Introduction
Tuberculosis (TB) is one of the leading infectious
diseases worldwide. The latest surveillance data by the World
Health Organization reveals that in 2006, there were 9.2 million
new cases and 1.7 million mortalities from TB (1). HIV co-infection markedly increases the
risk of developing active TB disease (2). Available antibiotic chemotherapy
regimens are becoming less effective in the face of emerging
multidrug-resistant M.tb strains (3). The most efficient way to control any
infectious disease is through prevention by a potent vaccine.
Bacille Calmette-Guerin (BCG) is the only currently
available vaccine against TB since being first introduced in 1921.
This vaccine has effective protection among children, particularly
against military and TB meningitis, but is ineffective in
protecting against adult pulmonary disease, particularly in TB
endemic regions (4). BCG vaccine has
failed to control TB epidemic after it has been used for 80 years.
Therefore, there is a need to develop better or improved TB vaccine
as an alternative to BCG. Subunit, DNA and virus vector vaccines,
auxotroph M.tbs and recombinant BCGs are the important novel
vaccine design strategies.
An effective vaccination strategy is the one that
has ability to elicit protective immune response (5). Important vaccination strategies involve
a prime-boost vaccination strategy (encompasses the benefits of
both types of candidates), a heterologous prime-boost regimen
comprising a prime with a viable vaccine candidate superior to BCG
and a boost with a subunit vaccine candidate is likely to produce
the most promising combination (6,7).
Heterologous prime-boost immunization regimes induce higher levels
of cellular immunity than homologous boosting with the same vaccine
(8). Recently, heterologous
prime-boost strategies based on the combination of DNA and protein
subunit vaccines, BCG, or live attenuated viruses have been
developed to improve the efficacy of vaccination against TB
(9).
Recombinant BCG co-expressing the Ag85B and ESAT-6
is regarded as one of the most promising candidate vaccines. Mice
vaccinated with rBCG have been observed to be better protected
against aerosol infection with virulent M.tb in comparison
to BCG (10). In the present study,
we developed an immunization strategy to prime recombinant BCG
encoding Ag85B-ESAT-6 (abbreviated as rBCG as below) along with
boost doses of Ag85B, ESAT-6 and Ag85B-ESAT-6 fusion protein. We
found that rBCG with increased doses of Ag85B-ESAT-6 fusion protein
induced efficient and long lasting T-helper (Th) 1 immune response
in comparison to rBCG alone or boost dose with single protein
(Ag85B or ESAT-6).
Materials and methods
BCG and rBCG
Mycobacterium bovis BCG obtained from Shanghai
Biological Products Institute Co., Ltd., Shanghai, China, rBCG was
constructed in our lab (11), coding
sequences for Ag85B and ESAT-6 were amplified from the M.tb
H37Rv genomics DNA. Ag85B and ESAT-6 coding regions were cloned
into the mycobacteral-E.coli shuttle vector PMV261, in which
gene expression is under the control of the strong M.bovis
HSP60 promoter. BCG was grown in Middlebrook 7H9 Medium (Difco
Laboratories; BD Biosciences, Detroit, MI, USA) supplemented with
0.5% glycerol, 0.05% Tween-80 and 10% ADC or on solid Middlebrook
7H11 Medium (Difco laboratories) supplemented with 0.5% glycerol
and 10% ADC. When the rBCG was cultured, the antibiotic kanamycin
was added to the same medium at a concentration of 25 µg/ml.
Ag85B, ESAT-6, Ag85B-ESAT-6 fusion
protein and DDA adjuvant
The Ag85B, ESAT-6 and Ag85B-ESAT-6 fusion proteins
were cloned and expressed as previously described (11–13).
Recombinant plasmid pQE30-ESAT-6, pET28a-Ag85B, and
pET28a-Ag85B-ESAT-6 separately carrying ESAT-6, Ag85B and
Ag85B-ESAT-6 gene as N-terminal histidine tagged fusion were
transformed into the host BL21 (DE3) strain of E.coli
(Novagen, Madison, WI, USA). Then they were induced for expression
by 1 mM Isopropyl β-D-1-thiogalactoside. Cells were lysed and the
lysate was applied to affinity chromatography using the His-Bind
column (Novagen) as the protocol. Endotoxin was measured using the
commercially available Quantitative Chromogenic End-point
Tachypleus Amebocyte Lysate reactivity endotoxin kit (Chinese
Horseshoe Crab Reagent Manufactory Co., Ltd., Xiamen, China). DDA
was mixed into sterile distilled water to a concentration of 2.5
mg/ml, heated to 80°C, cooled to 25°C before use and delivered at
250 µg/dose (14).
