Introduction
Intracranial aneurysms (IAs) are relatively common
vascular abnormalities of the cerebrum and are the third leading
cause of cerebrovascular accidents, accounting for ~3% in the
general population (1). Currently,
the incidence and operations of IAs have significantly increased,
and IA-associated clinical manifestations, such as intracranial
hypertension and hemorrhage, are considered to have a diameter of
≥0.6 cm for patients with increasing risk of rupture, which is
associated with significant morbidity and mortality (2,3).
Notably, subarachnoid hemorrhage remains lethal in up to 65% of
cases, and significantly disables 50% of those who survive
(4). Complex open surgical repair
and endovascular approaches have proven to be a good and potent
alternatives to open repair of these aneurysms for older and
high-risk patients as well as for aneurysms with optimal
morphological suitability (5).
However, intervention therapy has the risk of developing a
neurological injury (6–8). Nevertheless, the advances in effective
therapy for IAs have been limited due to inherited pathogenic
pathways remaining obscure. Therefore, identification of the
molecular mechanism for the IAs is required in order to develop an
effective treatment.
Particularly interesting Cys-His-rich protein
(PINCH), which is composed of 5 LIM domains arrayed in tandem, is
expressed in an ubiquitous manner in early embryonic development
and adult tissues and has been suggested to be important in
processes as diverse as migration, cell adhesion, differentiation,
proliferation and survival (9,10).
Functional studies have revealed that PINCH loss-of-function leads
to embryonic lethality and displays an abnormal epiblast polarity,
impaired cavitation, detachment of primitive endoderm (PrE) and
severe apoptosis of the PrE (11,12).
PINCH-1 promotes B-cell lymphoma (Bcl)-2-dependent survival
signalling and inhibits c-Jun N-terminal kinase (JNK)-mediated
apoptosis in the PrE (13). Although
the function of PINCH remains unknown, increasing numbers and
accumulating evidence indicates that PINCH has been correlated with
cancer development, invasion and metastasis in malignant cells,
including breast cancer (14),
pseudomyxoma peritonei (15),
gastric adenocarcinoma (16) and
rectal cancer (17). For example,
PINCH is demonstrated to protect tumor cells from apoptosis by
increasing the activity of the pro-survival protein extracellular
signal-regulated kinase (ERK)1/2 and Akt (18). In breast cancer cells, Ras
suppressor-1 induces apoptosis through the suppression of PINCH-1,
and the integrin-linked kinase/PINCH/α-parvin (IPP) complex as a
therapeutic target is downregulated in the chelidonine-treated
MDA-MB-231 cell line (14,19). To the best of our knowledge there is
no literature available regarding the association between PINCH
abnormal expression and IAs.
In the present study, an association between PINCH
and tumor size was revealed, and PINCH was highly expressed in IAs
(including unruptured and ruptured aneurysms), in which it was
predominantly localized to the lumen. Furthermore, the present
study sought to determine the regulatory events associated with the
expression of PINCH in ruptured and unruptured human cerebral
aneurysms. Moreover, the levels of PINCH were demonstrated to be
significantly higher in unruptured compared to ruptured aneurysms
as observed by immunohistochemistry and western blotting. In
addition, the rupture of aneurysms was associated with the
expression of PINCH in the cerebral aneurysms.
Materials and methods
Patients and specimens
A total of 31 IAs (including 19 unruptured and 12
ruptured cerebral aneurysms) were collected from Fu Xing Hospital
Affiliated to Capital Medical University (Beijing, China) between
Jan 2008 and June 2014. All the patients recruited in the present
study were not subjected to preoperative radiotherapy or
chemotherapy and were diagnosed with IAs based on histopathological
evaluation by digital subtraction angiography (DSA), as previously
described (20). The control
specimens were 5 intracranial cerebral arteries obtained from
patients who underwent surgery. All of the tissue samples collected
were immediately stored in liquid nitrogen for western blot
analysis or fixed in 10% formalin for immunohistochemical staining.
Human samples were obtained with written informed consent from all
patients. In addition, the study was approved by the Ethics
Committee of the Fu Xing Hospital Affiliated to Capital Medical
University (Beijing, China).
Histomorphology and
immunohistochemistry
Formalin-fixed and paraffin-embedded tumor tissues
were cut into 4 µm sections, which were stained with hematoxylin
and eosin (H&E) and visualized under a Leica DM 2500 microscope
(Leica Microsystems, Inc., Buffalo Grove, IL, USA).
