Introduction
Gastric carcinoma is one of the most common human
cancers and has a high mortality rate (1). Although significant progress in the
treatment of gastric carcinoma (involving surgical excision used in
combination with chemotherapy) has been achieved in recent years,
the median survival rate of gastric carcinoma remains low (2). The downregulation of tumor suppressor
genes has been implicated in the tumorigenesis of gastric carcinoma
(3,4). Therefore, elucidation of the molecular
mechanisms underlying gastric carcinoma is important for the
development of therapeutic strategies for this disease.
Receptor tyrosine kinase-like orphan receptor 2
(ROR2) is a member of the receptor tyrosine kinase family (5). It has been demonstrated that ROR2 is
involved in numerous cellular biological processes, including cell
proliferation, differentiation, apoptosis, adhesion and migration
(6–8). Furthermore, ROR2 is a receptor of the
non-canonical Wnt signaling pathway, and Wnt5a has been determined
to be the most important ligand of ROR2 (9). In a previous study, the expression
levels of ROR2 were observed to be recurrently downregulated in
various common human cancers, including esophageal, nasopharyngeal,
colorectal, gastric, hepatocellular, lung and breast cancer
(10). Accordingly, it has been
suggested that ROR2 may act as a tumor suppressor in human cancers.
However, ROR2 has been reported to have an oncogenic role in other
types of cancer (11,12). For instance, ROR2 was shown to be
upregulated in osteosarcoma tissues, and its expression level was
positively correlated with disease severity (11). In addition, ROR2 was reported to
increase the tumor growth potential in renal cell carcinoma (RCC)
(12). These findings suggest that
ROR2 may have dual roles in various cancer types. However, to the
best of our knowledge, the exact role of ROR2 in the regulation of
gastric carcinoma growth, as well as the underlying mechanism, have
yet to be investigated.
The present study aimed to investigate the role of
ROR2 in the regulation of the proliferation, apoptosis and cell
cycle progression of gastric carcinoma cells, as well as the
underlying molecular mechanisms.
Materials and methods
Tissue specimen collection
All protocols involved in the present study were
approved by the Ethics Committee of Shaanxi Provincial People's
Hospital (Xi'an, China). A total of 20 gastric carcinoma tissue
samples and their matched adjacent normal tissue samples were
collected from patients at the Shaanxi Provincial People's Hospital
between June 2013 and January 2014. Of the 20 patients, 13 were
male and 7 were female. The patients had a mean age of 53.7 years
(age range, 33–77 years). The patients did not receive radiation
therapy or chemotherapy prior to surgical resection. Informed
consent was obtained from all patients. The resected tissue samples
were immediately snap-frozen in liquid nitrogen and stored at −70°C
prior to use.
Cell culture
Four common human gastric cancer cell lines (BGC823,
SGC7901, HGC27 and AGS), and a normal gastric mucosa epithelial
cell line (GES-1), were obtained from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China). All cell
lines were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (both purchased from
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
incubator containing 5% CO2 at 37°C. Wnt5a was purchased
from R&D Systems, Inc. (Minneapolis, MN, USA), and 1 mM Wnt5a
was used to treat AGS cells transfected with pcDNA3.1-ROR2
plasmid.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from gastric
carcinoma tissues or cell lines, according to the manufacturer's
protocol. The concentration and quality of the RNA was analyzed
using Nanodrop 2000 ultramicro spectrophotometer (Thermo Fisher
Scientific, Inc.). Total RNA (500 ng) was reverse transcribed into
cDNA using the RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
qPCR was performed using the SYBR Green qPCR Assay kit (Toyobo Co.,
Ltd., Osaka, Japan), according to the manufacturer's protocol. The
PCR cycling conditions were 50°C for 2 min and 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 1 min. The specific primer pairs
were as follows: ROR2 sense, 5′-ATGGTTCACGACTGCGAATCC-3′ and
antisense, 5′-AATGGTCTTCATCCCGTTGGT-3′; and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) sense,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and antisense,
5′-GGCTGTTGTCATACTTCTCATGG-3′. GAPDH was used as an internal
control. cDNA was replaced with H2O for a negative
control. Independent experiments were repeated three times. The
relative mRNA expression levels of ROR2 were analyzed using the
2−∆∆Cq method (13).