Animal vaccination
Five-week-old female C57BL/6 mice (SLACCAS,
Shanghai, China) were used in the ABSL-2 animal facility at Second
Military Medical University (Shanghai, China). Mice received free
access to food and water throughout this study. All experiments
were performed in accordance to the local ethics committee. C57BL/6
mice (n=12 per group) were immunized subcutaneously at the dosage
5×106 CFU of BCG or rBCG in 200 µl phosphate-buffered
saline (PBS). After 4 weeks of the prime immunization, the C57BL/6
mice were immunized with 10 µg Ag85B+DDA, 10 µg ESAT-6+DDA or 10 µg
Ag85B-ESAT-6+DDA separately in the same way. Mice were sacrificed
via cervical dislocation to analyze the immune responses at 8 and
12 weeks after the protein immunization. As an additional control,
mice of the other group were injected with 250 µg of DDA adjuvant
only. The experiment was repeated twice. Six groups are described
in Table I.
 | Table I.Vaccination of the 6 groups. |
Table I.
Vaccination of the 6 groups.
| Group | Prime with | Increased with |
|---|
| PBS | PBS | PBS+DDA (250 µg) |
| BCG | BCG (5×106
CFU) | PBS+DDA (250 µg) |
| rBCG | rBCG: Ag85B-ESAT-6
(5×106 CFU) | PBS+DDA (250 µg) |
| rBCG/A | rBCG: Ag85B-ESAT-6
(5×106 CFU) | Ag85B (10 µg)+DDA
(250 µg) |
| rBCG/E | rBCG: Ag85B-ESAT-6
(5×106 CFU) | ESAT-6 (10 µg)+DDA
(250 µg) |
| rBCG/AE | rBCG: Ag85B-ESAT-6
(5×106 CFU) | Ag85B-ESAT-6 (10
µg)+DDA (250 µg) |
This study was approved by the Animal Ethics
Committee of Fudan University Animal Center.
ELISPOT assay for interferon (IFN)-γ
from spleen cell culture
Eight and twelve weeks after the boost vaccination,
mice were sacrificed, respectively, and their spleens removed
aseptically in RPMI-1640 medium containing 10% fetal calf serum, 2
mM glutamine, 50 µM β-mercaptoethanol, 100 µg/ml streptomycin and
100 U/ml penicillin. Spleens were gently ground through a 70 µm
cell strainer, and then single-cell suspensions were prepared with
Lympholyte-M density-gradient centrifugation (CedarLane Lab,
Burlington, NC, USA) according to the manufacturer's instructions.
We used the mouse IFN-γ ELISPOT kit (U-Cytech Biosciences, Utrecht,
The Netherlands) for detection of IFN-γ levels. Analyses were
conducted on the cells from 5 mice in each group. The cells were
diluted to the wells of the ELISPOT plate at 5×105 cells
per well in culture medium, as described above, containing purified
protein derivatives (PPD) 5 µg/ml, Ag85B (5 µg/ml), ESAT-6 (5
µg/ml) or phytohemagglutinin, 2 µg/ml, as positive control as
stimulus. The plate was incubated at 37°C, 5% CO2, 100%
humidity for 36 h and detected the IFN-γ secreting T cells as the
procedure. Spots were counted by use of an immunospot image
analyzer. Wells with <5 spots were not used for
calculations.
Enzyme-linked immunosorbent assay
(ELISA) analysis for IFN-γ, tumor necrosis factor (TNF)-α and
interleukin (IL)-4
Single-cell suspensions were obtained and dilution
of the cells in 2 ml culture medium contain the same concentration
of stimulus as described above in the 12-well plate at
1×106 cells per well. The plate was incubated at 37°C,
5% CO2, 100% humidity for 36 h. The suspensions were
collected of the cell culture for ELISA to detect the level of the
cytokines (IFN-γ, TNF-α and IL-4). The cell deposits were harvested
to prepare for flow cytometry. We used the mouse IFN-γ ELISA, TNF-α
ELISA and IL-4 ELISA kits (eBioscience, Inc., San Diego, CA, USA)
for detection of IFN-γ, TNF-α and IL-4. The concentration of the
cytokines (IFN-γ and TNF-α) in the suspension was calculated
according to the standards curve.
ELISA analysis for immunoglobulin
(Ig)G, IgG1, IgG2c
Sera were collected from the immunized animals to
monitor the antibody response by ELISA. Corning Costar 9018 ELISA
plates (Corning Costar, Inc., Corning, NY, USA) were coated with
Ag85B (5 µg/ml) or ESAT-6 (5 µg/ml). The plates were blocked with
PBS containing 1% bovine serum albumin (BSA) (Bovogen Biologicals
PTY., Ltd., VIC, Australia). Sera were added at serial 2-fold
dilution (beginning at a 1/100 dilution). After washing, and adding
horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1 and
IgG2c (SouthernBiotech, Birmingham, AL, USA) were diluted at
1/10,000, 1/1,000 and 1/1,000 separately in blocking buffer (PBS
containing 1% BSA). Plates displayed color by o-phenylenediamine
substrate. Antibody titers were expressed as reciprocal end point
titers.
