Introduction
The recurrent laryngeal nerve (RLN) can be damaged
during surgery of the head and neck (1). One of the most significant symptoms of
damage to this nerve is incomplete glottic closure, which can
profoundly decrease quality of life (2). Medialization procedures are commonly
carried out to treat unilateral vocal fold palsy, solely fulfilling
vocal fold medialization by static changes in the laryngeal
framework (3). Neurological
impairment can be compensated for by the regeneration of peripheral
nerves. However, experimental and clinical evidence has shown that,
particularly after severe injuries, regenerative ability is usually
limited with unsatisfactory results (1,4–6). Thus, in order to improve recovery
following nerve injury, one therapeutic option is to use natural
stimulatory reagents, such as neurotrophic factors.
Neurotrophic factors are able to enhance nerve
regeneration, and therefore may be useful as a molecular therapy.
Among all neurotrophic factors, brain-derived neurotrophic factor
(BDNF) and glial cell line-derived neurotrophic factor (GDNF) play
critical roles. BDNF promotes angiogenesis and neural regeneration,
as well as modulates local inflammatory processes (7). GDNF has been shown to boost nerve
regeneration after injury and exerts survival-promoting effects on
motor neurons in vivo and in vitro (8–11).
However, in practice, a large proportion of neurotrophic factors
are diffused away from the site of injury; thus, it is difficult to
retain effective concentrations of such factors due to their rapid
diffusion in extracellular fluids. One solution to this problem is
repeated injections of neurotrophic factors to the injured sites so
that therapeutic concentrations can be obtained; however, this
strategy poses unnecessary risks and costs (12). Thus, the discovery of a better
approach for targeting neurotrophic factors to wound sites is
imperative (13).
One method of overcoming the diffusion of
neurotrophic factors is by taking advantage of their binding
affinity to certain molecules. In doing so, these factors can be
immobilized at the site of injury. The glycoprotein, laminin,
appears to be an appropriate material for targeting damaged sites
because it is a major component of the extracellular matrix (ECM)
and is biocompatible (13). Laminin
is mainly produced by Schwann cells and is widespread in the
peripheral nervous system (PNS) (14,15).
Following injury to the PNS, laminin is upregulated and promotes
axonal regeneration (16,17). It has been reported that BDNF has an
affinity for laminin, but this affinity is low; more than half of
BDNF bound to laminin is quickly released within the first day of
injury (13). Thus, the native
affinity of BDNF for laminin appears to be insufficient for
therapeutic purposes.
Previous studies have demonstrated that the
N-terminal domain of agrin (NtA) has a high affinity to laminin
(18). Making use of this so-called
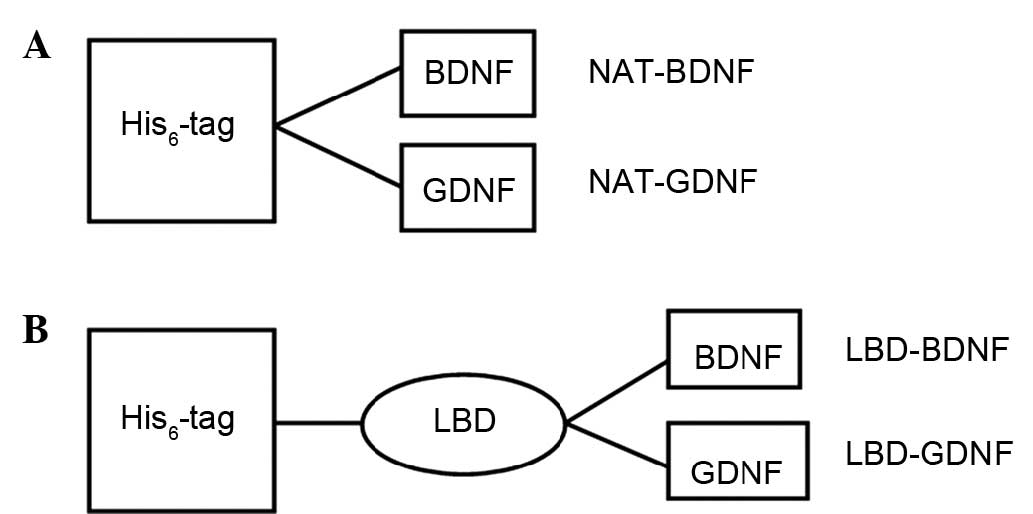
laminin-binding domain (LBD), a tripartite fusion protein, which
included a six-histidine purification tag, LBD, and the sequence of
native BDNF or GDNF was made in the present study. Thus, the two
fusion proteins that resulted were named LBD fused BDNF (LBD-BDNF)
and LBD fused GDNF (LBD-GDNF). A native BDNF and GDNF without NtA,
designated NAT-BDNF and NAT-GDNF, respectively, were prepared as
controls. The overall aim of this study was to utilize laminin as a
binding target so that a delivery system could be constructed to
maintain neurotrophic factor concentrations within certain regions
of interest.
Materials and methods
Preparation of recombinant
proteins
LBD-BDNF was prepared as previously described
(13), and LBD-GDNF was constructed
in an analogous manner. GDNF DNA, extracted from the rat cell line
PC12 [purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China)], was amplified by polymerase chain
reaction (PCR) with a KOD Plus polymerase kit (Toyobo Co., Ltd.,
Osaka, Japan). Primer sequences used were as follows: Forward,
GGTAGCGGCAGCGGTAGCACATGCCCGGAGCGCGCGCTG; reverse,
TACTCGAGTCAGATACATCCACACCTTTTAG. Reaction conditions for PCR were
as follows: Initial denaturation, 95°C for 5 min; 30 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 68°C for 30 sec; followed by a final extension at 68°C
for 5 min. and inserted into the vectors, pET-LBD and pET-28a
(Novagen; EMD Millipore, San Diego, CA, USA). Escherichia
coli BL21 (DE3) (Biovector Science Lab, Inc., Beijing, China)
was then transformed with the vectors, and proteins were induced
with 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) at 37°C for 5
h. Proteins were accumulated in inclusion bodies, and purification
of the solubilized proteins was performed under denaturing
conditions by nickel chelate chromatography, using
imidazole-containing buffer, with a HiTrap Chelating High
Performance column and an AKTA fast protein liquid chromatography
system (Amersham; GE Healthcare, Uppsala, Sweden). The purity of
the recombinant proteins was analyzed by 15% (wt/vol) sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), by
which 20 µg of protein was separated and stained with Coomassie
brilliant blue solution (Beyotime Institute of Biotechnology,
Haimen, China) for 1 h, then washed in saline, or transferred to a
PVDF membrane. This was followed by a block in 5% milk solution,
then western blotting with a mouse antibody against polyhistidine
(cat. no. ab18184; 1:2,000 dilution; Abcam, Cambridge, UK). This
was subsequently incubated with horseradish peroxidase-conjugated
goat anti-mouse antibody (cat. no. ab97040; 1:8,000 dilution;
Abcam) for 2 h at room temperature, following which bands were
visualized with enhanced chemiluminescence reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Purified proteins were then
dialyzed in a glutathione redox-refolding system by a
chromatographic method (19) and
stored at −80°C.
