Introduction
Vitiligo is a common skin disease, with an incidence
of 1–2% (1), in which melanocytes in
the diseased area disappear, resulting in depigmentation. The
pathomechanisms underlying vitiligo remain unclear, but may
include: Autoimmune, defect in neural regulation, over oxidation
caused by damage of reduction system, the recent emergence of the
formation of inclusion bodies caused by defect in melanocyte
absorption (2). It has been found
that components of immune reactions could mediate melanocyte lysis
in the serum of partial patients with vitiligo (3). IgG in the serum of patients with
vitiligo could penetrate cultured melanocytes in vitro,
causing apoptosis (4). These
findings indicate that humoral immunity and autoantibodies play an
important role in the occurrence and development of vitiligo.
Establishing a detection method for anti-melanocyte antibodies,
measuring antibody titers in the blood of patients with vitiligo
and observing its association with the disease are crucial for
understanding the pathogenesis of vitiligo, planning therapeutic
interventions and assessing curative effects (4,5). As
determined by the present study, immunofluorescence testing of
anti-melanocyte antibodies, melanocytes may be cultured in
vitro and pure cells harvested. An advantage of this method is
that there are sufficient uniform melanocytes for detecting the
antibodies in patient samples. The main drawbacks are that the
success rate of melanocyte culture is low, with a long cycle and
high cost, and it is easy to develop contamination of skin
fibroblasts and keratinocytes. On the basis of successfully
cultured melanocytes, an immunofluorescence assay of
anti-melanocyte antibodies may be conducted using the serum of
vitiligo patients. The present study demonstrated that vitiligo
patient serum may contain anti-melanocyte antibodies, and the
antibody positive fluorescent coloration is located in the
cytoplasm of melanocytes. The titer and the positive rate are
associated with the progression and stability of disease. Certain
drugs and physiotherapy, such as steroids and UVA, have poor
efficacy for treating vitiligo. Autologous skin grafting to
supplement melanocytes has been suggested to be an effective
treatment for vitiligo (6,7). However, it is difficult to treat cases
involving large lesions due to the limited availability of graft
skin. Sources of allogeneic melanocytes are more abundant; however,
there may be a rejection reaction. There are only individual
exploratory reports, with poor clinical results (8). Reports on transplantation of autologous
melanocytes cultured in vitro to treat vitiligo have been
published (9,10). An advantage of this approach is that
sufficient melanocytes can be harvested for a large area of the
transplant. The main drawbacks are that the success rate of culture
is low, with a long treatment cycle, and that a white border area
between the transplant and the normal skin area may develop.
Autologous melanocyte transplantation is suitable for patients in
with stable phase vitiligo, while those in development stage may be
vulnerable to relapse (11). In the
present study, the levels of each patients own immune fluorescent
antibody were initially detected, and transplantation was used to
treat the vitiligo patients in the stable stage with negative
autoantibody and a smaller number of vitiligo patients in the
development stage. The pigment of lesions was significantly
recovered, without white border areas between the transplanted area
and the normal skin. Moreover, there was no scarring or other
notable side effects. No recurrence was observed within five years
following transplantation. Thus, the present results indicate that
in negative anti-melanocyte autoantibody patients, transplantation
of cultured autologous melanocytes in vitro may be an
effective, safe and suitable treatment for those with large skin
lesions.
Materials and methods
Patient data
There were 36 patients (18 females and 18 males)
enrolled in the study, aged between 6 and 43 years. The disease
course ranged between 3 months and 20 years. The lesion in eight of
the cases was confined to one area, and more than two areas of
depigmentation were observed in 28 cases. Affected areas were
varied, including the limbs, trunk, face and shank. The skin lesion
area ranged from a minimum of 4 cm2 up to 70% of the
body surface. If the depigmentation area did not expand and change,
remaining stable for >3 years, it was identified as stable
stage, and otherwise identified as development stage. Among the 36
cases, there were 20 cases in development stage and 16 cases in the
stable stage. All patients had a history of drug or UVA treatment
which was ineffective. Written informed consent was obtained from
all patients. From each patient a 4-ml venous blood sample was
harvested, which stood for 2 h at room temperature, followed by
centrifugation at 4,000 × g for 10 min. The serum was then
placed in a blocking Eppendoff tube and preserved at −20°C. Normal
serum was also collected from 36 healthy subjects (18 males and 18
females) who were aged 6–43 years.