Flow cytometry analysis
Spleen tissue was obtained and prepared as single
cell suspension as described above in cell staining buffer
(BioLegend, Inc., San Diego, CA, USA). The debris was removed by
filtration of the cell suspension through 70-µm nylon mesh
strainer. Viable cells were counted and suspended in cell staining
buffer at 1×107 cells/ml. Cell suspensions (100 µl) were
distributed into aseptic Eppendorf plastic tubes. Cells were
blocked with PBS containing 1% BSA. Isotype controls of fluorescein
isothiocyanate (FITC) and phycoerythrin (PE) conjugated anti-mouse
IgG2b were used, 0.25 µg FITC anti-mouse CD4 and 0.25 µg PE
anti-mouse CD8 (eBioscience, Inc.) were added per million cells in
a 100 µl total staining volume followed by incubation in the dark
at 4°C for 20 min. Cell pellets were washed twice and resuspend in
0.5 ml of cell staining buffer for analyzing under the flow
cytometer (FACSCalibur; BD Biosciences, Detroit, MI, USA), with
appropriate machine settings. Ten thousand events were
collected.
Data analysis
Statistical significance was determined using
one-way ANOVA with Kruskal-Wallis tests and Dunnett tests of
GraphPad Prism 5.0 for Windows (GraphPad Software, Inc., La Jolla,
CA, USA). PBS group was regarded as negative control. The remaining
4 groups were compared with the rBCG group. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cytokine response
Single splenocyte suspensions from the 6 groups of
mice after boost at 8 and 12 weeks were obtained and assayed for
IFN-γ at 36 h post-stimulation with special antigen. An ELISPOT
assay was used to determine the relative numbers of IFN-γ
expressing cells in single splenocyte suspensions of mice immunized
with different groups. The numbers of such cells were shown by
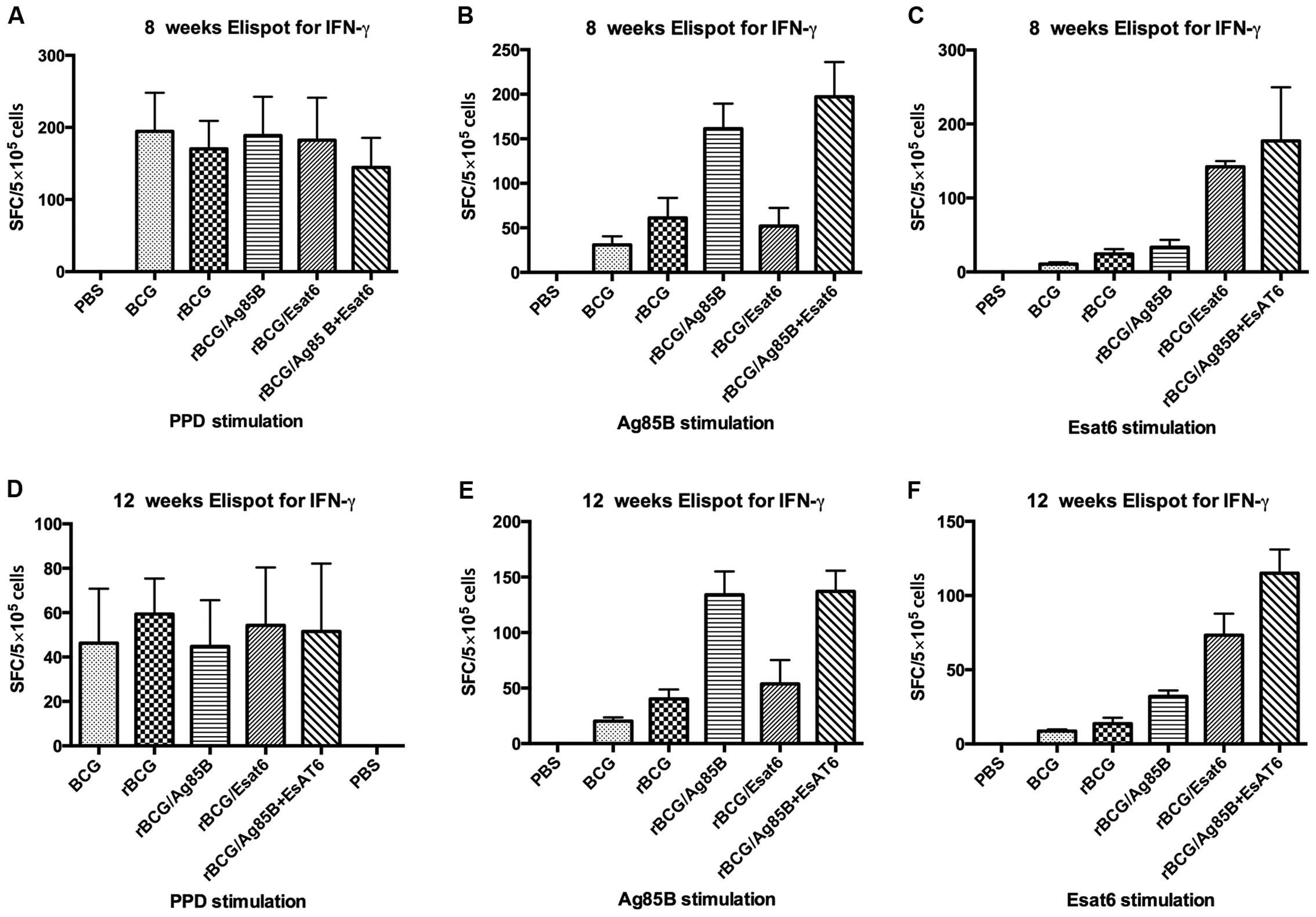
spot-forming units (Fig. 1).