In vitro laminin-binding assay
In order to measure laminin binding capacity, a
modified enzyme-linked immunosorbent assay (ELISA) technique was
employed in which laminin (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was added to 96-well plates (Costar). The
plates were then incubated at 4°C for 24 h, and ventilated to dry.
After washing the plates with phosphate-buffered saline (PBS; pH
7.3) five times, the plates were blocked with 2.5% (wt/vol) bovine
serum albumin (Wuhan Boster Biological Technology, Ltd.- Wuhan,
China) containing 0.1% (vol/vol) Tween 20 at 37°C for 2 h. The four
recombinant proteins with increasing concentrations (0–4 µM) were
added to the plates (100 µl/well) and incubated at 37°C for 2 h.
The plates were then washed five times with PBS to remove redundant
proteins. Proteins bound to laminin were tested using mouse
antibody against polyhistidine (cat. no. H1029; 1:1,000 dilution;
Sigma-Aldrich) for 1 h at 37°C, followed by goat antibody to mouse
IgG (alkaline phosphatase-conjugated; cat. no. P7998; 1:10,000;
Sigma-Aldrich) for 1 h at 37°C. Bound proteins were detected using
the alkaline phosphatase (AP) reaction with
para-nitrophenylphosphate (pNPP; Sigma-Aldrich) in AP buffer
(pH 9.6) for 15 min at 37°C; this reaction was stopped using 3 M
NaOH. Optical density at 405 nm (OD405) was quantified using an
ELISA reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
In vitro laminin releasing assay
Laminin loaded with 1.5 µmol/l NAT-BDNF or LBD-BDNF
or 3 µmol/l NAT-GDNF or LBD-GDNF was placed in a 48-well plate.
Laminin suspension was induced by the addition of 500 µl PBS and
incubation on a rocking platform (37°C, 80 rpm). PBS was changed
every 24 h, and at days 0–7, samples were collected and the four
proteins retained on the laminin were analyzed using the ELISA
assay.
In vitro bioactivity assay
The biological activity of NAT-BDNF, LBD-BDNF,
NAT-GDNF and LBD-GDNF was measured by neurite outgrowth and
survival of PC12 cells (20). A
density of 3×103 cells/well was seeded in
polylysine-treated 96-well plates (Costar) and cultured in
serum-free RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C for 1 h. Increasing concentrations of NAT-BDNF, LBD-BDNF,
NAT-GDNF or LBD-GDNF (0–800 ng) were then added to the plates.
After 24 h of culture, the percentage of cells with neurites and
the neurite length/cell diameter ratio of cells containing neurites
were determined. The number of surviving cells was determined using
a Cell Counting kit (CCK)-8 assay (Dojindo, Kumamoto, Japan) after
48 h of culture. Cells cultured under identical conditions without
recombinant proteins served as a control. The results were
evaluated in duplicate by two independent researchers.
In vitro bioactivity assay on
laminin
Recombinant proteins were separately added to a
96-well plate coated with laminin and incubated at 4°C for 12 h.
Plates were then washed three times to remove any unbound BDNF or
GDNF. PC12 cells were seeded at a density of 3×103
cells/well under the same conditions as described in the in
vitro bioactivity assay. After 24 h of incubation, the cells
were observed using phase contrast microscopy (Olympus Corporation,
Tokyo, Japan), and the percentage of cells with neurites and length
neurite/diameter of cells containing neurites were calculated. The
number of surviving cells was examined using a CCK-8 assay after 48
h of culture. For controls, cells were cultured under the same
conditions but without recombinant proteins.
Bioactivity of different proportions
of LBD-BDNF and LBD-GDNF on laminin in vitro
Different proportions of LBD-BDNF and LBD-GDNF (1:4,
2:3, 3:2 and 4:1) were added to a 96-well plate coated with
laminin, with the total protein added equaling 1,000 ng. The
remainder of the steps were carried out as previously described in
the bioactivity assay on laminin. Blank controls were manufactured
using the abovementioned conditions, with the exception that one of
the fusion factors was omitted.
Cytoskeletal staining
Different proportions of LBD-BDNF and LBD-GDNF (1:4,
2:3, 3:2 and 4:1) were added to a confocal dish coated with
laminin. PC12 cells were seeded at a density of 3×103
under the same conditions as described in the bioactivity assay on
laminin. The cells were washed three times with PBS and fixed with
4% paraformaldehyde for 2 h at room temperature. Cells were then
incubated with 0.5 µg/ml FITC-phalloidin (Sigma-Aldrich) for 20
min, followed by three washes with 0.3% Triton X-100. In order to
identify cell nuclei, 0.1 µg/ml 4′,6-diamidino-2-phenylindole
(DAPI; Sigma-Aldrich) staining was used. Finally, after three
washes in PBS to remove unbound DAPI, cells were observed using
confocal fluorescent microscopy (Olympus Corporation).
Statistical analysis
All data were summarized as the mean value ±
standard deviation. A student's t-test was performed to analyze
paired samples and one-way analysis of variance was performed to
analyze multiple comparison procedures using Statistical Package
for the Social Sciences (SPSS) version 20.0 software (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Fusion protein structures
The functional modules of NAT-BDNF, NAT-GDNF,
LBD-BDNF and LBD-GDNF are shown in Fig.