Transfection
AGS cells were cultured to 70% confluence and
resuspended in serum-free DMEM. Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) was used to transfect the AGS cells with
pcDNA3.1-ROR2 plasmid or blank pcDNA-3.1 vector as a negative
control (NC). Briefly, serum-free medium was used to dilute the
Lipofectamine 2000 or plasmid, after which the Lipofectamine 2000
and diluted plasmid were mixed. After incubation for 20 min at room
temperature, the mixture was added to the AGS cell suspension and
incubated at 37°C in 5% CO2 for 6 h, followed by
replacement of the medium with the normal serum-containing medium
and incubation for 48 h.
MTT assay
MTT assays were performed to determine the
proliferation of AGS cells. Briefly, 104 cells/well were
seeded onto a 96-well plate and incubated for 6, 12, 24 or 48 h at
37°C in 5% CO2. Subsequently, 5 mg/ml MTT (Thermo Fisher
Scientific, Inc.) was added to each well and the plates were
incubated for a further 4 h at 37°C in 5% CO2. The
supernatant, obtained by centrifugation at 1,000 × g for 3
min at room temperature, was removed and 100 µl dimethylsulfoxide
(Thermo Fisher Scientific, Inc.) was added to dissolve the
precipitate. The absorbance was detected at 492 nm using the
Bio-Tek™ ELX-800™ Absorbance Microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Cell cycle distribution analysis
AGS cells (5.0×106 cells/ml) were fixed
in 70% ethanol overnight at −20°C, followed by two rounds of
centrifugation at 1,000 × g for 5 min. The cell pellets were
then resuspended in 300 µl propidium iodide (PI) staining buffer
(Thermo Fisher Scientific, Inc.), and incubated for 30 min at room
temperature. DNA content analyses were performed by flow
cytometry.
Cell apoptosis assay
Cell apoptosis was determined using the Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD
Pharmingen, San Diego, CA, USA). Briefly, AGS cells were harvested
at 24 h post-transfection and washed twice with cold
phosphate-buffered saline (PBS). Subsequently, 106 cells
were resuspended in 200 µl binding buffer containing 10 µl Annexin
V-FITC and 5 µl PI-phycoerythrin, and incubated in the dark for 30
min at 4°C. Next, 300 µl binding buffer was added to the cells and
apoptosis was assessed by flow cytometry.
Western blotting
AGS cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.). Centrifugation was performed at 12,000 ×
g for 30 min at 4°C, and the supernatant was collected.
Protein concentration was determined using a Pierce BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Proteins (50 µg) were separated by 10%
SDS-PAGE (Pierce Biotechnology, Inc., Rockford, IL, USA), and
transferred onto polyvinylidene difluoride membranes (Pierce
Biotechnology, Inc.). The membranes were subsequently incubated
with rabbit anti-ROR2 polyclonal antibody (1:50; cat. no. ab137602;
Abcam, Cambridge, MA, USA), rabbit anti-c-Myc polyclonal antibody
(1:100; cat. no. ab39688; Abcam), rabbit anti-β-catenin antibody
(1:100; cat. no. ab32572) and rabbit anti-GAPDH polyclonal antibody
(1:200; cat. no. ab9485; Abcam) for 3 h at room temperature. After
washing three times with PBS containing Tween-20, the membranes
were incubated with horseradish peroxidase-conjugated mouse
anti-rabbit secondary antibody (1:10,000; cat. no. ab99697; Abcam)
for 1 h at room temperature. Detection was performed using an
Enhanced Chemiluminescence kit (Pierce Biotechnology, Inc.). The
relative protein expression levels were determined using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA),
and are presented as the density ratio versus GAPDH.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three experiments. Statistical analysis was performed
by one-way analysis of variance using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 were considered to indicate a
statistically significant difference.
Results
ROR2 is recurrently downregulated in
gastric carcinoma tissues and cell lines
First, the present study examined the mRNA
expression levels of ROR2 in gastric carcinoma tissues and cell
lines using RT-qPCR. As is shown in Fig.
1A, the mRNA expression levels of ROR2 were recurrently
downregulated in gastric carcinoma tissue samples, as compared with
their matched adjacent normal tissue samples (P<0.01). As is
shown in Fig. 1B and C, the mRNA and
protein expression levels of ROR2 were significantly decreased in
gastric carcinoma cell lines, as compared with GES1 cells
(P<0.01). These results suggest that downregulation of ROR2 may
be associated with the tumorigenesis of gastric carcinoma. Since
the AGS gastric carcinoma cells exhibited the lowest ROR2 protein
expression levels (Fig. 1C), AGS
cells were selected for use in the subsequent experiments.