Reagents
M2 melanocyte growth medium (DMEM/F12, containing 5
ng/ml basic fibroblast growth factor); anti-melanocyte
tyrosinase-related protein 75 (TRP-75) monoclonal antibody (cat no.
SAB2102617), S-100 monoclonal antibody (cat no. S2657), mouse
anti-human fibroblasts and keratinocytes monoclonal antibody (cat
no. C6909); FITC-labeled goat anti-mouse IgG (cat no. F9006). and
L-DOPA were purchased from Sigma-Aldrich.
Preparation of epidermal cell
suspensions
Normal eyelid skin was collected after surgery,
scissors were used to cut the epidermis, then samples were placed
in saline and digested with 0.25% trypsin at 37°C for 15 min or at
4°C overnight. Samples were subsequently centrifuged at 4,000 × g
for 10 min, then precipitate was collected and resuspended in
culture medium.
Melanocyte culture
According to previously described methods (11,12), the
epidermal melanocytes were suspended in M2 (DMEM/F12 medium
containing 5 ng/ml recombinant human basic fibroblast growth
factor; bFGF) melanocyte selective medium, which was changed once
every three days. After melanocytes fused, they were digested with
trypsin from culture flask. After the cells were cultured for 4 or
5 passages, melanocytes were digested with trypsin from the culture
flask, centrifuged for 10 min at 4,000 × g. The precipitate
was collected, resuspended in culture medium, and stored for
subsequent experiments.
Melanocyte identification
The morphology of the cultured cells was observed
using an inverted microscope. TRP-75 fluorescent immunostaining was
performed as previously described (13). Briefly, cells were seeded on glass
slides treated with gelatin. When the fusion rate reached 70%, the
cells were washed twice with phosphate-buffered saline (PBS), fixed
with cold acetone, added anti-TRP-75 monoclonal antibody at room
temperature for 1 h, added FITC-labeled goat anti-mouse secondary
antibody at room temperature 1 h. After staining with dopamine,
cells were fixed with cold methanol, put into 0.1% L-DOPA phosphate
buffer (pH 7.4) and incubated at 37°C, then observed for the degree
of coloration compared with normal skin.
Immunofluorescent staining of
anti-melanocyte antibody
After the slides were washed with distilled water
for 10 min and autoclaved 120°C for 30 min, coated with 0.2%
gelatin for 30 min and liquid was removed using a pipette. The
slides were fixed with 0.5% formaldehyde for 30 min, washed three
times with PBS (5 min each time), dried and set aside. A monolayer
of melanocytes in a culture flask was digested with trypsin and
counted with a hemocytometer by microscope, used culture medium to
prepare 1×105/ml cell suspension. The slides were marked
with 3 or 4 circles with a marker, and a 100-200-µl cell suspension
was inoculated into each circle, put in wet box and cultured in 5%
CO2 at 37°C. When the fusion rate reached 70%, after the
removal of liquid, the specimens were washed three times with PBS
(3 min each time), fixed with cold acetone, washed three times with
PBS, dried and set aside. Then, 10% rabbit serum was used to seal
the cells on glass slides for 60 min, which were then washed three
times with PBS (3 min each time). The patient or normal human
serum, after 1:10, 1:50 and 1:100 proportional dilution, was added
at a volume of 100 µl per well, PBS was added to a well as a
negative control, incubated for 1 h at room temperature and washed
three times with PBS (3 min each time). Next, FITC-labeled rabbit
anti-human IgG antibody (1:50) was added for 1 h at room
temperature, then washed three times with PBS (3 min each time).
Samples were blotted up PBS, sealed with mounting medium and
observed under a fluorescence microscope. When serum was diluted to
1:10, melanocytes that had immune fluorescence and intensity higher
than the PBS negative control were considered to be positive.
