Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE),
mostly caused by perinatal asphyxia, usually leads to brain damage,
manifested mainly by physical development retardation and
neurological system dysfunction and even mortality (1). There are various factors influencing
the occurrence of HIE, maternal factors are predominant and include
pregnancy complications such as advanced age, infections,
abnormalities in amniotic fluid, and other important factors such
as pre- and post-mature delivery, low birth weight, and birth
injury (2). It is difficult to
obtain an early diagnosis of HIE, because of the lack of specific
clinical manifestations and the imperfection in neonatal
neurological development. Moreover, the rates of misdiagnosis and
missed diagnosis by clinical observation of activity and
application of neonatal behavioral neurological assessment (NBNA)
scoring are relatively high, due to inaccurate NBNA scoring, and
the low positive diagnostic rates of EEG detection (3). Under hypoxic-ischemic conditions, the
brain cells exhibit poor tolerability, which leads to various
abnormally expressed proteins. Spectrin breakdown products (SBDPs)
of rats that facilitate the secretion of inflammatory factors and
transmitters have relatively high tissue specificity and are
consistently elevated during brain injury with higher levels
corresponding to more severe injury (4). The microtubule-associated Tau protein
levels in plasma are closely related to neuronal loss and cognitive
impairment (5). Among various
options for HIE treatment, mild hypothermia is one of the most
promising therapies (6), its
therapeutic mechanism, however, remains to be elucidated. The NBNA
scoring and the Gesell development scale are frequently utilized
tools in efficacy evaluation of the treatment for HIE.
Taking all this information into account, we
hypothesized that plasma and urine levels of SBDPs and Tau proteins
could be used for diagnosis of HIE. Furthermore, a mild hypothermia
therapy for HIE in newborns would be beneficial and would lower the
above factor levels. We set up groups of infant patients and
experiments to test these ideas observing the ethical clinical
conditions of our hospital.
Patients and methods
General considerations
In total 150 patients with neonatal HIE diagnosed
within the first 12 h after birth, in accordance with the revised
diagnostic criteria and clinical grading criteria of the Neonatal
Group of Chinese Pediatric Society of Chinese Medical Association,
were continuously selected from June 2014 to December 2015. There
were 30 cases with mild symptoms, 60 cases with moderate symptoms
and 60 cases with severe symptoms. During the same period, 30
infants admitted to this hospital for neonatal pneumonia were
enrolled for the control group. There were four main criteria for
exclusion from the study, listed as follows:
i) All newborns whose gestational age was <36
weeks or >40 weeks.
ii) Infants with congenital malformations,
intracranial hemorrhage, severe anemia (HB <120 g/l),
intracranial infection, congenital heart disease, hereditary
metabolic disease or intrauterine infection.
iii) Infants with maternal diseases such as
gestational hypertension, diabetes and abnormal placental
function.
iv) Infants with severe septicemia, coagulation
disorders or blood platelet counts <50×109/l.
The Ethics Committee of the Xuzhou Children's
Hospital approved this study, and all of the guardians of the
newborns signed informed consent. Baseline characteristics of
newborns in each conformed group were similar (Table I).
 | Table I.Comparison of neonatal baseline
materials in each group. |
Table I.
Comparison of neonatal baseline
materials in each group.
| Groups | Male/female | Average age in hours
(h) | Average of
gestational age (weight) | Birth weight
(kg) |
|---|
| Control group
(n=30) | 18/12 | 7.5±1.6 | 38.4±0.6 | 3.6±0.5 |
| Group with mild
symptoms (n=30) | 17/13 | 7.9±2.3 | 38.5±0.5 | 3.7±0.7 |
| Group with moderate
symptoms (n=60) | 34/26 | 7.6±1.5 | 38.3±0.7 | 3.8±0.6 |
| Group with severe
symptoms (n=60) | 35/25 | 8.3±2.1 | 38.2±0.8 | 3.5±0.5 |
Methods
Newborns treated with regular therapy included those
in the control and mild symptoms groups and 30 patients from the
moderate symptoms and 30 from the severe symptoms groups. Standard
guidelines were followed to maintain normal blood pressure and
acid-base balance, to decrease the intracranial pressure, providing
nutrition support and keeping the rectal temperature between 36 and
37°C, and for prophylaxis and management of convulsions. On the
1st, 3rd, 5th and 7th days after birth, blood and urine samples
were collected from each patient and preserved. The sera were
centrifuged for 5 min at 2,800 × g and the supernatants were saved
at −20°C for later assays. The expression levels of SBDPs and Tau
protein were measured by sandwich enzyme-linked immunosorbent
(ELISA) assay. ELISA kits were provided by Sigma-Aldrich (St.