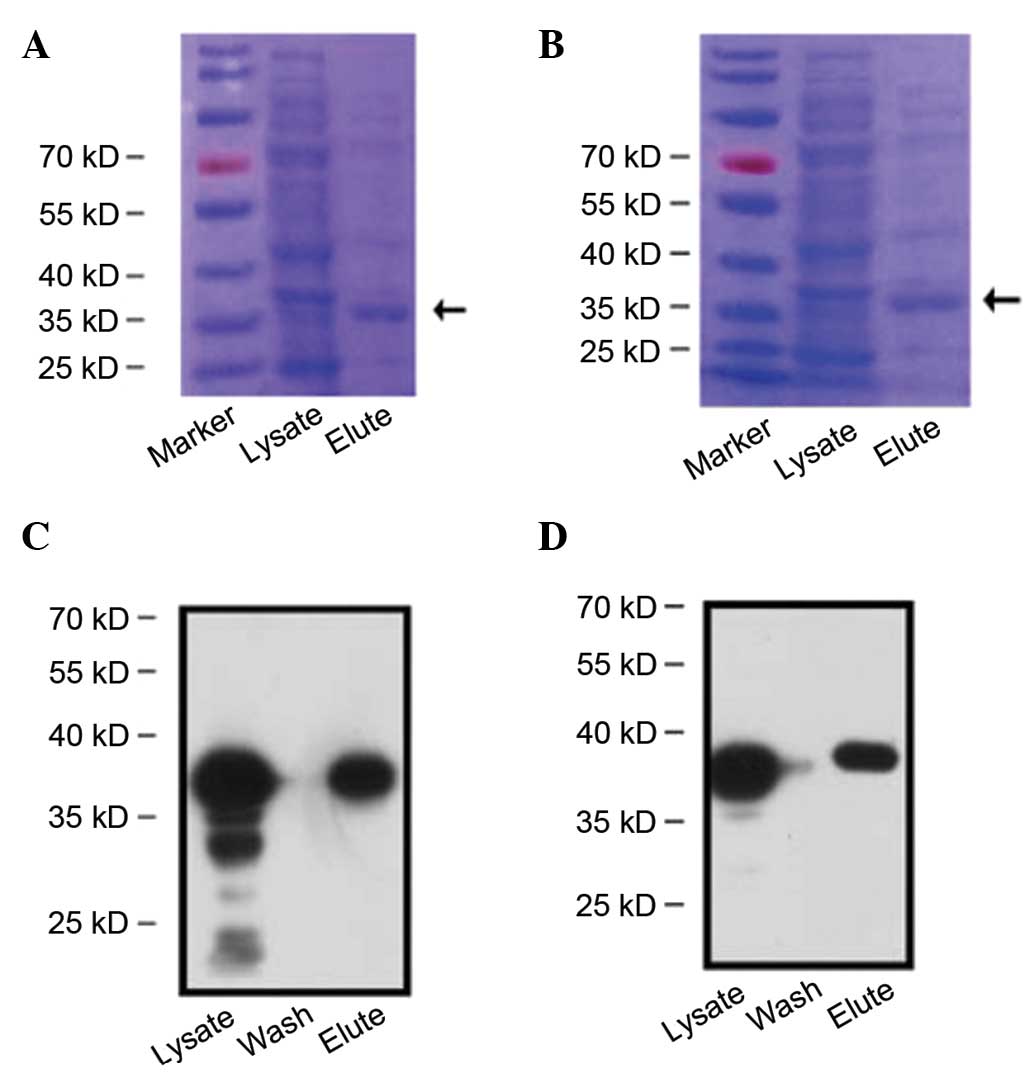
1. After being induced by IPTG, the recombinant proteins were
expressed, and identified by western blot analysis (Fig. 2).
LBD-BDNF and LBD-GDNF bind to laminin
and exhibit sustained released in vitro
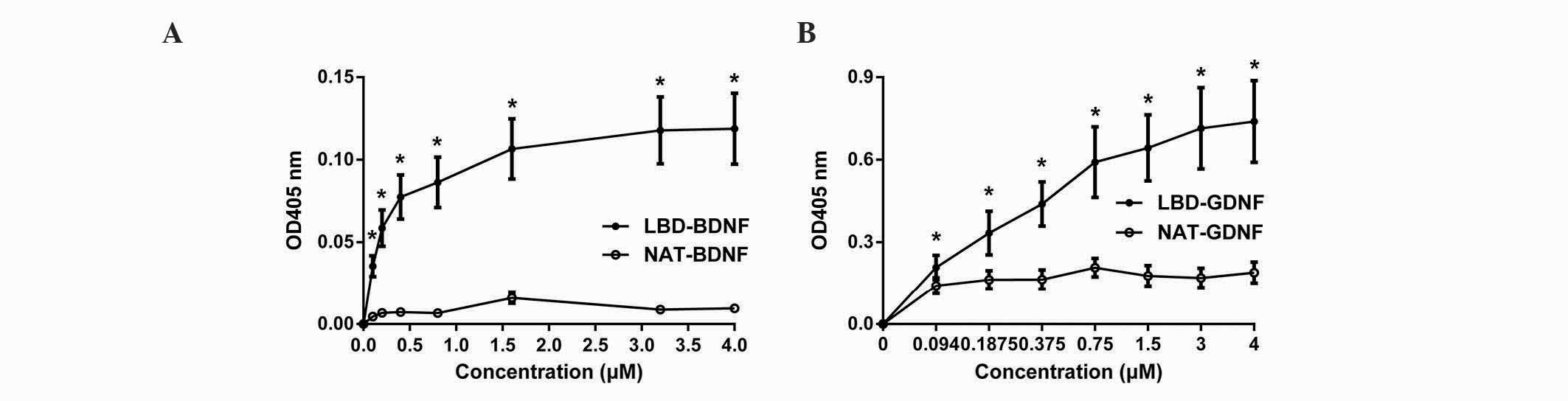
The binding of NAT-BDNF, LBD-BDNF, NAT-GDNF and
LBD-GDNF to laminin was measured in vitro through a modified
ELISA technique. From the results, it was concluded that the OD405
of neurotrophic factors with NtA (the LBD) was significantly higher
than that of neurotrophic factors without NtA at each indicated
point. These findings demonstrate that the retention of
neurotrophic factors with NtA on laminin was significantly greater
than that of neurotrophic factors without NtA (n=6, P<0.05;
Fig. 3). Thus, LBD-BDNF and LBD-GDNF
could specifically bind to laminin, and factors with NtA possessed
stronger laminin-binding capacity.
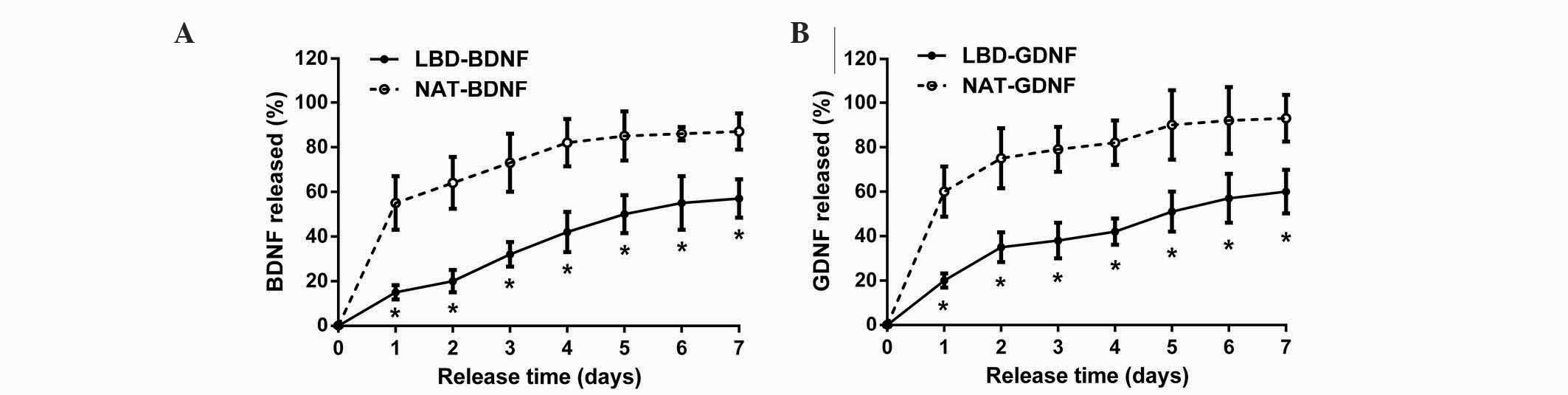
In the in vitro release experiment, sustained
release of neurotrophic factors was assessed for 7 days (Fig. 4). It was found that NAT-BDNF and
NAT-GDNF were quickly released on day 1, whereas LBD-BDNF and
LBD-GDNF were gradually released from day 1 to day 7. During the 7
days, the quantities of LBD-BDNF and LBD-GDNF retained on laminin
were significantly greater than those of NAT-BDNF and NAT-GDNF,
respectively (n=6, P<0.05). At day 7, the percentage of NAT-BDNF
and NAT-GDNF released from laminin was ~87 and 93%, respectively,
whereas the percentage of LBD-BDNF and LBD-GDNF released from
laminin was ~57 and 60%, respectively. These results suggest that
neurotrophic factors with a LBD can be retained on laminin for a
longer time in vitro than those without.