The 5 control artery and 31 aneurysm samples were
immunohistochemically evaluated using anti-human alpha-smooth
muscle actin (α-SMA; ab5694; 1:200; Abcam, Cambridge, MA, USA),
osteopontin (OPN; sc-20788; 1:100; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), matrix metalloproteinase (MMP) 9 (ab73734;
1:200; Abcam) and PINCH (sc-136299; 1:50, Santa Cruz Biotechnology,
Inc.). Tissues embedded in paraffin were cut into 4-µm sections,
mounted on glass slides and stained using indirect immunoperoxidase
(P0203; Beyotime Institute of Biotechnology, Haimen, China). The
paraffin sections were then baked in an oven at 65°C for 24 h, then
dewaxing to water, and were rinsed with PBS three times (5 min each
time). The sections that were washed well were placed in the EDTA
buffer for microwave antigen retrieval, boiled, then low heat
(100°C) to boil after an interval of 10 min. Following cooling, the
sections were washed with PBS 3 times. They were then placed in 3%
hydrogen peroxide solution and incubated at room temperature for 10
min, with the purpose of blocking any endogenous peroxidase. These
were then washed with PBS 3 times, and blocked with 5% bovine serum
albumin (BSA; ST023; Beyotime Institute of Biotechnology) for 20
min after drying (close charge). Following the removal of the BSA
solution, each section was incubated with 50 µl anti-α-SMA,
anti-OPN, anti-MMP9 and anti-PINCH primary antibodies overnight at
4°C, and then washed with PBS 3 times. Following the removal of PBS
solution, each slice was incubated with 50–100 µl goat anti-rabbit
IgG (A0208) and goat anti-mice IgG (A0216; both 1:5,000; both
Beyotime Institute of Biotechnology) secondary antibodies at 4°C
for 50 min, then washed thrice with PBS, and each slice was added
to 50–100 µl freshly prepared DAB solution with the help of a
microscope in order to observe color. After washing, the sections
were counterstained with hematoxylin, rinsed with tap water,
dehydrated and mounted. Six sections were randomly selected with no
overlapping area (magnification, ×200), were observed and
photographed. The tissue with brown color (except for the tissue
edges) were regarded as α-SMA, OPN, MMP9 and PINCH positive. Image
Pro-Plus 6 software (Media Cybernetics, Inc., Rockville, MD, USA)
was used for the analysis. The integrated optical density (IOD) was
respectively measured for the tumor tissues of immunohistochemical
positive staining. The higher the IOD, the higher the expression of
the corresponding protein and the lower the expression of the
corresponding protein was.
Western blot analysis
Tumor tissues were homogenized and NP-40 buffer
(P0013F; Beyotime Institute of Biotechnology) was used to extract
them, followed by 5–10 min of boiling and centrifugation at 7,200 ×
g for 15 min at 4°C in order to obtain the supernatant. A
10% SDS-PAGE gel was used to separate samples containing 50 µg
protein, which were then transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following
saturation with 5% (w/v) non-fat dry milk in Tris-buffered saline
and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with
the following antibodies: PINCH, OPN, Bcl-2 (sc-56015; 1:2,000),
Bcl-2-associated X protein (Bax; sc-20067; 1:2,000), caspase3
(sc-271759; 1:1,000), JNK (sc-137018; 1:500), p-JNK (sc-293136;
1:500), cyclin B1 (sc-7393; 1:1,000), cyclin D1 (sc-70899;
1:1,000), CDC2 (sc-137035; 1:1,000), ERK (sc-514302; 1: 2,000),
p-ERK (sc-101761; 1:1,000,), p38 (sc-4315; 1:1,000) and p-p38
(sc-101758; 1:500; all Santa Cruz Biotechnology, Inc.), α-SMA and
MMP9 (ab73734; 1:1,000; both Abcam) at 4°C overnight. Following
three washes with TBST, the membranes were incubated with goat
anti-rabbit IgG and goat anti-mice IgG (both 1:5,000) secondary
antibodies conjugated to 800CW Infrared IRDye, including donkey
anti-goat IgG and donkey anti-mouse IgG at a dilutions of
1:10,000–1:20,000. After a 1 h incubation at 37°C, the membranes
were washed three times with TBST and the blots visualized by the
Odyssey Infrared Imaging System (LI-COR Biotechnology, Lincoln, NE,
USA). The signals were assessed densitometrically (Odyssey
Application Software, version 3.0) and were normalized in order to
correct for unequal loading using the mouse monoclonal anti-β-actin
antibody (AP0060; 1:1,000; Bioworld Technology, Inc., St. Louis
Park, MN, USA).
Statistical analysis
All the data were expressed as the mean ± standard
deviation. The statistical analyses were performed using the SPSS
13.0 statistical software package (SPSS, Inc., Chicago, IL, USA),
and one-way analysis of variance was used to perform comparisons
among the different groups. P<0.05 was used to indicate a
statistically significant difference.
Results
Clinical characteristics of patients
with ruptured and unruptured aneurysms
No significant differences were observed in the mean
age (48.8 vs. 43.6 years old), gender distribution (men/women, 9:10
vs. 5:7) and location of aneurysm between patients with unruptured
and ruptured cerebral aneurysms (Tables
I and II). Furthermore, the
mean sizes of unruptured and ruptured aneurysms were 24.84 and
12.67 mm, respectively. However, the tumor size in the unruptured
aneurysms group was significantly higher compared to that of the
ruptured aneurysms group (P<0.05).
 | Table I.Expression of α-SMA, OPN, MMP9 and
PINCH in unruptured aneurysms. |
Table I.
