2005. View Article : Google Scholar : PubMed/NCBI
|