Louis, MO, USA) and microplate readers were provided by Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). All the procedures were
followed, adhering strictly to the manufacturer's instructions.
The other 60 patients, 30 from each the moderate
symptoms and severe symptoms groups, were treated with mild
hypothermia therapy, and the group with severe symptoms treated
with regular therapy. In these cases, selective head cooling
therapy (Olympic Cool-Cap system) was performed 6–12 h after birth,
and an intravenous infusion of saline at 4°C was set in place,
combined with a computer-controlled hypothermic blanket for
low-temperature inducement with a cooling rate of 0.5–1°C/h.
Finally, the rectal temperature was kept between 32 and 34°C for 24
h. Then, natural re-warming was applied during the course of 8 h,
and the temperature of newborns returned to levels between 37 and
37.5°C. On the 1st, 3rd, 5th and 7th days after birth, blood and
urine samples were collected and saved for the detection of
expression levels of SBDPs and Tau protein.
Detection method
For the sandwich ELISA tests, the samples were first
defrozen to room temperature. Then aliquots (100 µl) of coated
antibody were pipetted into the wells on a microplate, which was
covered by adhesive foil and kept for 24 h under constant
temperature (4°C) to coat the microplate. The plate was washed 3
times with a microplate washer. Standards were prepared by diluting
10 µl with 40 µl of dilution solution and added into each well, and
then each plate was covered with adhesive foil and incubated for 60
min at 43°C. For washing the plates the adhesive foils were opened
and the plates were washed 3 times with a microplate washer,
letting the plate stand for a full minute between washes. Then the
samples were mixed with a conjugate solution and 50 µl were added
to each well for incubation for 60 min at 43°C. After the
incubation time, the plates were washed 3 times with the microplate
washer letting the plates stand between washes for 40 sec. Finally,
for coloration and to stop the reaction, 50 µl substrate solution A
and 50 µl of solution B were pipetted into each standard well,
mixed, and placed in the dark for 15 min at 20°C. After that, 50 µl
of stopping solution were added. A microplate reader was used for
obtaining OD values after 15 min; the concentrations of
correspondent samples were calculated from a standards plot.
Indicators for follow-up
The clinical conditions after 28 days, 3, 6 and 12
months were evaluated. Comprehensive physical (weight, body length
and head circumference) and neurological development examinations
were performed. On the 28th day after birth, the NBNA scoring was
determined, including an assessment of behavioral ability (6
items), passive muscular tone (4 items), active muscular tone (4
items), primitive reflexes (3 items) and general evaluation (3
items). The total points for a perfect score were set at 40 (each
item was given 0–2 points). The assessment was performed during the
course of an hour after breast-feeding under quiet, semi-dark and
room temperature conditions. Any score <35 points indicated
brain injury, a score between 35 and 37 points indicated suspected
brain injury and a score of ≥37 points indicated normal
development. The developmental quotient (DQ) tests were performed
at 3, 6 and 12 months after birth using the Gesell development
scale. The test included adaptability, gross motor and fine
movement, language, and social contact. The results of each test
were presented as DQ, in which 55–75 points indicated mild
intelligence disability, 40–54 points indicated moderate
intelligence disability and points <40 indicated severe
intelligence disability.
Statistical analysis
SPSS 19.0 software (Chicago, IL, USA) was applied
for statistical data analysis. Quantitative data were presented as
mean ± standard deviation, t-test or analysis of variance (ANOVA)
were performed in the comparison among groups, LSD t-test was used
for comparisons between any two groups, and ANOVA of repeated
measurement data were carried out in comparisons of different
time-points within one group. Data are presented as percentages,
and χ2 test was applied in comparison among groups.
SBDPs and Tau, used as diagnostic criteria, were analyzed by a
receiver operating characteristic (ROC) curve. Differences with
P<0.05 were considered as statistically significant.