Splenocytes from mice vaccinated with PBS as the negative control
can hardly produce IFN-γ whether stimulated with PPD, Ag85B or
ESAT-6. Splenocytes from mice vaccinated with all the groups except
PBS produced IFN-γ at high level when stimulated with PPD at 8
weeks. They reduced from about 180 spot-forming cells (SFC) at 8
weeks to about 50 SFC at 12 weeks, but they showed no
difference.
When stimulated with Ag85B, the IFN-γ level of
rBCG/A and rBCG/AE induced 3-4-fold higher than that of rBCG
whether at 8 weeks or at 12 weeks. However, rBCG/E did not increase
the IFN-γ level. Compared BCG with rBCG, rBCG induced higher IFN-γ
level than BCG at 8 weeks, but at 12 week, they showed no
difference. When stimulated with ESAT-6, rBCG/AE and rBCG/E
increased >3-fold of rBCG at 8 weeks or 12 weeks. ESAT-6 is one
of the RD1 genes and deleted from BCG vaccine, thus IFN-γ
level in the BCG group can hardly be detected, whereas rBCG/E
showed no difference with rBCG.
ELISA assay for IFN-γ and TNF-α in the splenocyte
culture suspensions is shown in Table
II. Stimulated with Ag85B, the IFN-γ level of the rBCG/A and
rBCG/AE was nearly 9-fold higher than rBCG at 8 weeks. The IFN-γ
level decreased at 12 weeks, but the significant difference was
kept. rBCG/E and rBCG showed no difference of IFN-γ level at 8 and
12 weeks, respectively. When stimulated with ESAT-6, the IFN-γ
level of rBCG/AE was approximately double that of rBCG/E, while
they both much higher than rBCG at 8 weeks. rBCG/A induced higher a
IFN-γ level than rBCG at 8 weeks. The IFN-γ level of all the groups
decreased at 12 weeks, although the 3 groups were significant
higher than rBCG. The limit of sensitivity of the kit was 4 pg/ml,
and the IFN-γ level of the PBS and BCG was too low to be
detected.
 | Table II.Cytokine productiona (pg/ml). |
Table II.
Cytokine productiona (pg/ml).
|
| IFN-γ | TNF-α |
|---|
|
|
|
|
|---|
| Groups | 8 weeks | 12 weeks | 8 weeks | 12 weeks |
|---|
| Ag85B stimulus |
|
|
|
|
| PBS | <4b | <4 | 78.3±10.9 | 60.4±7.1 |
| BCG | <4 | <4 |
123.2±11.1c,d | 92.7±16.9 |
| rBCG | 94.6±4.6 | 20.1±5.5 | 252.95±11.5 | 132.7±11.7 |
|
rBCG/A |
909.2±116.7d |
262.8±49.6d | 239.1±21.5 |
191.0±26.0e |
|
rBCG/E | 121.0±17.6 | 35.2±7.0 |
180.2±11.5d | 147.4±9.3 |
|
rBCG/AE |
967.6±154.3d |
320.0±74.1d |
448.4±23.2d |
247.0±37.2d |
| ESAT-6
stimulus |
|
|
|
|
|
PBS | <4 | <4 | 18.5±3.6 | 16.2±3.4 |
|
BCG | <4 | <4 | 104.7±11.8 | 95.9±11.3 |
|
rBCG | 18.8±1.6 | <4 | 95.6±10.9 | 102.4±15.7 |
|
rBCG/A |
83.6±5.5f |
27.1±10.2e |
148.3±11.2e |
173.4±23.4d |
|
rBCG/E |
351.6±34.5d |
68.3±17.6d |
580.8±12.8d |
357.9±17.6d |
|
rBCG/AE |
836.4±86.6d |
234.2±39.3d |
585.1±39.7d |
503.9±28.3d |
When stimulated with Ag85B, rBCG/AE produced
approximately 1.8-fold TNF-α level of rBCG at 8 and 12 weeks.
rBCG/A showed no difference with rBCG at 8 weeks but a
significantly higher rBCG at 12 weeks. rBCG/E and BCG was
significantly lower than rBCG at 8 weeks, but they showed no
difference at 12 weeks. When stimulated with ESAT-6, rBCG/AE and
rBCG/E produced approximately 6-fold that of rBCG at 8 weeks. TNF-α
level of rBCG/A was also higher than rBCG. At 12 weeks, the 3
groups (rBCG/AE, rBCG/A and rBCG/E) were significantly higher than
rBCG.
The limit of sensitivity of the mouse IL-4 ELISA kit
was 4 pg/ml, and the level of production of IL-4 was too low to be
detected for all the groups. Thus, we did not include this in
Table II.