Evaluation of safety for tumorigenesis
and mycoplasma detection
Approximately 104 melanocytes were
suspended in 0.1 ml saline and injected (intracutaneously) into
nude mice. Subsequently, 105 melanocytes were suspended
in 0.2 ml saline and injected (subcutaneously) into the nude mice.
Each mouse was injected with two parts, a total of six mice. The
injection site was consecutively observed after three months,
dissected and the injection site and pathological morphology of
major organs were observed. Mycoplasma detection was conducted
using Hoechst 33258 staining.
Cultured autologous melanocytes of
vitiligo patients
Suction was applied to induce a blister using an
epidermal separator on the normal skin area of vitiligo patients.
Working pressure was 48 kPa, at 43°C for 1 h, using scissors to cut
the skin when blisters formed. Then, samples were placed in saline,
digested with 0.25% trypsin at 37°C for 15 min or 4°C overnight.
Samples were centrifuged at 4,000 × g for 10 min, the pellet was
collected then resuspended in culture medium. The epidermal
melanocytes were suspended in M2 (DMEM/F12 medium containing 5
ng/ml human recombinant bFGF) melanocyte selective medium. Medium
was changed once every three days, when melanocytes fused they were
digested with trypsin for subculture, as described in previous
studies (11,12). After subculture for 4 or 5
generations, digested melanocytes with trypsin from flasks, and the
melanoma cells were collected by centrifugation at 4,000 × g for 10
min, then suspended in medium. The concentration of cells was
7×104 melanocytes/ml.
Cell transplantation
The lesion area was sterilized. When the skin area
was larger, a surface anesthetic was applied, when the area was
smaller, used injection anesthesia was employed. The lesions were
gently wiped with a scalpel to avoid scarring, melanocytes