Results
Inter-group comparison of the levels
of SBDPs and of Tau protein at different time-points
At each time-point, the plasma levels of SBDPs and
Tau protein increased with the enhancement of HIE severity and
decreased with the prolongation in treatment duration in each group
with statistically significant differences between the averages in
all groups (P<0.05). For groups with moderate and severe
symptoms treated with mild hypothermia therapy, the levels of SBDPs
and Tau protein at any point in time were significantly lower than
those of groups treated with regular therapy (P<0.05) (Tables II and III).
 | Table II.Inter-group comparisons of the levels
of SBDPs at different time-points among groups (ng/ml). |
Table II.
Inter-group comparisons of the levels
of SBDPs at different time-points among groups (ng/ml).
| Groups | Before treatment | 1st day after
treatment | 3rd day after
treatment | 5th day after
treatment | 7th day after
treatment |
|---|
| Control group | 1.32±0.23 | 1.43±0.33 | 1.23±0.24 | 1.05±0.43 | 1.12±0.35 |
| Group with mild
symptoms | 3.26±0.45 | 3.02±0.52 | 2.86±0.63 | 2.75±0.42 | 2.66±0.37 |
| Group with moderate
symptoms treated by regular therapy | 4.68±0.68 | 4.56±0.67 | 4.42±0.59 | 4.27±0.54 | 4.03±0.53 |
| Group with moderate
symptoms treated by mild hypothermia therapy | 4.69±0.95 | 3.58±0.92 | 3.17±0.78 | 2.96±0.64 | 2.74±0.62 |
| Group with severe
symptoms treated by regular therapy | 6.85±1.23 | 6.23±1.15 | 6.10±1.05 | 5.89±0.98 | 5.76±0.93 |
| Group with severe
symptoms treated by mild hypothermia therapy | 6.86±1.32 | 5.62±1.25 | 5.30±1.13 | 5.12±1.10 | 4.76±0.86 |
 | Table III.Inter-group comparisons of levels of
Tau at different time-points (pg/ml). |
Table III.
Inter-group comparisons of levels of
Tau at different time-points (pg/ml).
| Groups | Before treatment | 1st day after
treatment | 3rd day after
treatment | 5th day after
treatment | 7th day after
treatment |
|---|
| Control group | 3.42±1.37 | 4.42±1.43 | 3.68±1.73 | 4.47±1.85 | 4.12±1.35 |
| Group with mild
symptoms | 7.35±1.24 | 7.12±1.33 | 6.89±1.27 | 6.72±1.05 | 6.43±0.89 |
| Group with moderate
symptoms treated by regular therapy | 9.24±1.43 | 8.97±1.46 | 8.46±1.53 | 8.20±1.13 | 7.85±1.22 |
| Group with moderate
symptoms treated by mild hypothermia therapy | 9.36±1.27 | 8.55±1.32 | 8.21±1.56 | 7.56±1.42 | 7.24±0.95 |
| Group with severe
symptoms treated by regular therapy | 13.52±2.52 | 13.20±2.34 | 12.86±2.52 | 12.66±2.37 | 11.85±2.74 |
| Group with severe
symptoms treated by mild hypothermia therapy | 14.26±2.85 | 12.87±2.54 | 11.85±2.49 | 10.75±2.49 | 10.49±2.48 |
Inter-group comparison of NBNA scores
during the follow-up period
During the follow-up period, the NBNA score
decreased with the increase of HIE severity. For groups with
moderate and severe symptoms treated by mild hypothermia therapy,
the NBNA score was significantly better than that of groups treated
with regular therapy (P<0.05) (Table
IV).
 | Table IV.Inter-group comparisons of NBNA scores
in follow-up period. |
Table IV.
Inter-group comparisons of NBNA scores
in follow-up period.
| Groups | Control group | Group with mild
symptoms | Group with moderate
symptoms treated by regular therapy | Group with moderate
symptoms treated by mild hypothermia therapy | Group with severe
symptoms treated by regular therapy | Group with severe
symptoms treated by mild hypothermia therapy |
|---|
| 28 days | 39.5±2.4 | 37.2±2.3 | 35.4±2.1 | 36.8±2.2 | 34.7±3.0 | 35.8±3.2 |
Inter-group comparison of DQ during
the follow-up period
At each time-point, DQ was worse with the
augmentation of HIE severity and improved with the prolongation in
treatment duration in each group, with statistically significant
differences between the averages of different groups (P<0.05).