LBD-BDNF and LBD-GDNF maintain higher
bioactivity on laminin in vitro
PC12 cells were used to test the bioactivity of
NAT-BDNF, LBD-BDNF, NAT-GDNF and LBD-GDNF. Fig. 5A shows PC12 cells cultured under
general conditions, while Fig. 5B
shows cells cultured with LBD-BDNF. PC12 cells were observed after
48 h incubation, and it was determined that the four factors
significantly promoted neurite outgrowth and neuronal survival
(Fig. 6), while there was no
significant difference between them at each concentration (Fig. 6). This implies that the fusion of
neurotrophic factors with a LBD moiety did not impair the inherent
activity of BDNF or GDNF.
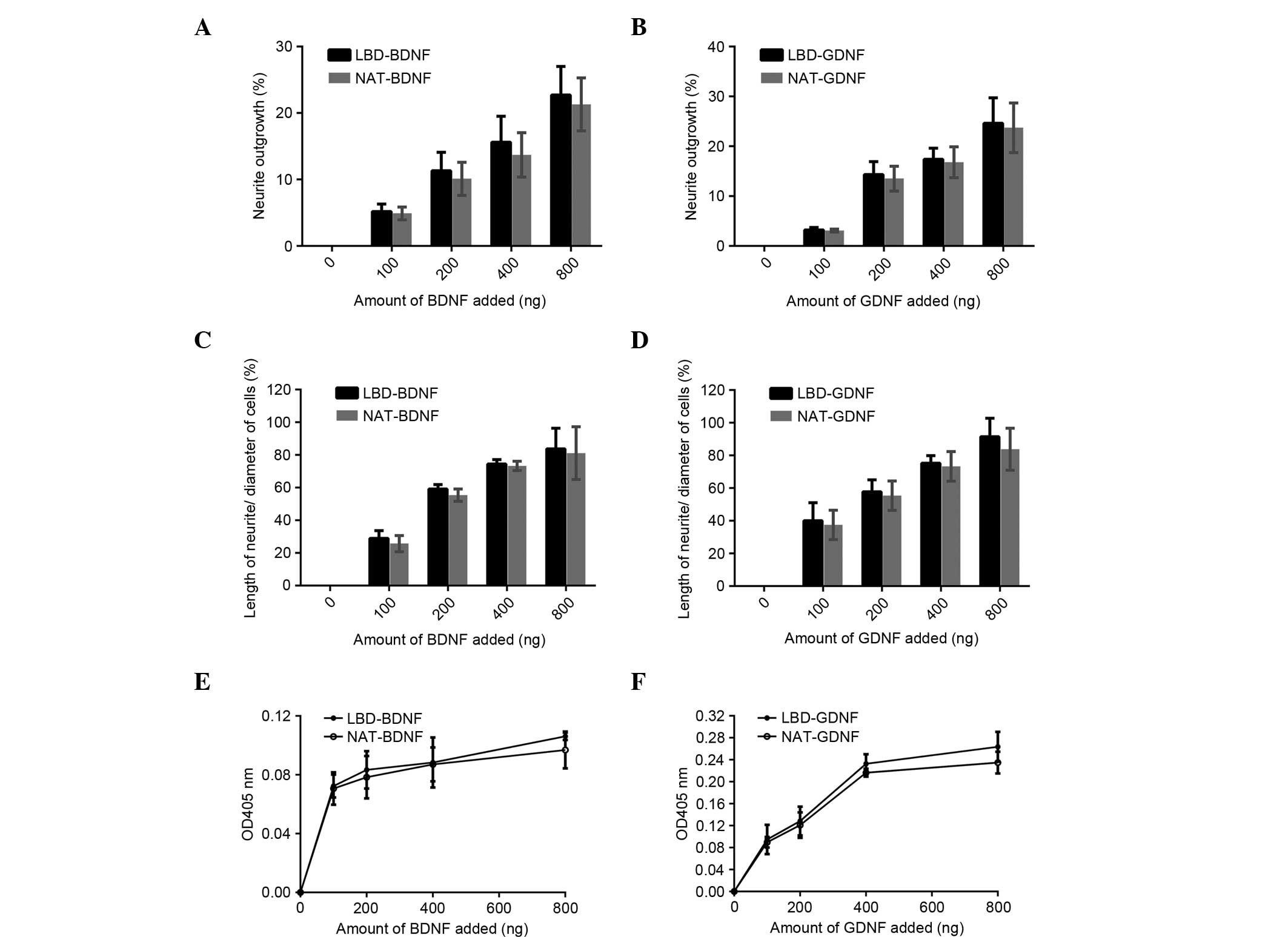 | Figure 6.Bioactivity comparison of recombinant
proteins in vitro. Effect of (A) LBD-BDNF, NAT-BDNF, (B)
LBD-GDNF and NAT-GDNF on neurite outgrowth from PC12 cells. Effect
of (C) LBD-BDNF, NAT-BDNF, (D) LBD-GDNF and NAT-GDNF on the ratio
of neurite length/cell diameter in PC12 cells. Effect of (E)
LBD-BDNF, NAT-BDNF, (F) LBD-GDNF and NAT-GDNF on PC12 cell survival
as determined by Cell Counting kit-8 assay. Data are presented as
mean ± standard deviation (n=6). NAT, native; LBD, laminin-binding
domain; BDNF, brain-derived neurotrophic factor; GDNF, glial cell
line-derived neurotrophic factor; OD, optical density. |
The bioactivities of BDNF and GDNF on laminin were
then measured in vitro (Fig.
7). When neurotrophic factors were incubated in laminin-coated
wells and PC12 cells were plated, a significant difference in
growth promotion (the percentage of cells with neurites) compared
with controls was observed (Fig. 7A and
B). Greater numbers of living cells and longer neurites were
also found for the neurotrophic factors with the LBD NtA (n=6,
P<0.05; Fig. 7C-F). LBD-BDNF and
LBD-GDNF maintained higher bioactivities, as well as greater
concentrations on laminin than did the native controls. These
results suggest that LBD-BDNF and LBD-GDNF can target laminin, and
that these fusion proteins could be useful in nerve injury repair.