suspended in a small amount of medium were transferred to the
transplant area with 7,000 cell/cm2, evenly coated over
the skin surface using a sterile glass rod. The lesion area was
covered with layer of Vaseline gauze, and covered with sterile
gauze, let stand for 1 h.
Results
Cell culture
After epidermal cells were seeded in culture bottles
for 4 h, they began to exhibit adherence. After 2–3 days,
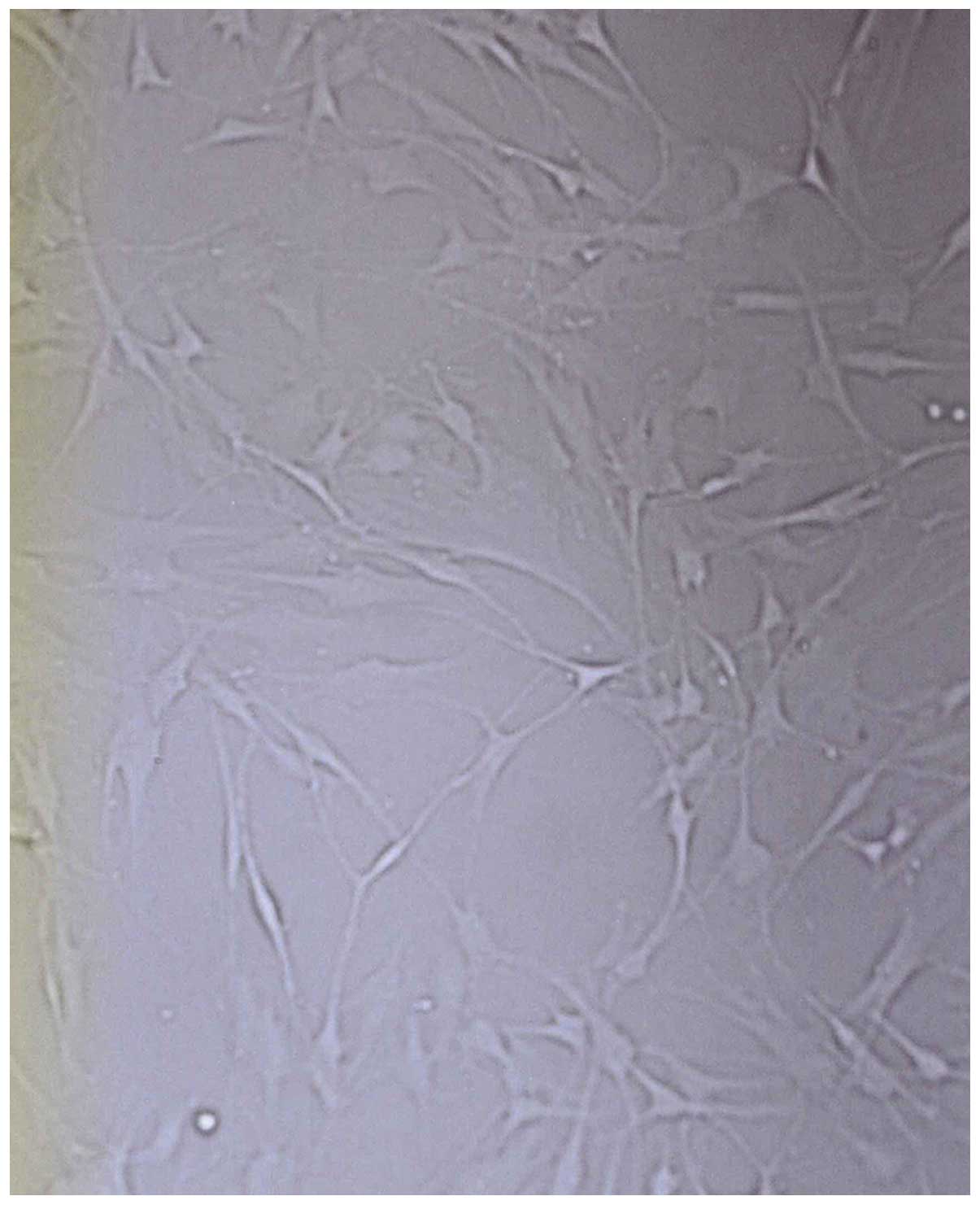
melanocytes were stretched in length, pseudopodia and branches
appeared. After seven days, the cells showed typical morphology of
melanocytes, primarily fusiform bipolar type cells with branches
(Fig. 1). After 4 or 5 generations
(~40 days) cultured generations reached the required
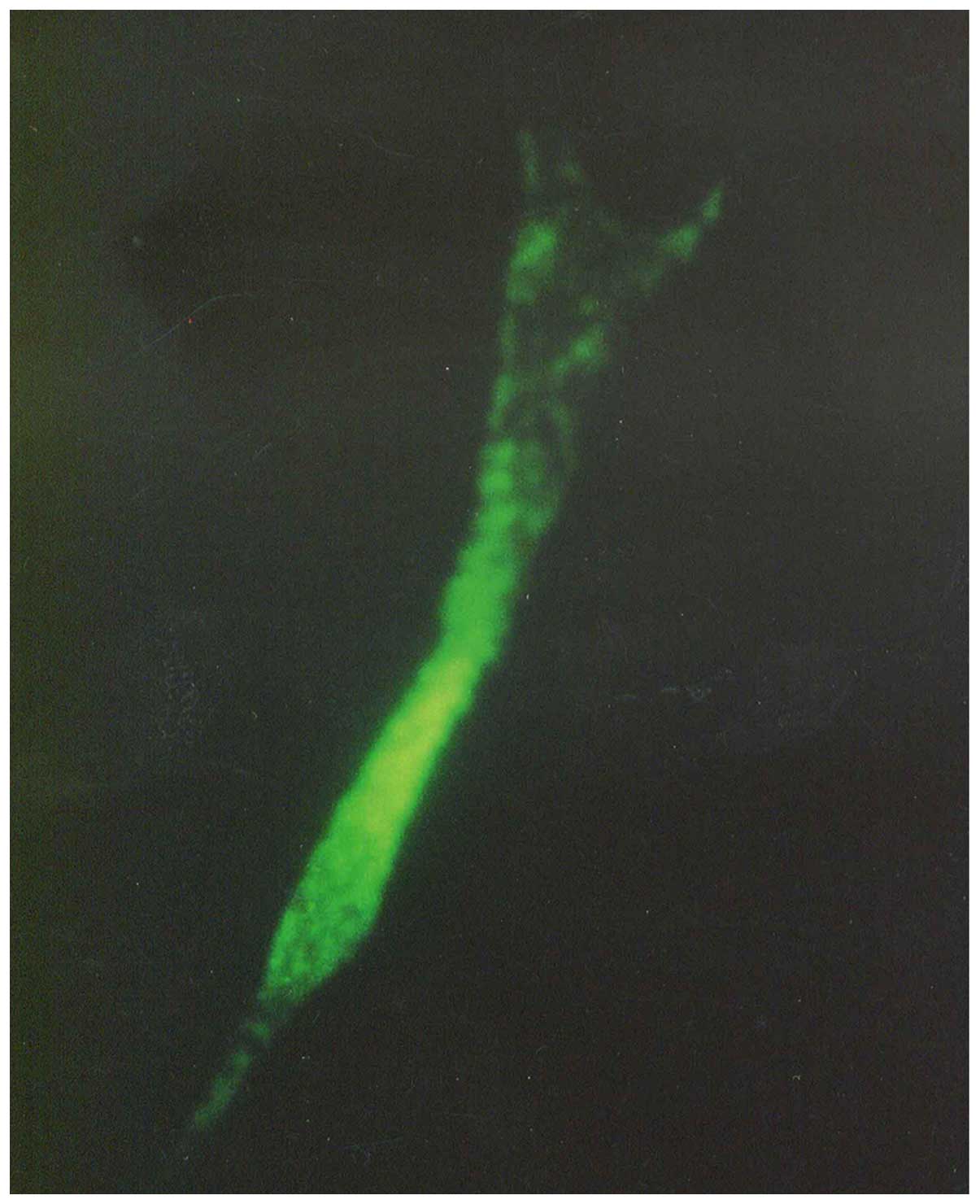
transplantation quantity of 7,000 cell/cm2. Anti-TRP-75
fluorescence staining was used to display cytoplasmic morphology at
high magnification (Fig. 2).