For groups with moderate and severe symptoms treated by mild
hypothermia therapy, DQ at any point in time was significantly
better than that of groups treated with regular therapy (P<0.05)
(Table V).
 | Table V.Inter-group comparison of DQ in
follow-up period. |
Table V.
Inter-group comparison of DQ in
follow-up period.
| Groups | 3rd month | 6th month | 12th month |
|---|
| Control group | 82.4±3.6 | 83.5±4.7 | 84.2±5.3 |
| Group with mild
symptoms | 77.2±4.3 | 78.3±4.6 | 79.4±5.2 |
| Group with moderate
symptoms treated with regular therapy | 72.4±6.3 | 73.6±6.6 | 75.4±6.8 |
| Group with moderate
symptoms treated with mild hypothermia therapy | 72.3±6.5 | 74.8±6.3 | 75.8±6.2 |
| Group with severe
symptoms treated with regular therapy | 67.5±8.3 | 68.3±8.5 | 71.2±8.7 |
| Group with severe
symptoms treated with mild hypothermia therapy | 67.2±7.3 | 71.3±7.4 | 73.5±7.7 |
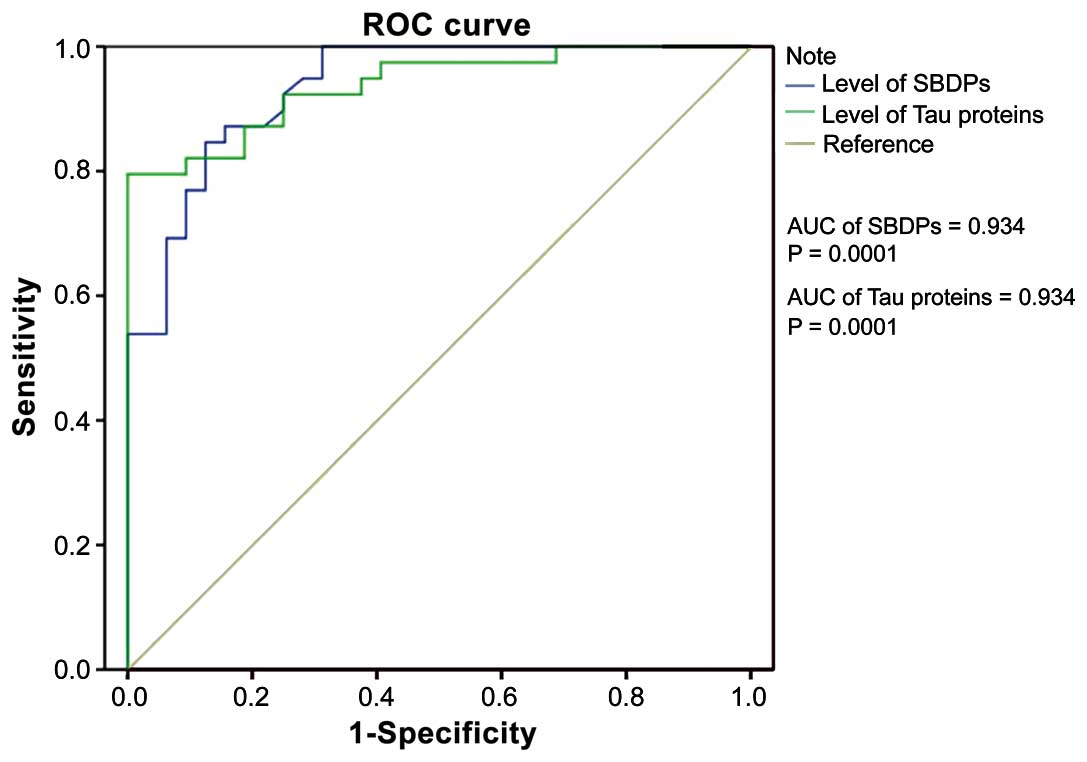
Analysis of ROC curve for diagnosis of
HIE according to the levels of SBDPs and Tau protein
The area under the curve (AUC) of ROC for HIE
diagnosis in accordance with the level of SBDPs was 0.934
(P=0.0001). The sensitivity and specificity of diagnosis reached
84.6 and 87.5%, respectively, when the critical point was 1.58
ng/ml (Fig. 1).