Expression of α-SMA, OPN, MMP9 and
PINCH in unruptured aneurysms.
| No. | Age | Gender | Location | Size (mm) | α-SMA | MMP9 | OPN | PINCH |
|---|
| 1 | 55 | M | R-MCA | 23 | 0.201 | 0.294 | 0.144 | 0.287 |
| 2 | 64 | M | L-PCA | 12 | 0.307 | 0.165 | 0.115 | 0.228 |
| 3 | 56 | M | R-PCA | 27 | 0.158 | 0.276 | 0.133 | 0.186 |
| 4 | 22 | M | R-MCA | 16 | 0.231 | 0.285 | 0.265 | 0.273 |
| 5 | 61 | M | R-VA | 27 | 0.192 | 0.253 | 0.243 | 0.256 |
| 6 | 6 | M | L-PCA | 15 | 0.134 | 0.221 | 0.259 | 0.269 |
| 7 | 51 | M | L-VA | 22 | 0.145 | 0.287 | 0.184 | 0.228 |
| 8 | 54 | M | R-MCA | 12 | 0.178 | 0.211 | 0.213 | 0.249 |
| 9 | 52 | M | L-ICA | 35 | 0.211 | 0.235 | 0.356 | 0.348 |
| 10 | 50 | F | L-ICA | 12 | 0.309 | 0.178 | 0.174 | 0.203 |
| 11 | 69 | F | L-ICA | 30 | 0.236 | 0.168 | 0.194 | 0.232 |
| 12 | 44 | F | L-MCA | 34 | 0.314 | 0.259 | 0.184 | 0.238 |
| 13 | 56 | F | L-ICA | 7 | 0.073 | 0.252 | 0.283 | 0.131 |
| 14 | 12 | F | L-PCA | 30 | 0.238 | 0.104 | 0.236 | 0.245 |
| 15 | 52 | F | R-ICA | 40 | 0.173 | 0.146 | 0.189 | 0.253 |
| 16 | 62 | F | R-ICA | 15 | 0.138 | 0.257 | 0.324 | 0.278 |
| 17 | 52 | F | R-MCA | 40 | 0.221 | 0.231 | 0.341 | 0.397 |
| 18 | 62 | F | R-ICA | 25 | 0.066 | 0.182 | 0.195 | 0.307 |
| 19 | 48 | F | R-MCA | 50 | 0.296 | 0.388 | 0.429 | 0.394 |
 | Table II.Expression of α-SMA, OPN, MMP9 and
PINCH in ruptured aneurysms. |
Table II.
Expression of α-SMA, OPN, MMP9 and
PINCH in ruptured aneurysms.
| No. | Age | Gender | Location | Size (mm) | α-SMA | MMP9 | OPN | PINCH |
|---|
| 1 | 48 | M | R-ACA | 9 | 0.261 | 0.312 | 0.172 | 0.214 |
| 2 | 40 | M | L-MCA | 20 | 0.173 | 0.326 | 0.224 | 0.135 |
| 3 | 15 | M | L-ACA | 6 | 0.277 | 0.288 | 0.108 | 0.113 |
| 4 | 39 | M | L-PICA | 7 | 0.13 | 0.203 | 0.192 | 0.187 |
| 5 | 31 | M | R-MCA | 7 | 0.315 | 0.273 | 0.094 | 0.229 |
| 6 | 56 | F | R-MCA | 5 | 0.253 | 0.219 | 0.149 | 0.198 |
| 7 | 62 | F | R-ICA | 15 | 0.24 | 0.366 | 0.197 | 0.213 |
| 8 | 34 | F | L-ICA | 20 | 0.081 | 0.383 | 0.146 | 0.211 |
| 9 | 37 | F | L-ICA | 5 | 0.293 | 0.404 | 0.139 | 0.216 |
| 10 | 39 | F | L-ICA | 15 | 0.349 | 0.282 | 0.188 | 0.269 |
| 11 | 58 | F | L-ICA | 30 | 0.236 | 0.297 | 0.253 | 0.307 |
| 12 | 64 | F | L-ICA | 13 | 0.145 | 0.171 | 0.186 | 0.227 |
Histological assessment was performed in the normal
control (n=5), unruptured (n=19) and ruptured (n=12) cerebral
aneurysms. The size of the IAs was distinguished using DSA, and the
size of small, large and giant IAs had a diameter <12, 12–25 and
>25 mm respectively (Fig. 1A).
Moreover, as shown in Tables I and
II, the location of IAs was
separated by DSA. In the normal control group, H&E staining
demonstrated that organized smooth muscle cells and continuous
endothelial cells were arranged in the cerebral vessel wall
(Fig. 1B). In IAs, there were layers
of discontinuous endothelial cells and scattered smooth muscle
cells in unruptured aneurysms (Fig.
1B). Fresh or organizing thrombosis lined the luminal, and an
extremely thin thrombosis-lined hypocellular wall was observed in
the ruptured aneurysms (Fig.
1B).