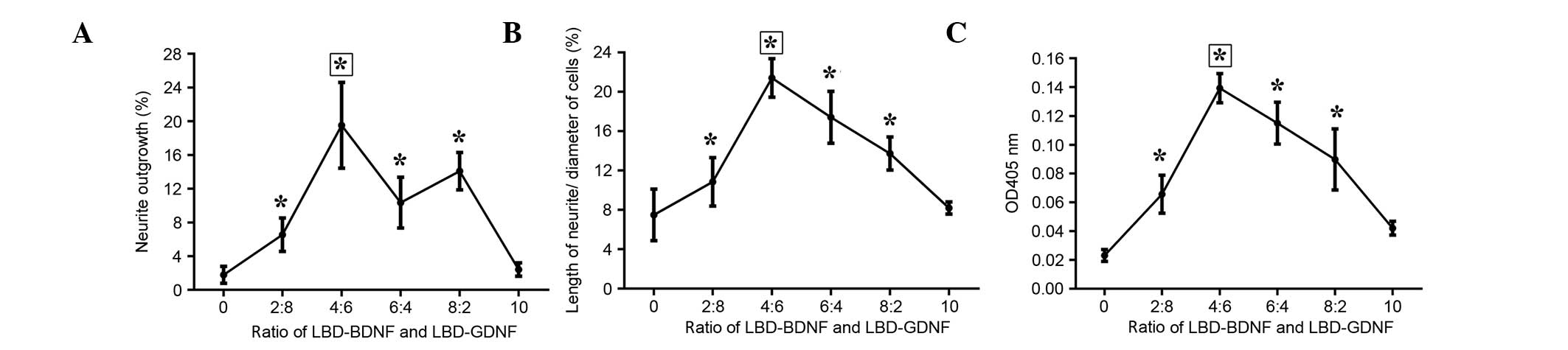
Furthermore, when the ratio of LBD-BDNF and LBD-GDNF was 4:6, the
ability to promote neurite growth was superior to that of the other
ratios examined (Fig. 8).
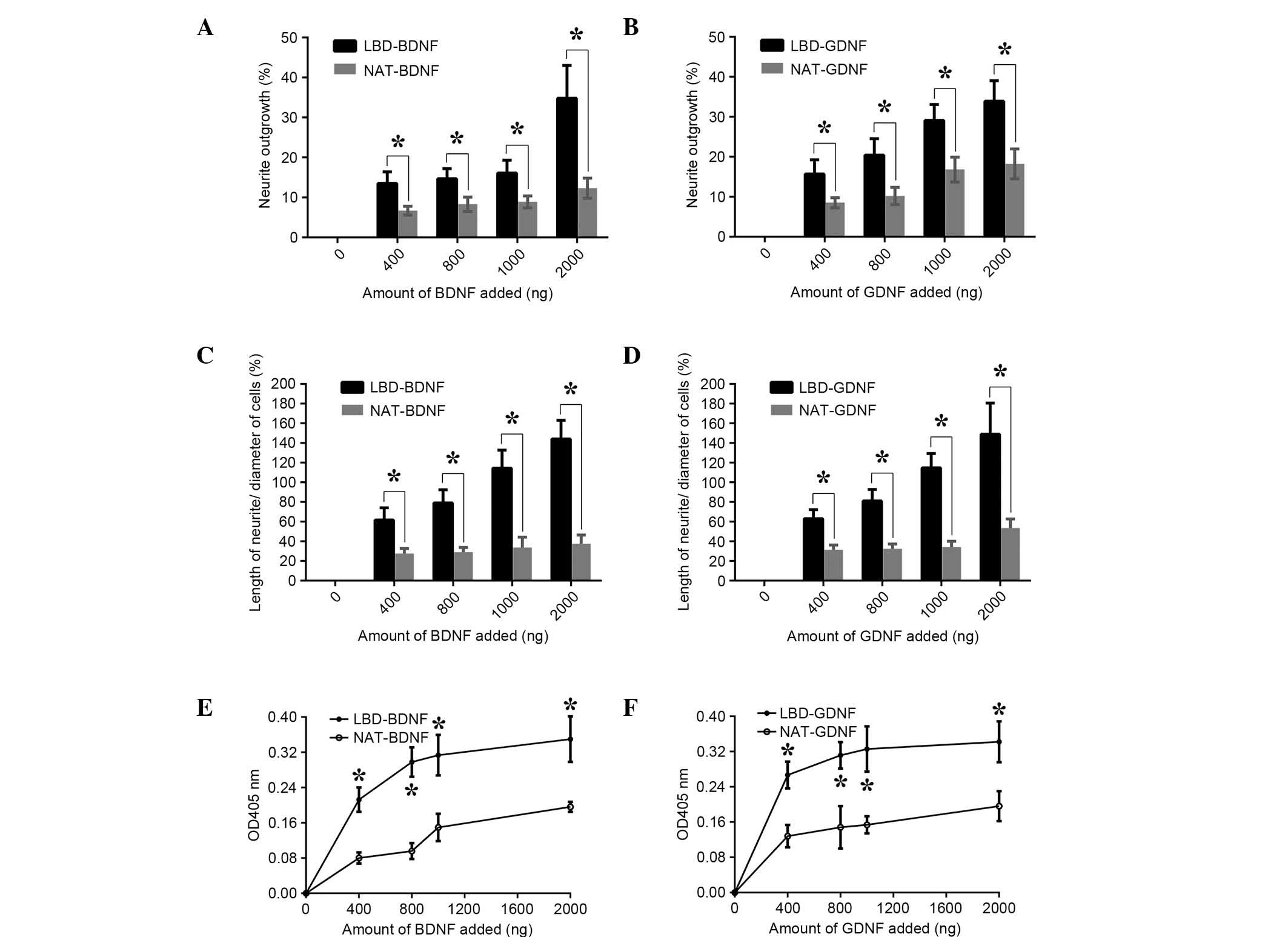 | Figure 7.Bioactivity comparison of recombinant
proteins on laminin in vitro. Effect of (A) LBD-BDNF,
NAT-BDNF, (B) LBD-GDNF and NAT-GDNF on neurite outgrowth from PC12
cells. Effect of (C) LBD-BDNF, NAT-BDNF, (D) LBD-GDNF and NAT-GDNF
on the ratio of neurite length/cell diameter in PC12 cells. Effect
of (E) LBD-BDNF, NAT-BDNF, (F) LBD-GDNF and NAT-GDNF on PC12 cell
survival as determined by Cell Counting kit-8 assay. Data are
presented as mean ± standard deviation (n=6). *P<0.05 the NAT
control. NAT, native; LBD, laminin-binding domain; BDNF,
brain-derived neurotrophic factor; GDNF, glial cell line-derived
neurotrophic factor; OD, optical density. |
Cytoskeletal staining
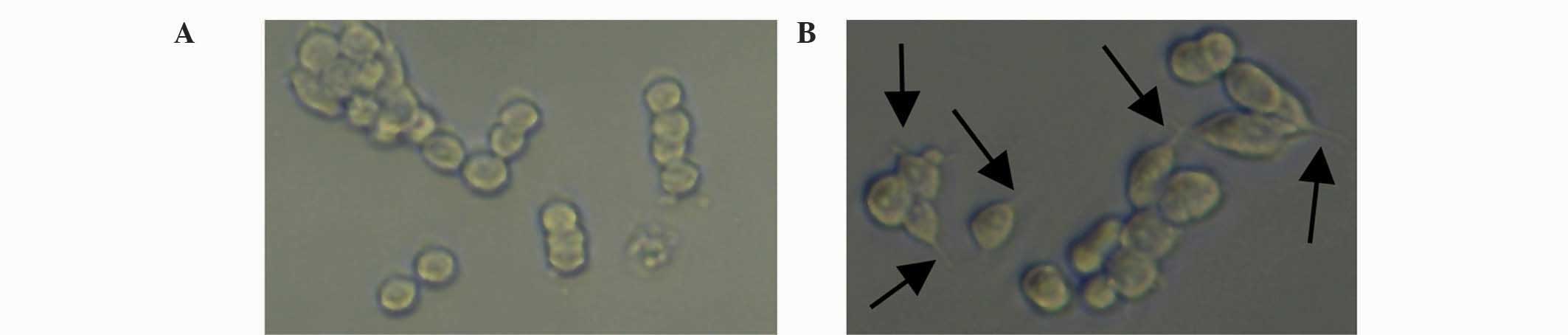
As shown in Fig. 9,
the cytoskeleton, nucleus and neurites of the PC12 cells are
clearly presented. Although the entire cell skeleton cannot be