Overexpression of ROR2 inhibits the
proliferation of AGS gastric carcinoma cells independently of
Wnt5a
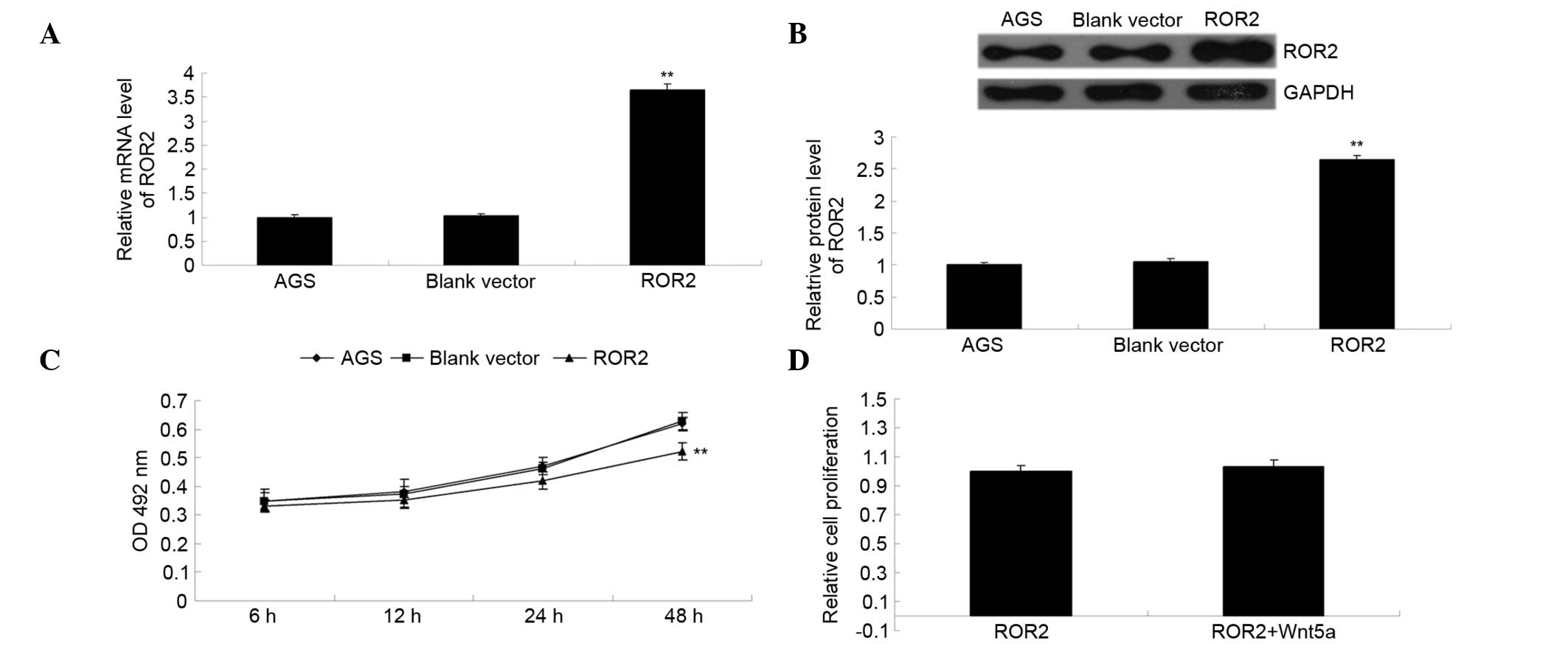
The ROR2 plasmid was transfected into gastric
carcinoma AGS cells. As is shown in Fig.
2A and B, the relative mRNA and protein expression levels of
ROR2 were significantly increased following transfection, as
compared with the control group (P<0.01), indicating that the
transfection had been successful. The MTT assay was used to
investigate the effect of ROR2 overexpression on AGS cell
proliferation. As is shown in Fig.
2C, overexpression of ROR2 significantly inhibited the
proliferation of AGS cells (P<0.01). However, addition of Wnt5a,
the ligand of ROR2, did not affect the proliferation of
ROR2-overexpressing AGS cells (P>0.05; Fig. 2D), thus suggesting that
overexpression of ROR2 inhibits AGS cell proliferation
independently of Wnt5a.