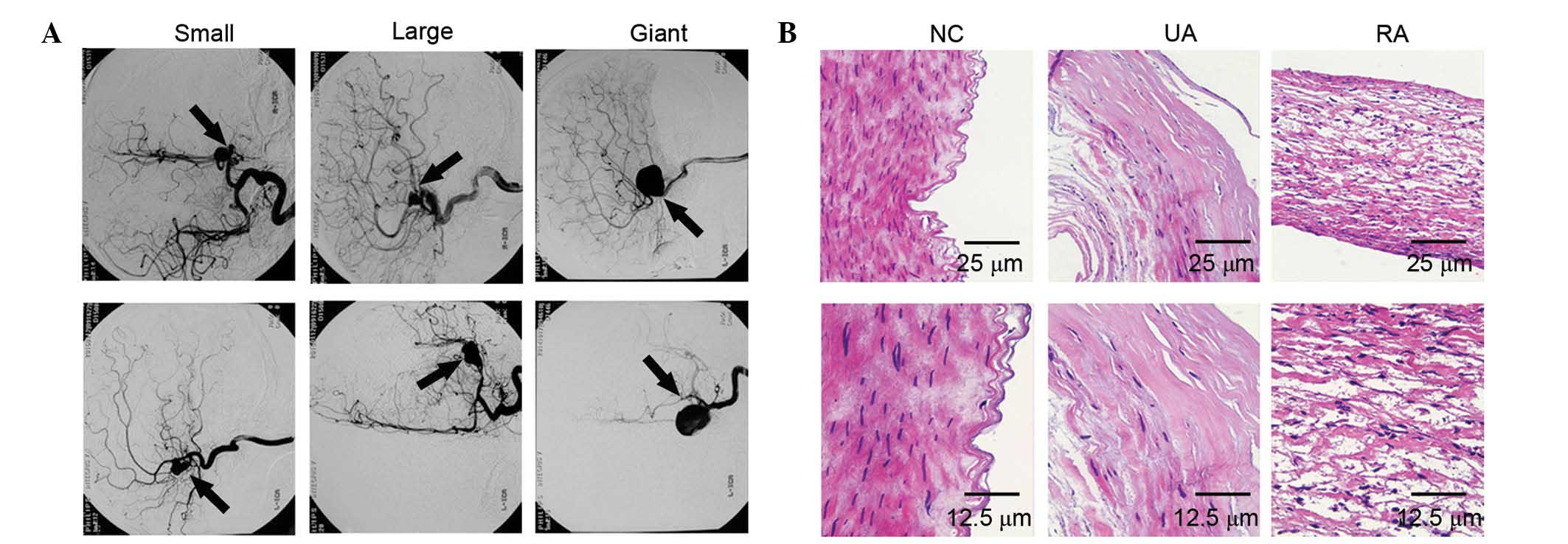 | Figure 1.(A) Front view (top) and lateral view
(bottom) of the 2D digital subtraction angiogram were applied to
depict small (diameter, <12 mm), large (diameter range, 12–25
mm) and giant (diameter, >25 mm) intracranial aneurysms. (B)
Pathological staining in NC, UAs and RAs by hematoxylin & eosin
staining (top, magnification, ×200; scale bar, 25 µm; bottom,
magnification, ×400; scale bar, 12.5 µm). NC, normal control; UAs,
unruptured intracranial aneurysms; RAs, ruptured intracranial
aneurysms. |
Expression of α-SMA, OPN, MMP9 and
PINCH in IAs
α-SMA positive cells were densely distributed in the
control group. However, the levels of α-SMA were significantly
decreased in unruptured (P<0.05) and ruptured (P<0.05)
cerebral aneurysms compared to those of the control group, and no
difference in α-SMA was noted between patients with unruptured and
ruptured cerebral aneurysms (Fig. 2A and
B). Moreover, the protein expression levels of OPN, MMP9 and
PINCH (all P<0.05) in the UA and RA group were significantly
higher than those of the control group (Fig. 2A and B). In addition, the level of
MMP9 (P<0.05) was significantly increased in the RA compared to
the UA group. However, OPN and PINCH levels were decreased in the
RA group compared to those of the UA group (both P<0.05)
(Fig. 2A and B). In general, these
results suggested that OPN and PINCH tend to show a higher
expression in the unruptured cerebral aneurysms compared to
ruptured cerebral aneurysms.
 | Figure 2.(A) Expression of α-SMA, OPN, MMP9 and
PINCH was measured by immunohistochemical staining in the NC, UA
and RA (magnification, ×400; scale bar, 12.5 µm). (B) IOD was
respectively measured for the tumor tissues of immunohistochemical
positive staining. Values were expressed as the mean ± standard
deviation. *P<0.05 vs. NC group, #P<0.05 vs. RA
group. NC, normal control; UA, unruptured intracranial aneurysm;
RA, ruptured intracranial aneurysm; HE, hematoxylin and eosin;
α-SMA, α-smooth muscle actin; OPN, osteopontin; MMP9, matrix
metalloproteinase 9; PINCH, particularly interesting Cys-His-rich
protein; IOD, integrated optical density. |
In order to examine whether there was a correlation
between PINCH and tumor size as well as between PINCH and OPN,
which was measured in tumor tissues from the same individuals. As
shown in Fig. 3A, measurements
obtained from the same individuals were strongly correlated between
PINCH and tumor size (r=0.650 and P=0.0026) as well as
between PINCH and OPN (r=0.639 and P=0.0033) in the
unruptured cerebral aneurysms. However, the correlation between
PINCH and tumor size (r=0.450, P=0.1393) and between PINCH
and OPN (r=0.366 and P=0.2426) revealed no obvious
difference in the ruptured cerebral aneurysms (Fig. 3B). Consistent with
immunohistochemical methods, the western blot results demonstrated
that the protein expression of OPN, MMP9 and PINCH in the UA and RA
group was markedly higher than those of the control group (all
P<0.05), and OPN and PINCH (both P<0.05) were significantly
decreased in the RA group as compared to those of the UA group.