visualized, the featured images were chosen for their clear
presentations of the neurites. After combining LBD-BDNF and
LBD-GDNF, a greater effect was observed than when they were applied
separately. Through these results, it may be concluded that when
the ratio of mixing of LBD-BDNF and LBD-GDNF was 4:6, their ability
to promote growth was superior to that at other ratios,
demonstrating a synergetic effect (Fig.
8).
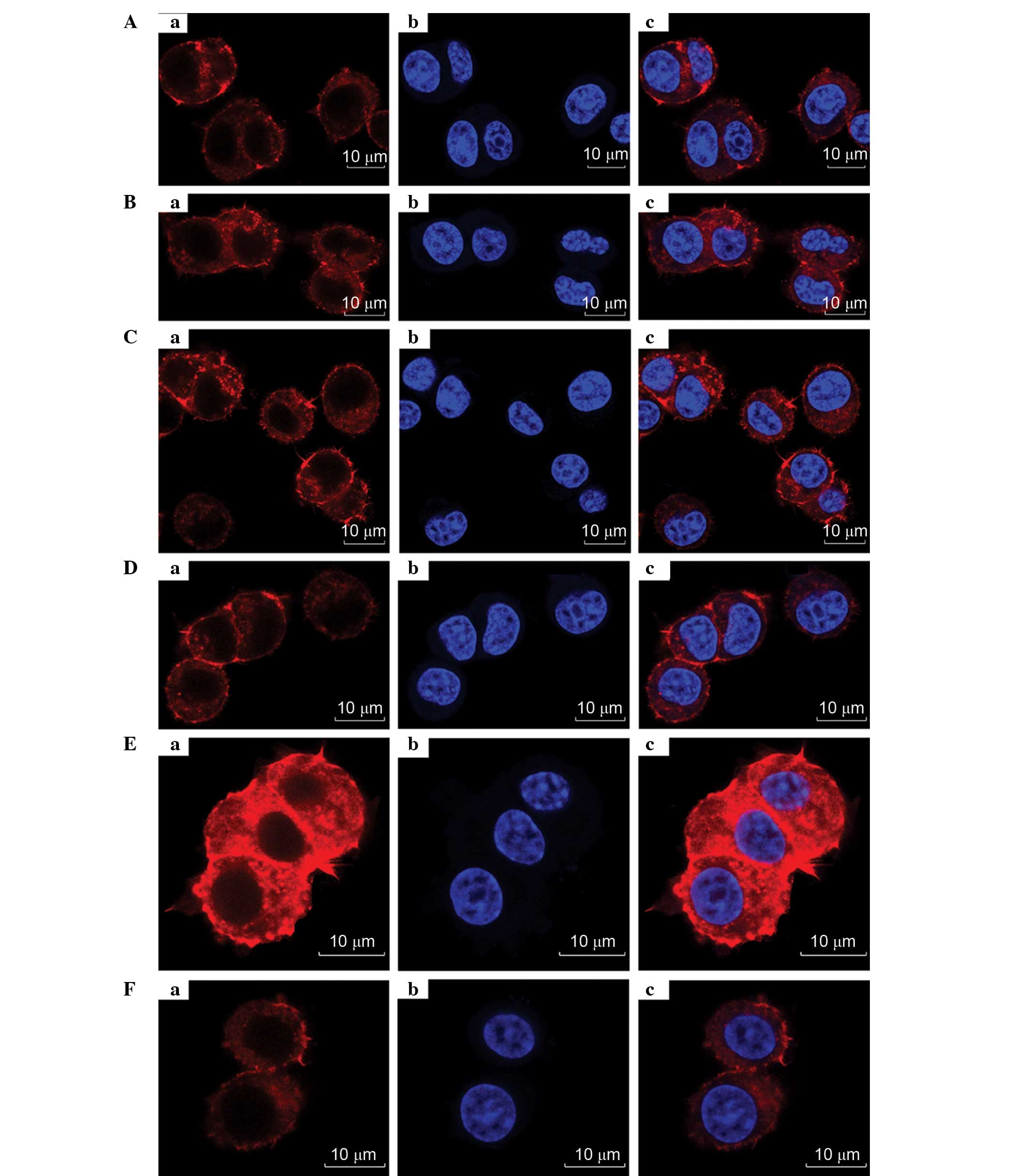 | Figure 9.Images of PC12 cell (Aa)
cytoskeletons; (Ab) nucleim, or (Ac) overlays, viewed by confocal
fluorescent microscopy following treatment with different
proportions of LBD-BDNF and LBD-GDNF. (A) Only LBD-BDNF, LBD-GDNF
and LBD-BDNF in a (B) 1:4 ratio, (C) 2:3 ratio, (D) 3:2 ratio and
(E) 4:1 ratio, and (F) only LBD-GDNF. NAT, native; LBD,
laminin-binding domain; BDNF, brain-derived neurotrophic factor;
GDNF, glial cell line-derived neurotrophic factor. |
Discussion
Peripheral nerve injury often causes a loss of
function, and the current gold standard for repairing such damage
is autologous nerve grafting (21).