The AUC of ROC for HIE diagnosis according with the
level of Tau was 0.934 (P=0.0001). The sensitivity and specificity
of diagnosis reached 79.5 and 96.9%, respectively, when the
critical point was 4.76 pg/ml (Fig.
1).
Discussion
The pathogenesis of HIE is complicated, it can
include calcium overload, a cytokine storm, reperfusion injury,
cellular apoptosis and dysfunction of energetic metabolism and
others (7). Under normal conditions,
the rates of formation and clearance of oxygen-free radicals remain
in dynamic equilibrium (8). However,
this equilibrium becomes disrupted when the brain tissues are under
hypoxic ischemic states, forming enormous quantities of oxygen-free
radicals and enhancing the response to oxidative stress. Newborns
are usually highly susceptible to oxidative injury due to a high
content of polyunsaturated fatty acids in their brain. However, in
most cases of moderate and severe HIE, patients suffer from
neurological sequelae (9).
Regular symptomatic treatment of HIE includes
maintaining adequate heart and ventilation rates, lowering
intracranial pressure, keeping pH and electrolyte balances and
prophylaxis against convulsions. In addition, some drugs such as
allopurinol, anti-apoptosis drugs and deferoxamine are sometimes
used to promote the synthesis of EPO and inhibit the process of
cellular apoptosis (10). Through
mild hypothermia therapy, the head of a newborn is kept in a
low-temperature environment (27°C), resulting in a decrease in the
release of excitatory neurotransmitters, with a decrease in the
rate of formation of oxygen-free radicals and inhibition of
apoptosis of neurons (11). The
underlying mechanisms are not fully understood but include a
decrease in the concentration of SBDPs (12). By suppressing neuronal apoptosis, the
large release of caspase-3 is blocked, leading to decreases in
SBDPs. Downregulation of the activity of glial cells helps to avoid
the excessive activation of inflammatory factors, protecting the
nerve and preventing the exacerbation of brain injury (13). The low-temperature stabilizes the
plasmalemma of brain cells, alleviating the effect of peroxidation
reactions, regulating the phosphorylation of Tau proteins, reducing
the possibility of breakdown of microtubules, maintaining the
regular morphology of neurons and axonal transport and mitigating
brain injuries (14).
Usually, SBDPs exists in the neuron axon and
presynaptic terminals. At the beginning of HIE, large amounts of
calpain-2 and caspase-3 are activated and accelerate apoptosis and
necrosis of brain cells, leading to brain injuries. However, to
hydrolyze calpain-2 and caspase-3, mass SBDPs are produced, which
can affect the distribution of SBDPs in neuronal axons, resulting
in axotomy. This process is irreversible. Thus, SBDPs do not only
indicate brain injuries, but also contribute to the pathological
mechanism of apoptosis and necrosis of brain cells (12,15).
Under normal circumstances, Tau proteins have two forms,
dephosphorylated form and phosphorylated, in a balanced state.
During the progress of HIE, the Tau protein gets excessively
phosphorylated, which affects its normal physiological functions.
The Tau protein accumulates in neurons, altering the normal
formation of microtubules. Besides, it also splits the normal
microtubule-related proteins from the microtubule, leading to the
collapse of microtubule, and creating large amounts of deposited
matter inside the neurons damaging them (16,17).
In this study, at any time, the mild hypothermia
therapy improved the levels of SBDPs and Tau proteins and also the
NBNA scores and DQ values. The levels of SBDPs and Tau proteins
showed good sensitivity, specificity and accuracy in diagnosis of
HIE. Thus, early diagnosis of HIE using SBDPs and Tau protein
levels should lead to prompt treatment with mild hypothermia for
improving neurological function development and prognosis of HIE
infants.
References
|
1
|
Boix H: Are we doing our best for our
patients with hypoxic-ischaemic encephalopathy? An Pediatr (Barc).