Humoral response
To evaluate humoral immune response after the
vaccination, specific antibodies were determined in mice immunized
with the antigen. Eight and 12 weeks following the last
immunization, serum IgG1, IgG2c and total IgG antibody levels were
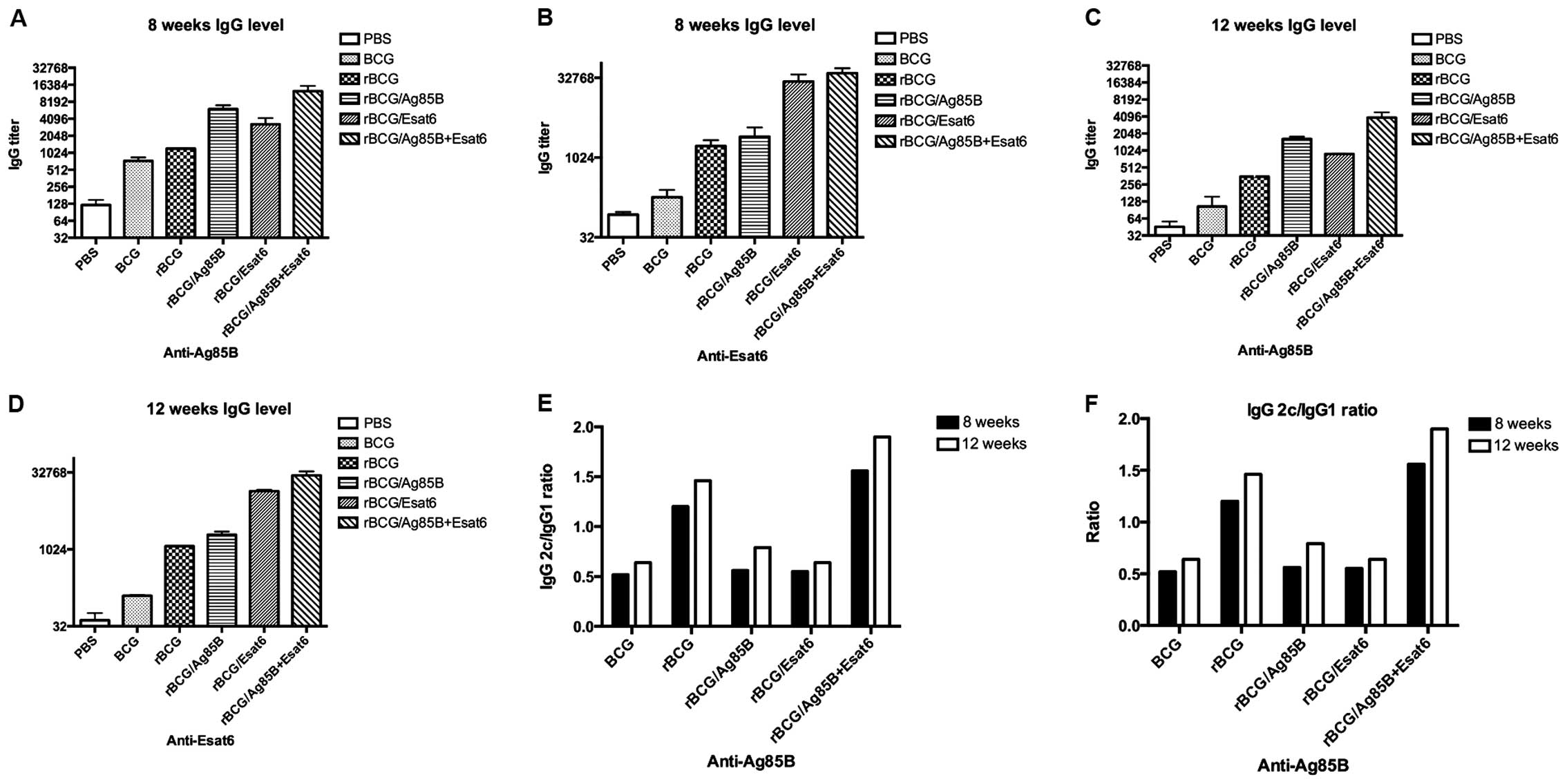
measured by ELISA. Fig. 2A-D shows
the relative concentrations of protein-specific total IgG
antibodies in the sera of mice. Total IgG titers for Ag85B of the
rBCG/A and rBCG/AE were higher than rBCG at 8 and 12 weeks. rBCG/E
and BCG showed no difference with rBCG. Total IgG titers for ESAT-6
of the rBCG/E and rBCG/AE were enhanced >100-fold that of rBCG
at 8 and 12 weeks. rBCG/A showed no difference with rBCG. The
ESAT-6 gene was one of the RD1 regions of the BCG and did
not exist in the BCG. Thus, we detected the ESAT-6 special antibody
titer at a very low level.
In C57BL/6 mice, the gene coding for IgG2a is
deleted. Therefore, in the absence of a functional IgG2a
gene, the IgG2c isotype was used as an indicator of a T-helper
(Th)-type 1 response. The ratios of IgG2c/IgG1 were calculated to
determine the induction of Th1 or Th2 responses in animals
(Fig. 2E and F). As a result in
response to Ag85B, the IgG2c/IgG1 ratios of the rBCG and rBCG/AE
were higher than that of BCG, rBCG/A or rBCG/E and above to 1.2. At
12 weeks the IgG2c/IgG1 ratios of rBCG and rBCG/AE also kept higher
than the other 3 groups.