However, the clinical application of autologous nerve grafting is
hampered due to limited donor sites, extra incisions required, and
a possible loss of function at the donor site (21). One potential therapeutic strategy
that could address these issues is the application of natural
stimulatory factors, such as neurotrophic factors, to the site of
injury.
Neurotrophic factors are produced by organisms to
promote neural cell survival, growth and differentiation.
Neurotrophic factors are not only able to reduce nerve degeneration
and prevent the progression of disease, but are also able to
stimulate axonal growth and promote functional regeneration
(22). Researchers have studied
several fusion neurotrophic factors, including native human nerve
growth factor (NGF)-β fused with a collagen-binding domain (CBD)
(23–25), fibronectin fused with CBD (26), BDNF fused with CBD (7,27–32),
NGF-β fused with LBD (20), ciliary
neurotrophic factor fused with LBD (33), and BDNF fused with LBD (13); however, to the best of our knowledge,
to date there have been no studies investigating an LBD-GDNF fusion
protein.
BDNF and GDNF play important roles in the repair of
nerve injury. However, it is difficult to retain them at injury
sites because they easily diffuse. In order to maintain active
concentrations, previous studies have employed multiple injections,
adenoviral vectors overexpressing BDNF or GDNF, or fusion proteins
such as CBD-BDNF and LBD-BDNF (3,7,13,34,35).
However, the risk of surgery, immunological rejection, and costs
with these methods would all be increased. Moreover, the presence
of redundant neurotrophic factors could cause adverse effects
(7).
Laminin, a critical component of the ECM in the PNS,
has been shown to affect neuronal behavior, including migration,
neurite outgrowth, proliferation, and central synaptic
differentiation (20,36). A previous study has shown that
LBD-BDNF can support marked neuroprotective function following
middle cerebral artery occlusion (13). Therefore, in an attempt to retain
neurotrophic factors at the site of injury and enhance nerve
regeneration, laminin was chosen as a target for BDNF and GDNF in
the present study. Whether LBD-BDNF and LBD-GDNF had synergistic
effects was also evaluated.
Agrin is a synapse organizer that promotes
acetylcholine receptor clustering at the neuromuscular junction.
Research has shown that NtA has high affinity with the coiled-coil
domain of laminin (18). Thus, in
order to target BDNF and GDNF to laminin, NtA was fused to the two
neurotrophic factors to create LBD-BDNF and LBD-GDNF in the present
study. As expected, compared with NAT-BDNF and NAT-GDNF, LBD-BDNF
and LBD-GDNF presented comparable laminin-binding ability and
demonstrated neuroprotective activities. In the laminin-binding
assay of LBD-BDNF and LBD-GDNF, neurotrophic factors with NtA
showed stronger binding abilities to laminin compared with those
without NtA. At the same concentration, neurotrophic factors with
NtA could be targeted to laminin, and effectively avoided being
washed away or extensively diluted by extracellular fluids. Thus,
LBD-BDNF or LBD-GDNF served as targeted proteins for maintaining
effective concentrations of neurotrophic factors.
Next, PC12 cells were used for evaluating the
bioactivities of LBD-BDNF and LBD-GDNF through neurite outgrowth
and the CCK-8 assay. The results showed that LBD-BDNF and LBD-GDNF
maintained the bioactivities of BDNF and GDNF. Additionally,
neurotrophic factors with NtA retained higher concentrations and
bioactivities compared with those without NtA. It may be concluded
that this was due to a difference in laminin-binding affinity
between these proteins. Further, these findings showed that NtA
enabled BDNF and GDNF target laminin.
Finally, LBD-BDNF and LBD-GDNF were mixed together
in varying ratios to detect which ratio of mixing was the most
conducive for the growth of PC12 cells. Optimal results were
observed when LBD-BDNF and LBD-GDNF were mixed in a ratio of 4:6.
Moreover, it was found that a mixture of LBD-BDNF and LBD-GDNF
promoted the growth of PC12 cells to a greater extent than the use
of either LBD-BDNF or LBD-GDNF alone at the corresponding
concentration.
The present in vitro experiments clearly
showed that these fusion proteins had certain advantages over
native ones. Thus, the above findings provide evidence for the use
of fusion proteins to immobilize a molecule, such as BDNF or GDNF,
on a laminin-coated surface.
There are many methods for the application of
exogenous neurotrophic factors; however, it appears that the most
effective approaches for maintaining the survival of motor neurons
and promoting their axonal regeneration is via routes that ensure
sufficient concentrations and the continuous function of
neurotrophic factors. The disadvantages of locally injecting
neurotrophic factors include poor outcome, short-lived benefits,
and side effects (30). If the
administration of such factors could be controlled effectively and
accurately at the injured area, they could be more safe and
effective. In addition, there are few studies that have reported on
the application of neurotrophic factors to regeneration of the RLN.
Further basic research and advancements in the clinical development
of RLN repair technology should allow for a better understanding of
this approach. Moreover, clinical and basic research of the
administration of neurotrophic factors via this technology is
likely to promote the treatment of RLN injury and other diseases of
the nervous system.
In summary, in the current study, the efficacy of a
laminin-targeting peripheral nerve injury repair system was
evaluated. It was found that this system maintained high
concentrations and bioactivities of neurotrophic factors when
compared with non-targeted controls. Moreover, when the ratio of
mixing of LBD-BDNF and LBD-GDNF was 4:6, the ability of these
factors to promote growth was superior to the combination of these
factors in other ratios; this finding might be important when
developing therapeutic strategies for repairing peripheral nerve
injury.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81271067).
References
|
1
|
Tessema B, Roark RM, Pitman MJ, Weissbrod
P, Sharma S and Schaefer SD: Observations of recurrent laryngeal
nerve injury and recovery using a rat model. Laryngoscope.
119:1644–1651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi JS, Oh SH, An HY, Kim YM, Lee JH and
Lim JY: Functional regeneration of recurrent laryngeal nerve injury
during thyroid surgery using an asymmetrically porous nerve guide
conduit in an animal model. Thyroid. 24:52–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araki K, Shiotani A, Watabe K, Saito K,
Moro K and Ogawa K: Adenoviral GDNF gene transfer enhances
neurofunctional recovery after recurrent laryngeal nerve injury.
Gene Ther. 13:296–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navarro X, Vivó M and Valero-Cabré A:
Neural plasticity after peripheral nerve injury and regeneration.
Prog Neurobiol. 82:163–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lundborg G: A 25-year perspective of
peripheral nerve surgery: Evolving neuroscientific concepts and
clinical significance. J Hand Surg Am. 25:391–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woodson GE: Spontaneous laryngeal
reinnervation after recurrent laryngeal or vagus nerve injury. Ann
Otol Rhinol Laryngol. 116:57–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan J, Zhang B, Zhang J, Ding W, Xiao Z,
Zhu Z, Han Q, Wu C, Sun Y, Tong W, et al: Nerve regeneration and
functional recovery by collagen-binding brain-derived neurotrophic
factor in an intracerebral hemorrhage model. Tissue Eng Part A.