However, protein expression of MMP9 was not changed in the RA group
compared to the UA group (Fig. 3C).
These results indicated that PINCH was highly expressed in the
unruptured IAs, which may be a critical factor for preventing
aneurysmal rupture.
Diagram depicting the possible
regulation mechanism of PINCH in the tumorigenesis of IAs
There are several molecules that have been
demonstrated to interact with PINCH signaling. The function of the
ternary complex of IPP as a signalling platform is achieved by
directly interacting with factors that function as upstream
regulators of numerous different signalling pathways (21). The present study summarized the known
binding partners of PINCH signaling, which are important in
processes as diverse as cell adhesion, migration, proliferation,
differentiation, survival and apoptosis (22,23). The
results demonstrated a decrease in the pro-apoptotic proteins Bax
and caspase3 and an increase in the anti-apoptotic protein Bcl-2 in
the RA and UA groups compared to those of the control group
(Fig. 4A). Moreover, the JNK
signaling pathway was inhibited in unruptured and ruptured cerebral
aneurysms, and JNK and p-JNK protein expression were significantly
lower in the RA and UA groups compared to the control groups
(Fig. 4A). In addition, the
steady-state levels of proteins involved in the cell cycle
checkpoint were analyzed. The results revealed that cyclin B1,
cyclin D1 and CDC2 were upregulated in the RA and UA groups
compared to those of the NC group (Fig.
4A). In order to further investigate the potential mechanism
that may be involved in the PINCH-associated progression of IAs,
the protein expression levels of ERK1/2, p-ERK1/2, p38 and p-p38 in
tumor tissues were examined. Western blotting revealed that the
protein expression levels of ERK1/2, p-ERK1/2, p38 and p-p38 were
markedly reduced in the RA and UA groups compared with the NC group
(Fig. 4A). Due to the main role of
JNK and ERK signaling in carcinogenesis and maintenance of common
cancers, the dysregulated expression of JNK and ERK signaling also
affects the expression of its potential downstream targets, which
are responsible for a wide range of biological processes, including
cell proliferation and differentiation, cell cycle and survival and
apoptosis. Thus, the results of the present study indicated that
PINCH may facilitate IA progression, at least partially, through
the activation of ERK signaling and the suppression of JNK
signaling (Fig. 4B).
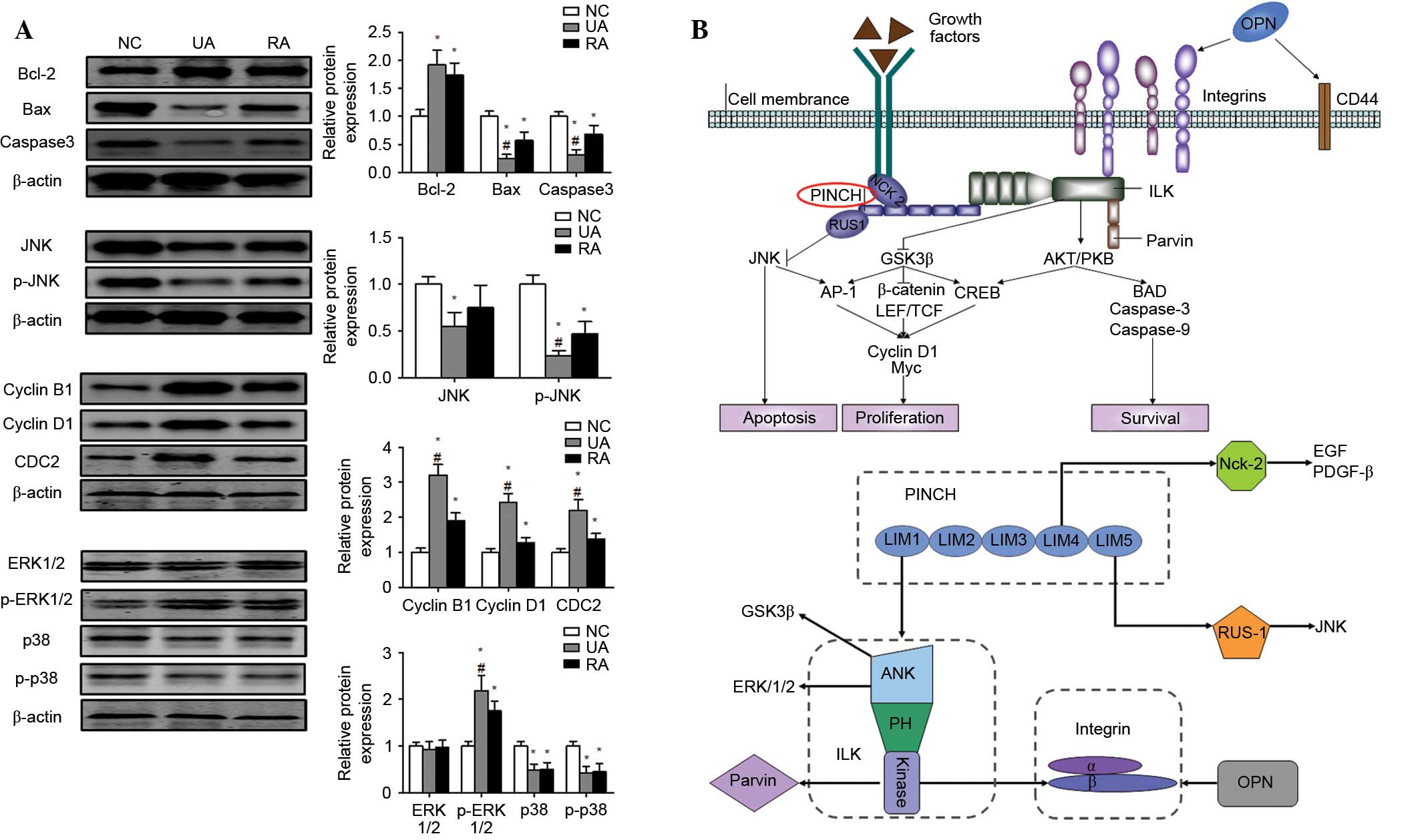 | Figure 4.(A) Bcl-2, Bax, caspase3, JNK, p-JNK,
cyclin B1, cyclin D1, CDC2, ERK, p-ERK, p38 and p-p38 protein
levels were measured by western blotting in tumor tissues. (B)
Diagram depicting the possible molecular mechanism of PINCH in the
tumorigenesis of intracranial aneurysms. Values were expressed as
the mean ± standard deviation, *P<0.05 vs. NC group,
#P<0.05 vs. RA group. Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; NC, normal control; UA, unruptured
intracranial aneurysm; RA, ruptured intracranial aneurysm; JNK,
c-Jun N-terminal kinases; ERK, extracellular signal-regulated