In response to ESAT-6, the ratios of rBCG and
rBCG/AE groups were higher than that of rBCG/A or rBCG/E. There
were no obvious IgG1 and IgG2c titers detected in the mice
immunized with BCG.
CD4+ T cell and
CD8+T cell analyze
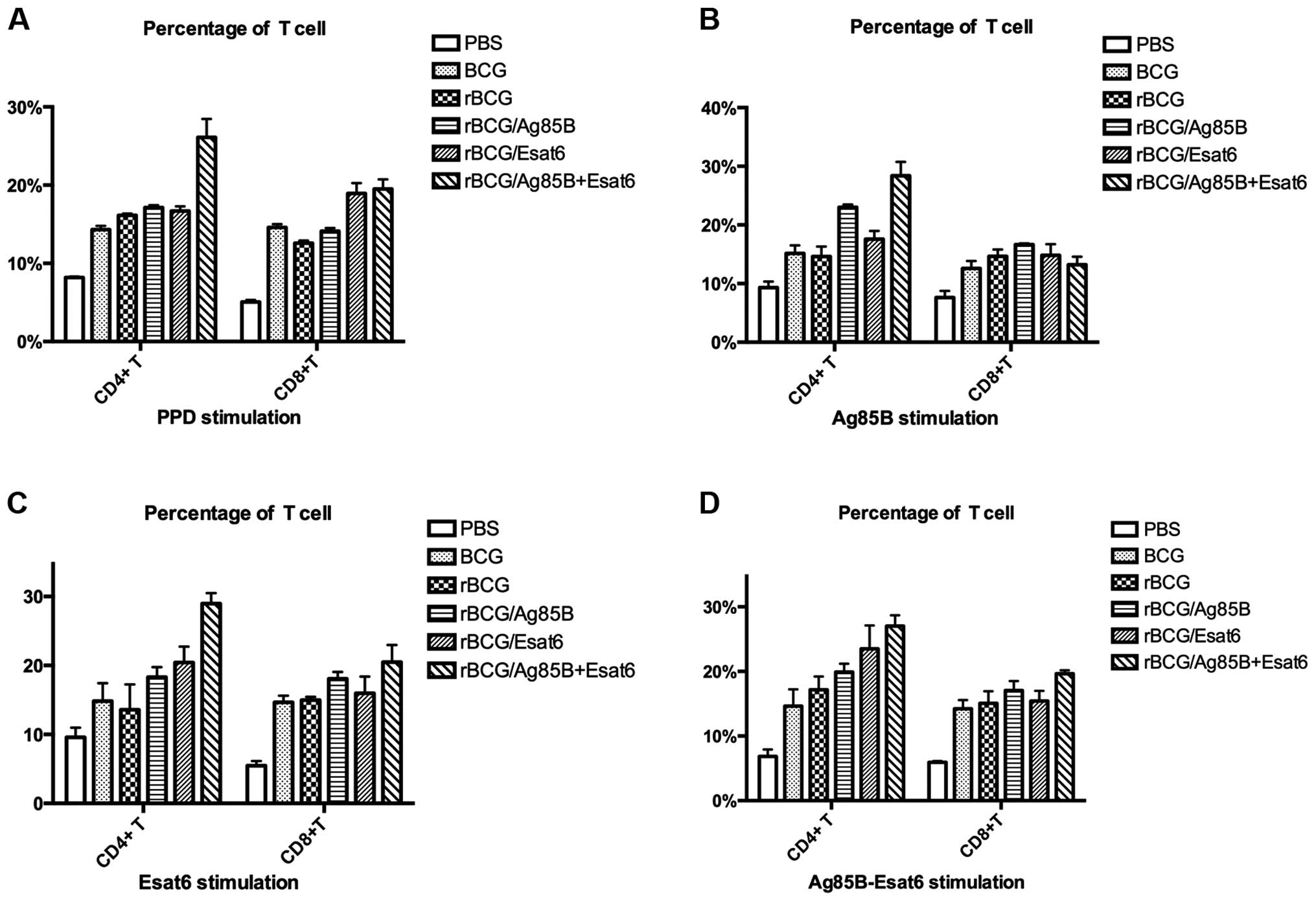
To investigate the alteration in the proportions of
lymphoid cells in the spleen after vaccination, the T cells were
stained with the cell surface markers for flow cytometry analysis.
The CD4+ T-cell and CD8+ T-cell levels
stimulated by different special antigen are shown in Fig. 3A-D.
The splenocytes were prepared from the mice at 8
weeks after the boost. When stimulated with PPD, rBCG/AE improved
CD4+ T cells compared to rBCG. The CD8+
T-cell levels of the 4 groups (BCG, rBCG/A, rBCG/E and rBCG/AE)
were higher than that of rBCG. Stimulated with Ag85B-ESAT-6, rBCG/E
and rBCG/AE enhanced CD4+ T-cell level compared to rBCG.
Only rBCG/AE enhanced CD8+ T-cell level as rBCG.