21:62–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henderson CE, Phillips HS, Pollock RA,
Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA,
Simpson LC; corrected to, ; Simmons L, et al: GDNF: A potent
survival factor for motoneurons present in peripheral nerve and
muscle. Science. 266:1062–1064. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Wu W, Lin LF, Lei M, Oppenheim RW
and Houenou LJ: Rescue of adult mouse motoneurons from
injury-induced cell death by glial cell line-derived neurotrophic
factor. Proc Natl Acad Sci USA. 92:9771–9775. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan Q, Matheson C and Lopez OT: In vivo
neurotrophic effects of GDNF on neonatal and adult facial motor
neurons. Nature. 373:341–344. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakamoto T, Watabe K, Ohashi T, Kawazoe Y,
Oyanagi K, Inoue K and Eto Y: Adenoviral vector-mediated GDNF gene
transfer prevents death of adult facial motoneurons. Neuroreport.
11:1857–1860. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yanamoto H, Nagata I, Sakata M, Zhang Z,
Tohnai N, Sakai H and Kikuchi H: Infarct tolerance induced by
intra-cerebral infusion of recombinant brain-derived neurotrophic
factor. Brain Res. 859:240–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Q, Li B, Feng H, Xiao Z, Chen B, Zhao
Y, Huang J and Dai J: The promotion of cerebral ischemia recovery
in rats by laminin-binding BDNF. Biomaterials. 32:5077–5085. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Longo FM, Hayman EG, Davis GE, Ruoslahti
E, Engvall E, Manthorpe M and Varon S: Neurite-promoting factors
and extracellular matrix components accumulating in vivo within
nerve regeneration chambers. Brain Res. 309:105–117. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lander AD, Fujii DK and Reichardt LF:
Purification of a factor that promotes neurite outgrowth: Isolation
of laminin and associated molecules. J Cell Biol. 101:898–913.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martini R: Expression and functional roles
of neural cell surface molecules and extracellular matrix
components during development and regeneration of peripheral
nerves. J Neurocytol. 23:1–28. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu SY and Gordon T: The cellular and
molecular basis of peripheral nerve regeneration. Mol Neurobiol.
14:67–116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mascarenhas JB, Rüegg MA, Winzen U,
Halfter W, Engel J and Stetefeld J: Mapping of the laminin-binding
site of the N-terminal agrin domain (NtA). EMBO J. 22:529–536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Z, Weidenhaupt M, Ivanova N, Pavlov M,
Xu B, Su ZG and Janson JC: Chromatographic methods for the
isolation of, and refolding of proteins from, Escherichia coli
inclusion bodies. Protein Expr Purif. 25:174–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun W, Sun C, Zhao H, Lin H, Han Q, Wang
J, Ma H, Chen B, Xiao Z and Dai J: Improvement of sciatic nerve
regeneration using laminin-binding human NGF-beta. PLoS One.
4:e61802009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang XN, Jin YQ, Bi H, Wei W, Cheng J, Liu
ZY, Shen Z, Qi ZL and Cao Y: Peripheral nerve repair with epimysium
conduit. Biomaterials. 34:5606–5616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levi-Montalcini R: The nerve growth
factor: Thirty-five years later. Biosci Rep. 7:681–699. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun W, Lin H, Chen B, Zhao W, Zhao Y, Xiao
Z and Dai J: Collagen scaffolds loaded with collagen-binding
NGF-beta accelerate ulcer healing. J Biomed Mater Res A.
92:887–895. 2010.PubMed/NCBI
|
|
24
|
Sun W, Lin H, Chen B, Zhao W, Zhao Y and
Dai J: Promotion of peripheral nerve growth by collagen scaffolds
loaded with collagen-targeting human nerve growth factor-beta. J
Biomed Mater Res A. 83:1054–1061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H,
Chen B, Xiao Z and Dai J: The effect of collagen-binding NGF-beta
on the promotion of sciatic nerve regeneration in a rat sciatic
nerve crush injury model. Biomaterials. 30:4649–4656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitajima T, Terai H and Ito Y: A fusion
protein of hepatocyte growth factor for immobilization to collagen.
Biomaterials. 28:1989–1997. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han Q, Sun W, Lin H, Zhao W, Gao Y, Zhao
Y, Chen B, Xiao Z, Hu W, Li Y, et al: Linear ordered collagen
scaffolds loaded with collagen-binding brain-derived neurotrophic
factor improve the recovery of spinal cord injury in rats. Tissue
Eng Part A. 15:2927–2935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong
J, Wu J, Liang W, Chen L, Zhao Y, et al: The promotion of neural
regeneration in an extreme rat spinal cord injury model using a
collagen scaffold containing a collagen binding neuroprotective
protein and an EGFR neutralizing antibody. Biomaterials.
31:9212–9220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang W, Han Q, Jin W, Xiao Z, Huang J, Ni
H, Chen B, Kong J, Wu J and Dai J: The promotion of neurological
recovery in the rat spinal cord crushed injury model by
collagen-binding BDNF. Biomaterials. 31:8634–8641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan J, Tong W, Ding W, Du S, Xiao Z, Han
Q, Zhu Z, Bao X, Shi X, Wu C, et al: Neuronal regeneration and
protection by collagen-binding BDNF in the rat middle cerebral
artery occlusion model. Biomaterials. 33:1386–1395. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han S, Wang B, Jin W, Xiao Z, Chen B, Xiao
H, Ding W, Cao J, Ma F, Li X, et al: The collagen scaffold with
collagen binding BDNF enhances functional recovery by facilitating
peripheral nerve infiltrating and ingrowth in canine complete
spinal cord transection. Spinal Cord. 52:867–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han S, Wang B, Jin W, Xiao Z, Li X, Ding
W, Kapur M, Chen B, Yuan B, Zhu T, et al: The linear-ordered
collagen scaffold-BDNF complex significantly promotes functional
recovery after completely transected spinal cord injury in canine.
Biomaterials. 41:89–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao J, Sun C, Zhao H, Xiao Z, Chen B, Gao
J, Zheng T, Wu W, Wu S, Wang J and Dai J: The use of laminin
modified linear ordered collagen scaffolds loaded with
laminin-binding ciliary neurotrophic factor for sciatic nerve
regeneration in rats. Biomaterials. 32:3939–3948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moro K, Shiotani A, Watabe K, Takeda Y,
Saito K, Mori Y and Ogawa K: Adenoviral gene transfer of BDNF and
GDNF synergistically prevent motoneuron loss in the nucleus
ambiguus. Brain Res. 1076:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiotani A, Saito K, Araki K, Moro K and
Watabe K: Gene therapy for laryngeal paralysis. Ann Otol Rhinol
Laryngol. 116:115–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen ZL and Strickland S: Laminin gamma 1
is critical for Schwann cell differentiation, axon myelination, and
regeneration in the peripheral nerve. J Cell Biol. 163:889–899.
2003. View Article : Google Scholar : PubMed/NCBI
|