Overexpression of ROR2 induces the
apoptosis of AGS gastric carcinoma cells independent of Wnt5a
Cell apoptosis assays were performed to investigate
the effect of ROR2 overexpression on AGS cell apoptosis. As is
shown in Fig. 3A, the apoptotic
level of AGS cells transfected with ROR2 plasmid was significantly
increased, as compared with the control groups (P<0.01).
Furthermore, the addition of Wnt5a did not affect the apoptosis
level of ROR2-overexpressing AGS cells (P>0.05; Fig. 3B), thus suggesting that
overexpression of ROR2 induces the apoptosis of AGS cells
independently of Wnt5a.
Overexpression of ROR2 induces cell
cycle arrest in AGS gastric carcinoma cells
Wnt signaling has previously been demonstrated to
participate in the regulation of cell cycle progression (14). Therefore, the cell cycle distribution
of AGS gastric carcinoma cells was examined for each group. As is
shown in Fig. 4, ROR2-overexpressing
AGS cells accumulated at the G1 phase, as compared with the control
groups (P<0.01). These results suggest that overexpression of
ROR2 induces an arrest in cell cycle progression at the G1 phase,
and indicate that the induction of cell cycle arrest may be
responsible for the reduced proliferation of ROR2-overexpressing
AGS cells.
Overexpression of ROR2 suppresses the
activation of canonical Wnt signaling
ROR2 is a receptor of the non-canonical Wnt
signaling pathway (15). Recently,
ROR2 has been suggested to have an inhibitory effect on the
activation of the canonical Wnt signaling pathway, which is
involved in the regulation of cell survival and proliferation
(5,16). Since the present study demonstrated
that overexpression of ROR2 inhibited AGS cell proliferation while
inducing cell apoptosis and cell cycle arrest, it was hypothesized
that overexpression of ROR2 may inhibit the activation of the
canonical Wnt signaling pathway in AGS cells. To verify this,
western blotting was performed to investigate the activation of the
canonical Wnt signaling pathway, as well as c-Myc, which is an
important downstream effecter responsible for the regulation of
cell proliferation (17). As is
shown in Fig. 5, overexpression of
ROR2 significantly inhibited the protein expression levels of
β-catenin in the nucleus, as well as the protein expression levels
of c-Myc in AGS cells (P<0.01); thus suggesting that the
activation of the canonical Wnt signaling pathway was inhibited in
ROR-overexpressing cells. Theses results suggest that the
inhibitory effect of ROR2 overexpression on AGS cell proliferation
may be due to the suppression of canonical Wnt signaling.
Discussion
ROR2 is a type I transmembrane protein that belongs
to the ROR subfamily of cell surface receptors (18). Previous studies have suggested that
the deregulation of ROR2 is a key factor during tumorigenesis, and
that ROR2 is involved in the development of some common types of
human cancers (5,11,12). For
instance, the expression levels of ROR2 were associated with the
expression of genes involved in the extracellular matrix, including
Twist and matrix metalloproteinase-2, in RCC (12). In addition, knockdown of ROR2
inhibited RCC cell migration, as well as anchorage-independent
growth in soft agar and growth in an orthotopic xenograft model
(12); thus suggesting that ROR2 has
an oncogenic role in RCC. Furthermore, ROR2 was reported to promote
the development of various other types of cancer, including
melanoma and oral squamous cell carcinoma (15,19).
Conversely, ROR2 has been reported to exert a
suppressive role in other types of human cancers. Li et al
(10) demonstrated that ROR2 was
recurrently silenced by CpG methylation at its promoter in
nasopharyngeal, esophageal, colorectal, gastric, hepatocellular,
lung and breast cancer cell lines. The present study demonstrated
that the expression levels of ROR2 were recurrently reduced in
gastric carcinoma tissues, as compared with their matched normal
adjacent tissues. In addition, ROR2 expression was significantly
decreased in several common gastric carcinoma cell lines, as
compared with normal gastric epithelial cells. These results
suggested that the aberrant downregulation of ROR2 may be
associated with the development and progression of gastric
carcinoma.
In the present study, AGS gastric carcinoma cells
were transfected with an ROR2 plasmid, and the expression levels of
ROR2 were observed to have been successfully increased following
transfection. Overexpression of ROR2 markedly inhibited AGS cell
proliferation, while inducing cell apoptosis and cell cycle arrest
at the G1 phase. Similarly, Li et al (10) reported that ectopic expression of
ROR2 inhibited tumor cell growth and induced cell cycle arrest and
apoptosis. Furthermore, ROR2 was revealed to suppress
epithelial-mesenchymal transition, migration and invasion (10).