kinase; PINCH, particularly interesting Cys-His-rich protein. |
Discussion
Functional studies reveal that the interaction of
PINCH1 with integrin-linked kinase (ILK) is a prerequisite in order
to locate both proteins to integrin-mediated adhesion sites and to
prevent their proteasome-mediated degradation (24,25).
Further studies indicate that PINCH1 regulates the cell-matrix,
cell polarity and cell-cell adhesion during the implanting of mouse
embryos (12). Moreover, PINCH1 and
ILK function together in controlling cell behavior, including cell
shape modulation, motility and survival (12,24).
Expression profiles and roles of PINCH in tumor tissues have been
previously reported (15,17–19).
However, the exact role of PINCH signaling in IA progression and
rupture is not clear yet. Therefore, the aim of the present study
was to investigate the function of PINCH signaling in maintaining
the development of IAs and preventing aneurysmal rupture.
In the present study several important observations
were made. Initially, the basic expression levels of α-SMA, MMP9,
PINCH and OPN in 19 unruptured and 12 ruptured cerebral aneurysms
were studied. Compared to the normal control, the α-SMA, MMP9,
PINCH and OPN expression levels were significantly elevated in
tumor tissues detected by immunohistochemical staining and western
blotting; however, the expression levels of PINCH and OPN were
different between unruptured and ruptured aneurysms. Next, the
correlation of PINCH with tumor size and OPN was confirmed, and the
linear correlation plot of protein expression between PINCH and
tumor size, as well as between PINCH and OPN showed a strong
positive correlation in the unruptured aneurysms. Furthermore, the
possible molecular mechanism of PINCH in the tumorigenesis of IAs
was analyzed and it was revealed that PINCH may facilitate IA
progression, at least partially, through the activation of ERK
signaling and suppression JNK signaling. This could regulate the
downstream regulators, which induce cell proliferation, survival
and apoptosis (21).
OPN, which is an important immunomodulator, is a
matricellular protein that is highly expressed in the aortic wall
and was demonstrated to be targeted for cleavage by MMP (26). OPN promotes atherosclerosis through
its functions in smooth muscle cell survival, adhesion and
migration, and promotes inflammation of carotid plaques in
hypertensive patients (27).
Furthermore, it mitigates vascular calcification (28) and contributes to the increased
amounts of MMP-9 in cardiac and skeletal muscle of mdx mice
(29). The present study revealed
that the protein levels of MMP9 and OPN were significantly
increased in unruptured aneurysms, and the immunohistochemical
staining was consistent with the with western blot analysis. It is
noteworthy that measurements obtained from the same individuals
were strongly correlated between PINCH and OPN (r=0.639 and
P=0.0033) in the unruptured cerebral aneurysms. However, the
correlation between PINCH and OPN (r=0.366 and P=0.2426)
showed no obvious difference in the ruptured cerebral aneurysms.