May 24–2016.(In Spanish) (Epub ahead of print).
|
|
2
|
Xu J, Gang QQ, Hao P and Zhang JN:
Pathological and magnetic resonance imaging findings in a neonatal
Tibet minipig model of hypoxic-ischemic encephalopathy. Nan Fang Yi
Ke Da Xue Xue Bao. 36:705–709. 2016.(In Chinese). PubMed/NCBI
|
|
3
|
Rumajogee P, Bregman T, Miller SP, Yager
JY and Fehlings MG: Rodent hypoxia-ischemia models for cerebral
palsy research: a systematic review. Front Neurol. 7:572016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Shi Q, Zheng S, Luo L, Yuan S,
Wang X, Cheng Z and Zhang W: Role of α-II-spectrin breakdown
products in the prediction of the severity and clinical outcome of
acute traumatic brain injury. Exp Ther Med. 11:2049–2053.
2016.PubMed/NCBI
|
|
5
|
Lv H, Wang Q, Wu S, Yang L, Ren P, Yang Y,
Gao J and Li L: Neonatal hypoxic ischemic encephalopathy-related
biomarkers in serum and cerebrospinal fluid. Clin Chim Acta.
450:282–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elbahtiti A, Aly NY, Abo-Lila R and
Al-Sawan R: Therapeutic hypothermia for infants with hypoxic
ischemic encephalopathy: a five years' single center experience in
Kuwait. J Neonatal Perinatal Med. 9:179–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demarest TG, Schuh RA, Waddell J, McKenna
MC and Fiskum G: Sex-dependent mitochondrial respiratory impairment
and oxidative stress in a rat model of neonatal hypoxic-ischemic
encephalopathy. J Neurochem. 137:714–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar A, Mittal R, Khanna HD and Basu S:
Free radical injury and blood-brain barrier permeability in
hypoxic-ischemic encephalopathy. Pediatrics. 122:e722–e727. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fisher PG: Are neonatal stroke and
hypoxic-ischemic encephalopathy related? J Pediatr. 173:1–3. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YW, Mathur AM, Chang T, McKinstry RC,
Mulkey SB, Mayock DE, Van Meurs KP, Rogers EE, Gonzalez FF,
Comstock BA, et al: High-dose erythropoietin and hypothermia for
hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics.
137:123–124. 2016. View Article : Google Scholar
|
|
11
|
Sabir H, Osredkar D, Maes E, Wood T and
Thoresen M: Xenon combined with therapeutic hypothermia is not
neuroprotective after severe hypoxia-ischemia in neonatal rats.
PLoS One. 11:e01567592016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobeissy FH, Liu MC, Yang Z, Zhang Z,
Zheng W, Glushakova O, Mondello S, Anagli J, Hayes RL and Wang KK:
Degradation of βII-spectrin protein by calpain-2 and caspase-3
under neurotoxic and traumatic brain injury conditions. Mol
Neurobiol. 52:696–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gabriel ML, Braga FB, Cardoso MR, Lopes
AC, Piatto VB and Souza AS: The association between pro- and
anti-inflammatory cytokine polymorphisms and periventricular
leukomalacia in newborns with hypoxic-ischemic encephalopathy. J
Inflamm Res. 9:59–67. 2016.PubMed/NCBI
|
|
14
|
Liu F, Yang S, Du Z and Guo Z: Dynamic
changes of cerebral-specific proteins in full-term newborns with
hypoxic-ischemic encephalopathy. Cell Biochem Biophys. 66:389–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Witek MA and Fung LW: Quantitative studies
of caspase-3 catalyzed αII-spectrin breakdown. Brain Res.
1533:1–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ojo JO, Mouzon B, Algamal M, Leary P,
Lynch C, Abdullah L, Evans J, Mullan M, Bachmeier C, Stewart W, et
al: Chronic repetitive mild traumatic brain injury results in
reduced cerebral blood flow, axonal injury, gliosis, and increased
T-Tau and Tau oligomers. J Neuropathol Exp Neurol. 75:636–655.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Chen Y, Xu Y and Pi G: Effects of
PTEN inhibition on the regulation of Tau phosphorylation in rat
cortical neuronal injury after oxygen and glucose deprivation.
Brain Inj. 30:1150–1159. 2016. View Article : Google Scholar : PubMed/NCBI
|