Lu et al (11)
demonstrated that the expression levels of ROR2 and Wnt5a were
significantly upregulated in osteosarcoma, and that their
expression levels were associated with the malignant progression.
Therefore, the present study investigated the effect of Wnt5a on
the proliferation and apoptosis of gastric carcinoma cells.
However, it was demonstrated that the addition of Wnt5a did not
affect the proliferation or the apoptosis of AGS cells transfected
with an ROR2 plasmid; thus suggesting that the inhibitory effects
of ROR2 overexpression on gastric carcinoma may be independent of
Wnt5a.
The canonical Wnt signaling pathway has been
observed to be involved in embryonic development, cell
differentiation and tumorigenesis (20–22). In
addition, aberrant activation of the canonical Wnt signaling
pathway has been demonstrated in a variety of human malignancies,
including gastric carcinoma (23–27).
Therefore, inhibition of canonical Wnt signaling may be a promising
strategy for the treatment of gastric carcinoma. The present study
investigated whether overexpression of the non-canonical Wnt
receptor ROR2 was able to alter the activation of the canonical Wnt
signaling pathway. It was demonstrated that overexpression of ROR2
significantly decreased the protein expression level of β-catenin
in the nucleus of AGS cells, as well as the protein expression
levels of c-Myc, an important downstream effector in the canonical
Wnt signaling pathway (17). These
results suggested that activation of non-canonical Wnt signaling
may inhibit the activity of the canonical Wnt signaling pathway in
AGS gastric carcinoma cells.
In addition to gastric carcinoma, ROR2 expression
levels were reported to be significantly decreased in
hepatocellular carcinoma (HCC) tissues, and this was associated
with a poor prognosis (28); thus
suggesting that ROR2 may act as a tumor suppressor in HCC.
Therefore, ROR2 appears to exert dual roles as an oncogene or tumor
suppressor depending on the tumor type (5).
The main limitation of the present study was that
the sample volume of gastric cancer tissues was not sufficient to
examine the association between the expression levels of ROR2 and
Wnt5a, and the clinical characteristics of patients with gastric
cancer, such as tumor grade, TNM stage and metastasis. The results
of the present study suggested that ROR2 induced the apoptosis of
AGS cells independently of Wnt5a. Therefore, future studies are
required to elucidate the underlying mechanism by which ROR2
affects gastric cancer cell apoptosis.
In conclusion, the present study demonstrated that
ROR2 was recurrently downregulated in gastric carcinoma tissues and
cell lines. Furthermore, overexpression of ROR2 significantly
inhibited the proliferation and induced the apoptosis and cell
cycle arrest of gastric carcinoma cells. An investigation of the
underlying molecular mechanism demonstrated that ROR2
overexpression inhibited the activity of the canonical Wnt
signaling pathway. The results of the present study suggested that
ROR2 may emerge as a potential candidate for the treatment of
gastric carcinoma.
References
|
1
|
Shi J, Qu YP and Hou P: Pathogenetic
mechanisms in gastric cancer. World J Gastroenterol.
20:13804–13819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.PubMed/NCBI
|
|
3
|
Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y,
Liu C, Song W, Wang F, Zhang J, et al: DNA methylation
downregulated mir-10b acts as a tumor suppressor in gastric cancer.
Gastric Cancer. 18:43–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang
Z, Zhu W, Shu Y and Liu P: MiR-145, miR-133a and miR-133b inhibit
proliferation, migration, invasion and cell cycle progression via
targeting transcription factor Sp1 in gastric cancer. FEBS Lett.
588:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ford CE, Ma SS Qian, Quadir A and Ward RL:
The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in
human carcinogenesis. Int J Cancer. 133:779–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundaram MV: Canonical RTK-Ras-ERK
signaling and related alternative pathways. WormBook. 1–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jimenez G, Shvartsman SY and Paroush Z:
The Capicua repressor-a general sensor of RTK signaling in
development and disease. J Cell Sci. 125:1383–1391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Batchu SN and Korshunov VA: Novel tyrosine
kinase signaling pathways: Implications in vascular remodeling.