Anti-fibroblast and keratinocyte-specific antigen staining resulted
in no coloration, indicating that there was no contamination of
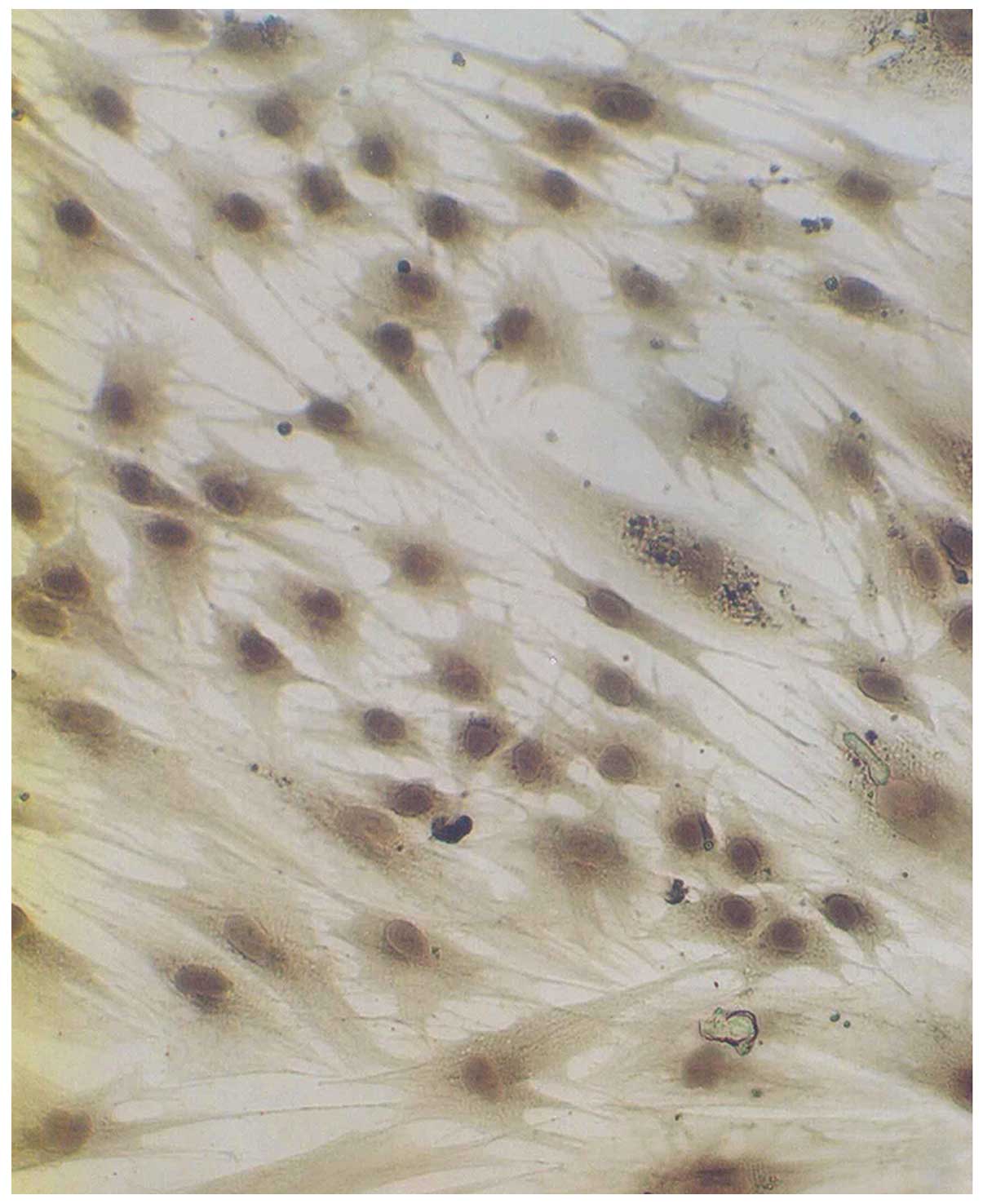
both types of the cells. Dopamine cytoplasmic staining showed as
brown or dark gray (Fig. 3). The
mycoplasma of cell cultures exhibited no contamination.
Subsequently, 104- and 105-melanocyte
suspensions were injected into nude mice. The mice were
continuously observed for three months, at which point the
injection sites showed no lumps. After dissection, injection sites
and organs showed no pathological changes.
Detection of anti-melanocyte
antibodies of vitiligo serum
A total of 36 cases of vitiligo disease and
immunofluorescent titer of anti-melanocyte antibody in serum are
shown in Table I. A total of 20
cases of patients' immunofluorescent titer in serum antibody were
>1:10 among 30 cases, the total positive rate was 56%.