These results suggested that PINCH and OPN offered a mechanism of
facilitating IA progression, which may be closely associated with
resistance of aneurysmal rupture.
Previous studies suggest that PINCH-1 is likely to
have a function in the suppression of apoptosis. For example,
depletion of PINCH-1 from human HeLa cervical carcinoma cells
promotes apoptosis, and an increase of apoptotic endodermal cells,
mouse embryos and embryonic neural crest cells are observed with
PINCH loss-of-function (10,11,18). A
prior study suggested that PINCH-1 regulates the anti- and
pro-apoptotic pathways and contributes to apoptosis resistance in
all types of cancer cells (18). The
apoptosis signaling pathway is activated by the loss of PINCH-1,
and the level of Bim is significantly increased in response to loss
of PINCH-1 in several types of PINCH-1-dependent cancer cells.
However, Bim depletion completely blocks the increase of apoptosis
induced by the loss of PINCH-1 (18). Moreover, activation of the
phosphorylation of the Src family kinase and ERK1/2 is promoted by
PINCH-1 (18). In the PrE, PINCH-1
is a pro-survival factor, which prevents apoptosis of PrE cells by
modulating two independent signalling pathways; PINCH-1 inhibits
JNK-mediated apoptosis by stabilizing the PINCH-1 binding protein
RSU-1 and promotes Bcl-2-dependent pro-survival signalling
downstream of integrins (13). In
the present study, a correlation was identified between the
activation of ERK signaling and the suppression of JNK signaling
with the expression of PINCH in IAs. The downstream regulators,
including anti-apoptotic (Bcl-2), pro-apoptotic proteins (Bax and
caspase3) and cell cycle proteins (cyclin B1, cyclin D1 and CDC2)
were involved in PINCH-mediated tumorigenesis and progression in
IAs.
To the best of our knowledge, this is the first
study to report that PINCH is highly expressed in IAs and resists
aneurysmal rupture. The study also demonstrated that PINCH may
facilitate IA progression, at least partially, through the
activation of ERK and suppression of JNK signaling, which could
regulate the downstream regulators including anti- and
pro-apoptotic protein and cell cycle proteins.
Acknowledgements
The present study was supported by the Basic and
Clinical Research Mutual Foundation of the Capital Medical
University (grant no. 15JL65).
References
|
1
|
Chalouhi N, Ali MS, Jabbour PM,
Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ and Dumont AS:
Biology of intracranial aneurysms: Role of inflammation. J Cereb
Blood Flow Metab. 32:1659–1676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orz Y and AlYamany M: The impact of size
and location on rupture of intracranial aneurysms. Asian J
Neurosurg. 10:26–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Starke RM, Chalouhi N, Jabbour PM,
Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K,
Hasan DM, Greig NH, Owens GK and Dumont AS: Critical role of TNF-α
in cerebral aneurysm formation and progression to rupture. J
Neuroinflammation. 11:772014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordon IM, Hinchliffe RJ, Holt PJ, Loftus
IM and Thompson MM: Review of current theories for abdominal aortic
aneurysm pathogenesis. Vascular. 17:253–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raux M, Patel VI, Cochennec F,
Mukhopadhyay S, Desgranges P, Cambria RP, Becquemin JP and
LaMuraglia GM: A propensity-matched comparison of outcomes for
fenestrated endovascular aneurysm repair and open surgical repair
of complex abdominal aortic aneurysms. J Vasc Surg. 60:858–863.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe Y, Kuratani T, Shirakawa Y,
Torikai K, Shimamura K and Sawa Y: Hybrid endovascular repair of a
dissecting thoracoabdominal aortic aneurysm with stent graft
implantation through the false lumen. J Vasc Surg. 59:264–267.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wiebers DO, Whisnant JP, Huston J III,
Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols
D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman
GL and Torner JC: International Study of Unruptured Intracranial
Aneurysms Investigators: Unruptured intracranial aneurysms: Natural
history, clinical outcome, and risks of surgical and endovascular
treatment. Lancet. 362:103–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molyneux AJ, Kerr RS, Birks J, Ramzi N,
Yarnold J, Sneade M and Rischmiller J: ISAT Collaborators: Risk of
recurrent subarachnoid haemorrhage, death, or dependence and
standardised mortality ratios after clipping or coiling of an
intracranial aneurysm in the international subarachnoid aneurysm
trial (ISAT): Long-term follow-up. Lancet Neurol. 8:427–433. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Dai C, Wu C and Liu Y: PINCH-1
promotes tubular epithelial-to-mesenchymal transition by
interacting with integrin-linked kinase. J Am Soc Nephrol.
18:2534–2543. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang X, Sun Y, Schneider J, Ding JH,
Cheng H, Ye M, Bhattacharya S, Rearden A, Evans S and Chen J:
Pinch1 is required for normal development of cranial and cardiac
neural crest-derived structures. Circ Res. 100:527–535. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton
N, Ross J Jr and Chen J: PINCH1 plays an essential role in early
murine embryonic development but is dispensable in ventricular
cardiomyocytes. Mol Cell Biol. 25:3056–3062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Bordoy R, Stanchi F, Moser M, Braun
A, Kudlacek O, Wewer UM, Yurchenco PD and Fässler R: PINCH1
regulates cell-matrix and cell-cell adhesions, cell polarity and
cell survival during the peri-implantation stage. J Cell Sci.