Curr Opin Nephrol Hypertens. 21:122–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enomoto M, Hayakawa S, Itsukushima S, Ren
DY, Matsuo M, Tamada K, Oneyama C, Okada M, Takumi T, Nishita M and
Minami Y: Autonomous regulation of osteosarcoma cell invasiveness
by Wnt5a/Ror2 signaling. Oncogene. 28:3197–3208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Ying J, Tong X, Zhong L, Su X, Xiang
T, Shu X, Rong R, Xiong L, Li H, et al: Epigenetic identification
of receptor tyrosine kinase-like orphan receptor 2 as a functional
tumor suppressor inhibiting β-catenin and AKT signaling but
frequently methylated in common carcinomas. Cell Mol Life Sci.
71:2179–2192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu BJ, Wang YQ, Wei XJ, Rong LQ, Wei D,
Yan CM, Wang DJ and Sun JY: Expression of WNT-5a and ROR2
correlates with disease severity in osteosarcoma. Mol Med Rep.
5:1033–1036. 2012.PubMed/NCBI
|
|
12
|
Wright TM, Brannon AR, Gordan JD, Mikels
AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen
E, et al: Ror2, a developmentally regulated kinase, promotes tumor
growth potential in renal cell carcinoma. Oncogene. 28:2513–2523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katoh M: WNT signaling pathway and stem
cell signaling network. Clin Cancer Res. 13:4042–4045. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Connell MP, Fiori JL, Xu M, Carter AD,
Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier
M, et al: The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A
signaling in metastatic melanoma. Oncogene. 29:34–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Green J, Nusse R and van Amerongen R: The
role of Ryk and Ror receptor tyrosine kinases in Wnt signal
transduction. Cold Spring Harb Perspect Biol. 6:2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heo SH, Jeong ES, Lee KS, Seo JH, Jeong
DG, Won YS, Kwon HJ, Kim HC, Kim DY and Choi YK: Canonical Wnt
signaling pathway plays an essential role in N-methyl-N-nitrosurea
induced gastric tumorigenesis of mice. J Vet Med Sci. 75:299–307.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stricker S and Mundlos S: FGF and ROR2
receptor tyrosine kinase signaling in human skeletal development.
Curr Top Dev Biol. 97:179–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi M, Shibuya Y, Takeuchi J, Murata
M, Suzuki H, Yokoo S, Umeda M, Minami Y and Komori T: Ror2
expression in squamous cell carcinoma and epithelial dysplasia of
the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
107:398–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lerner UH and Ohlsson C: The WNT system:
Background and its role in bone. J Intern Med. 277:630–649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones AE, Price FD, Le Grand F, Soleimani
VD, Dick SA, Megeney LA and Rudnicki MA: Wnt/β-catenin controls
follistatin signalling to regulate satellite cell myogenic
potential. Skelet Muscle. 5:142015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He H, Chen K, Wang F, Zhao L, Wan X, Wang
L and Mo Z: miR204-5p promotes the adipogenic differentiation of
human adipose-derived mesenchymal stem cells by modulating DVL3
expression and suppressing Wnt/beta-catenin signaling. Int J Mol
Med. 35:1587–1595. 2015.PubMed/NCBI
|
|
23
|
Chang B, Tessneer KL, McManus J, Liu X,
Hahn S, Pasula S, Wu H, Song H, Chen Y, Cai X, et al: Epsin is
required for dishevelled stability and Wnt signalling activation in
colon cancer development. Nat Commun. 6:63802015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie J, Zhang Y, Hu X, Lv R, Xiao D, Jiang
L and Bao Q: Norcantharidin inhibits Wnt signal pathway via
promoter demethylation of WIF-1 in human non-small cell lung
cancer. Med Oncol. 32:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashihara E, Takada T and Maekawa T:
Targeting the canonical Wnt/β-catenin pathway in hematological
malignancies. Cancer Sci. 106:665–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ilmer M, Boiles AR, Regel I, Yokoi K,
Michalski CW, Wistuba II, Rodriguez J, Alt E and Vykoukal J: RSPO2
enhances canonical wnt signaling to confer stemness-associated
traits to susceptible pancreatic cancer cells. Cancer Res.
75:1883–1896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang F, Zhao WY, Li RK, Yang XM, Li J, Ao
JP, Jiang SH, Kong FZ, Tu L, Zhuang C, et al: Silencing of WISP3
suppresses gastric cancer cell proliferation and metastasis and
inhibits Wnt/β-catenin signaling. Int J Clin Exp Pathol.
7:6447–6461. 2014.PubMed/NCBI
|
|
28
|
Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ
and Liu XH: Loss of Wnt5a and Ror2 protein in hepatocellular
carcinoma associated with poor prognosis. World J Gastroenterol.
18:1328–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|