Discussion
Data from humans and several animal models have
suggested that Th1 subset and IFN-γ are involved in the development
of protective immunity against M.tb. Thus, increase in Th1
response or induction of higher levels of IFN-γ should lead to
increased anti-mycobacterial activity (15,16).
TNF-α has been shown to be critical for granuloma formation in mice
(17), and in humans, targeted
anti-TNF-α therapy for chronic inflammatory conditions could lead
to reactivation of latent TB (18).
IFN-γ and TNF-α contribute to the recruitment of monocytes and
granulocytes (19) and activate the
antimicrobial activity of macrophages (15). In the present study, we compared the
immunogenicity of several heterologous prime-boost combinations
based on rBCG and different protein subunit vaccines. rBCG showed
higher IFN-γ and TNF-α levels than BCG at early time (<12 weeks
after vaccination) as observed in our earlier study, but no
statistical significant difference was observed after 12 weeks of
vaccination. Additionally, the immunogenicity of rBCG gradually
decreased with time. On the other hand, rBCG, along with boost
doses of subunit protein vaccine kept levels of IFN-γ and TNF-α
higher for a long period of time.
Vaccination with BCG or rBCG did not induce very
high special antigen IgG titer at 12 weeks, but the boost with
subunit vaccine, the special antigen IgG titer was expressed higher
and sustained for a long time. rBCG/AE showed higher IgG titer than
rBCG/A or rBCG/E for the same special antigen. This showed Ag85B
has the ability to synergize with ESAT-6 in order to improve the
IgG titer.
The ratios of IgG2c/IgG1 were calculated to
determine the levels of induction of the Th1/Th2 responses in
animals. The increasing ratio of IgG2c/IgG1 revealed the ability of
induction of Th1 protection immune response. rBCG/AE showed the
highest ratio of IgG2c/IgG1, followed by rBCG, and rBCG/E and
rBCG/A, which were almost the same as BCG. This result showed Ag85B
may have coordinated well with ESAT-6 to promote the immune
response to Th1-type, while rBCG/A or rBCG/E could not improve the
ratio of IgG2c/IgG1 ideally. The above observation could be
justified by the fact that with rBCG with single protein, whether
Ag85B or ESAT-6 may break the balance of the antigen-induced immune
response; thus, failing to drive the immune response to the
Th1-type. The result clearly showed that rBCG/AE facilitated the
Th1 immune response as compared with BCG, rBCG, rBCG/A or
rBCG/E.
Studies over the past few years of anti-TB immunity
in mice concluded that immunity is mediated predominantly by CD4
Th1 cells with the aid of CD8 T cells. It is known that immune
responses against M.tb are mediated by CD4+ T
cells, although recent evidence indicates that CD8+ T
cells also contribute to antimycobacterial immunity (15). CD8+ T cells appear to
mediate immune surveillance of latent TB infection (20) and to be involved in macrophage
activation (21). Using rBCG as the
priming immunization in C57BL/6 mice and then boosting these mice
with Ag85B-ESAT-6 fusion protein induced higher levels of both
antigen specific CD4+ T and CD8+ T cells.
rBCG/A or rBCG/E did not improve CD4+ T and
CD8+ T cells significantly.
Ag85B and ESAT-6 are very promising vaccine
candidate molecules for several reasons: i) They are strongly
recognized by T-cell antigens in the first phase of infection
(22,23); ii) they have demonstrated protective
efficacy in animal models (10,24); and
iii) they contained numerous well-characterized epitopes recognized
in TB patients. Additionally, the present study showed that the
boost with Ag85B-ESAT-6 fusion protein dose performed best in
induction of immune response.
In summary, comprehensive qualitative assessments of
the cellular immune response may allow more accurate identification
of protective T-cell populations (25,26).
Thus, the present study concludes that heterologous prime-boost
vaccination schedules based on rBCG and Ag85B-ESAT-6 fusion protein
subunit vaccine holds strong potential for future immune therapies
against TB.
Acknowledgements
The present study was supported by the National 11.5
(grant nos. 2008ZX-103-013 and 2008ZX-103-011).
References
|
1
|
World Health Organization (WHO), . Global
tuberculosis control: Surveillance, planning, financing: WHO report
2008. WHO; Geneva: 2008
|
|
2
|
Maher D, Watt CJ, Williams BG, Raviglione
M and Dye C: Tuberculosis deaths in countries with high HIV
prevalence: what is their use as an indicator in tuberculosis
programme monitoring and epidemiological surveillance? Int J Tuberc
Lung Dis. 9:123–127. 2005.PubMed/NCBI
|
|
3
|
Cohn DL, Bustreo F and Raviglione MC:
International Union Against Tuberculosis and Lung Disease:
Drug-resistant tuberculosis: Review of the worldwide situation and
the WHO/IUATLD Global Surveillance Project. Clin Infect Dis.
24:(Suppl 1). S121–S130. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fine PE: Variation in protection by BCG:
Implications of and for heterologous immunity. Lancet.
346:1339–1345. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaufmann SHE: Envisioning future
strategies for vaccination against tuberculosis. Nat Rev Immunol.
6:699–704. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaufmann SHE: Recent findings in
immunology give tuberculosis vaccines a new boost. Trends Immunol.