118:2913–2921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montanez E, Karaköse E, Tischner D,
Villunger A and Fässler R: PINCH-1 promotes Bcl-2-dependent
survival signalling and inhibits JNK-mediated apoptosis in the
primitive endoderm. J Cell Sci. 125:5233–5240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giotopoulou N, Valiakou V, Papanikolaou V,
Dubos S, Athanassiou E, Tsezou A, Zacharia LC and Gkretsi V: Ras
suppressor-1 promotes apoptosis in breast cancer cells by
inhibiting PINCH-1 and activating p53-upregulated-modulator of
apoptosis (PUMA); verification from metastatic breast cancer human
samples. Clin Exp Metastasis. 32:255–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andréasson H, Wanders A, Sun XF, Willén R,
Graf W, Nygren P, Glimelius B, Zhang ZY and Mahteme H:
Histopathological classification of pseudomyxoma peritonei and the
prognostic importance of PINCH protein. Anticancer Res.
32:1443–1448. 2012.PubMed/NCBI
|
|
16
|
Zhu ZL, Yan BY, Zhang Y, Yang YH, Wang ZM,
Zhang HZ, Wang MW, Zhang XH and Sun XF: PINCH expression and its
clinicopathological significance in gastric adenocarcinoma. Dis
Markers. 33:171–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holmqvist A, Gao J, Holmlund B, Adell G,
Carstensen J, Langford D and Sun XF: PINCH is an independent
prognostic factor in rectal cancer patients without preoperative
radiotherapy-a study in a Swedish rectal cancer trial of
preoperative radiotherapy. BMC Cancer. 12:652012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen K, Tu Y, Zhang Y, Blair HC, Zhang L
and Wu C: PINCH-1 regulates the ERK-Bim pathway and contributes to
apoptosis resistance in cancer cells. J Biol Chem. 283:2508–2517.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim O, Hwangbo C, Kim J, Li DH, Min BS and
Lee JH: Chelidonine suppresses migration and invasion of MDA-MB-231
cells by inhibiting formation of the integrin-linked
kinase/PINCH/α-parvin complex. Mol Med Rep. 12:2161–2168.
2015.PubMed/NCBI
|
|
20
|
Zheng SF, Yao PS, Yu LH and Kang DZ:
Keyhole approach combined with external ventricular drainage for
ruptured, poor-grade, anterior circulation cerebral aneurysms.
Medicine (Baltimore). 94:e23072015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Legate KR, Montañez E, Kudlacek O and
Fässler R: ILK, PINCH and parvin: The tIPP of integrin signalling.
Nat Rev Mol Cell Biol. 7:20–31. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang LY, Tang ZJ and Han YZ:
Neuroprotective effects of caffeic acid phenethyl ester against
sevofluraneinduced neuronal degeneration in the hippocampus of
neonatal rats involve MAPK and PI3K/Akt signaling pathways. Mol Med
Rep. 14:3403–3412. 2016.PubMed/NCBI
|
|
23
|
Cui ZW, Xie ZX, Wang BF, Zhong ZH, Chen
XY, Sun YH, Sun QF, Yang GY and Bian LG: Carvacrol protects
neuroblastoma SH-SY5Y cells against Fe(2+)-induced apoptosis by
suppressing activation of MAPK/JNK-NF-kappaB signaling pathway.
Acta Pharmacol Sin. 36:1426–1436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukuda T, Chen K, Shi X and Wu C: PINCH-1
is an obligate partner of integrin-linked kinase (ILK) functioning
in cell shape modulation, motility, and survival. J Biol Chem.
278:51324–51333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Chen K, Guo L and Wu C:
Characterization of PINCH-2, a new focal adhesion protein that
regulates the PINCH-1-ILK interaction, cell spreading, and
migration. J Biol Chem. 277:38328–38338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papke CL, Yamashiro Y and Yanagisawa H:
MMP17/MT4-MMP and thoracic aortic aneurysms: OPNing new potential
for effective treatment. Circ Res. 117:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wolak T, Sion-Vardi N, Novack V, Greenberg
G, Szendro G, Tarnovscki T, Nov O, Shelef I, Paran E and Rudich A:
N-terminal rather than full-length osteopontin or its C-terminal
fragment is associated with carotid-plaque inflammation in
hypertensive patients. Am J Hypertens. 26:326–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jono S, Peinado C and Giachelli CM:
Phosphorylation of osteopontin is required for inhibition of
vascular smooth muscle cell calcification. J Biol Chem.
275:20197–20203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dahiya S, Givvimani S, Bhatnagar S,
Qipshidze N, Tyagi SC and Kumar A: Osteopontin-stimulated
expression of matrix metalloproteinase-9 causes cardiomyopathy in
the mdx model of Duchenne muscular dystrophy. J Immunol.
187:2723–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|


