26:660–667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McShane H, Pathan AA, Sander CR,
Goonetilleke NP, Fletcher HA and Hill AV: Boosting BCG with MVA85A:
The first candidate subunit vaccine for tuberculosis in clinical
trials. Tuberculosis (Edinb). 85:47–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goonetilleke NPMH, McShane H, Hannan CM,
Anderson RJ, Brookes RH and Hill AV: Enhanced immunogenicity and
protective efficacy against Mycobacterium tuberculosis of bacille
Calmette-Guérin vaccine using mucosal administration and boosting
with a recombinant modified vaccinia virus Ankara. J Immunol.
171:1602–1609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferraz JC, Stavropoulos E, Yang M, Coade
S, Espitia C, Lowrie DB, Colston MJ and Tascon RE: A heterologous
DNA priming-Mycobacterium bovis BCG boosting immunization strategy
using mycobacterial Hsp70, Hsp65, and Apa antigens improves
protection against tuberculosis in mice. Infect Immun.
72:6945–6950. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palendira U, Spratt JM, Britton WJ and
Triccas JA: Expanding the antigenic repertoire of BCG improves
protective efficacy against aerosol Mycobacterium tuberculosis
infection. Vaccine. 23:1680–1685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Y, Zhu B, Wang Q, Chen J, Qie Y, Wang J
and Wang H, Wang B and Wang H: Recombinant BCG coexpressing Ag85B,
ESAT-6 and mouse-IFN-γ confers effective protection against
Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol Med
Microbiol. 51:480–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang BL, Xu Y, Wu CQ, Xu YM and Wang HH:
Cloning, expression, and refolding of a secretory protein ESAT-6 of
Mycobacterium tuberculosis. Protein Expr Purif. 39:184–188. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Wang B, Chen J, Wang Q, Zhu B, Shen
H, Qie Y, Wang J and Wang H: Chimaeric protein improved
immunogenicity compared with fusion protein of Ag85B and ESAT-6
antigens of Mycobacterium tuberculosis. Scand J Immunol.
64:476–481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romano M, Rindi L, Korf H, Bonanni D,
Adnet PY, Jurion F, Garzelli C and Huygen K: Immunogenicity and
protective efficacy of tuberculosis subunit vaccines expressing
PPE44 (Rv2770c). Vaccine. 26:6053–6063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flynn JL and Chan J: Immunology of
tuberculosis. Annu Rev Immunol. 19:93–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung YJ, LaCourse R, Ryan L and North RJ:
Evidence inconsistent with a negative influence of T helper 2 cells
on protection afforded by a dominant T helper 1 response against
Mycobacterium tuberculosis lung infection in mice. Infect Immun.
70:6436–6443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roach DR, Bean AG, Demangel C, France MP,
Briscoe H and Britton WJ: TNF regulates chemokine induction
essential for cell recruitment, granuloma formation, and clearance
of mycobacterial infection. J Immunol. 168:4620–4627. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keane J, Gershon S, Wise RP,
Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN and Braun
MM: Tuberculosis associated with infliximab, a tumor necrosis
factor alpha-neutralizing agent. N Engl J Med. 345:1098–1104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pfeffer K: Biological functions of tumor
necrosis factor cytokines and their receptors. Cytokine Growth
Factor Rev. 14:185–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tully G, Kortsik C, Höhn H, Zehbe I,
Hitzler WE, Neukirch C, Freitag K, Kayser K and Maeurer MJ: Highly
focused T cell responses in latent human pulmonary Mycobacterium
tuberculosis infection. J Immunol. 174:2174–2184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brookes RH, Pathan AA, McShane H, Hensmann
M, Price DA and Hill AV: CD8+ T cell-mediated
suppression of intracellular Mycobacterium tuberculosis growth in
activated human macrophages. Eur J Immunol. 33:3293–3302. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brodin P, Rosenkrands I, Andersen P, Cole
ST and Brosch R: ESAT-6 proteins: Protective antigens and virulence
factors? Trends Microbiol. 12:500–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Souza S, Rosseels V, Romano M, Tanghe A,
Denis O, Jurion F, Castiglione N, Vanonckelen A, Palfliet K and
Huygen K: Mapping of murine Th1 helper T-Cell epitopes of mycolyl
transferases Ag85A, Ag85B, and Ag85C from Mycobacterium
tuberculosis. Infect Immun. 71:483–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brandt L, Elhay M, Rosenkrands I, Lindblad
EB and Andersen P: ESAT-6 subunit vaccination against Mycobacterium
tuberculosis. Infect Immun. 68:791–795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Rosa SC, Lu FX, Yu J, Perfetto SP,
Falloon J, Moser S, Evans TG, Koup R, Miller CJ and Roederer M:
Vaccination in humans generates broad T cell cytokine responses. J
Immunol. 173:5372–5380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Makedonas G and Betts MR: Polyfunctional
analysis of human t cell responses: Importance in vaccine
immunogenicity and natural infection. Springer Semin Immunopathol.
28:209–219. 2006. View Article : Google Scholar : PubMed/NCBI
|