Furthermore, 16 cases were antibody positive among 20 cases of
anti-melanocyte patients in the development stage, the antibody
positive rate was 80%; 11 cases of which were >1:50 and five
cases of antibody titer were 1:10. Four cases were antibody
positive among 16 cases of anti-melanocyte patients in stable
stage, and the antibody positive rate was 25%. A single case of
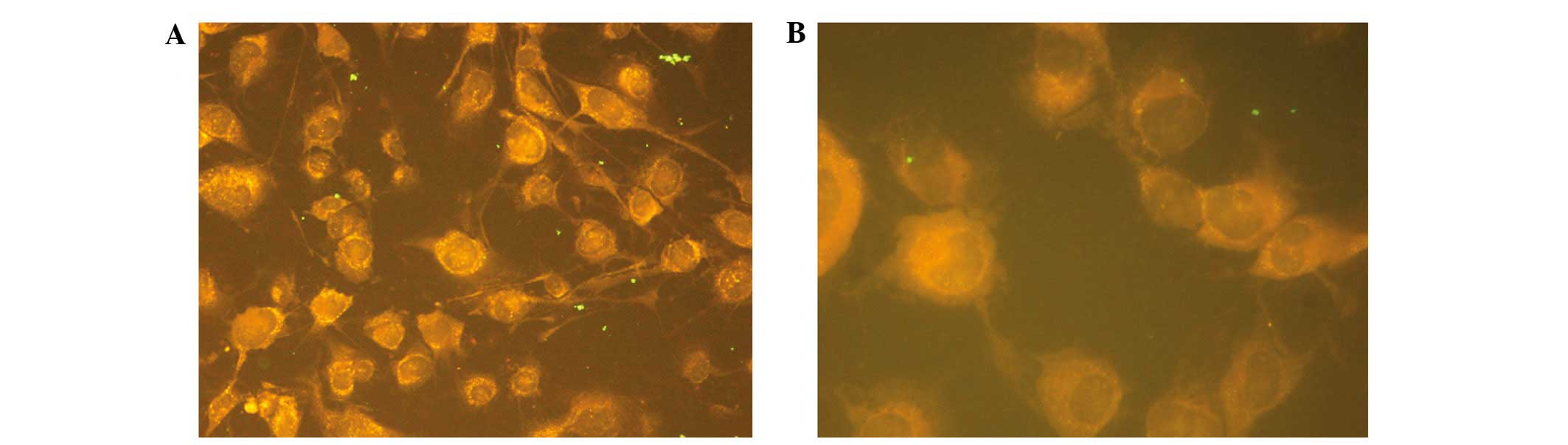
antibody titer was 1:50. Three cases of antibody titer were 1:10
fluorescence of antibody positive located in the cytoplasm of
melanocytes. (Fig. 4A and B). Normal
serum controls were collected from 36 healthy subjects (18 males
and 18 females) who were aged 6–43 years, which were diluted in
1:10. The immunofluorescence intensity of melanocytes was no higher
than the PBS-treated negative control.
 | Table I.Feature of skin area and effect of
autologous melanocytes transplantation of 16 cases of vitiligo. |
Table I.
Feature of skin area and effect of
autologous melanocytes transplantation of 16 cases of vitiligo.
| No. | Gender | Age (years) | Sites | Skin lesions (n) | Size
(cm2) | Degree of
recovery |
|---|
| 1 | Male | 28 | Leg | 1 | 16 | Good |
| 2 | Female | 19 | Leg | 2 | 15 | Good |
| 3 | Male | 25 | Face | 1 | 12 | Good |
| 4 | Male | 29 | Shoulder, ear | 3 | 9 | Good |
| 5 | Male | 20 | Face | 4 | 200 | Good |
| 6 | Female | 21 | Neck | 1 | 9 | Bad |
| 7 | Female | 18 | Chest, neck | 3 | 18 | Good |
| 8 | Female | 16 | Leg | 1 | 11 | Good |
| 9 | Male | 34 | Eyelid | 1 | 4 | Bad |
| 10 | Female | 41 | Face | 1 | 2 | Good |
| 11 | Female | 16 | Neck | 1 | 3 | Medium |
| 12 | Male | 21 | Forehead | 3 | 23 | Medium |
| 13 | Female | 24 | Forehead, eyelid | 2 | 8 | Medium |
| 14 | Female | 25 | Leg | 4 | 50 | Good |
| 15 | Male | 21 | Neck | 1 | 11 | Medium |
| 16 | Female | 40 | Neck | 1 | 18 | Medium |
Transplantation effect
The serum was diluted by 1:10 in 16 cases with
negative serum anti-melanocyte antibody, including 12 cases in
stable stage and four cases in development stage, which accepted
transplantation of autologous melanocytes (Table I). After 7–10 days, wound healing was
observed at the transplantation site. After 30 days, the
transplantation site turned red and slight pigmentation appeared.
At the edge of transplantation, there was a white dividing line
with shallow junction of normal skin. At 3–5 months after
transplantation, the pigmentation was deepened and the border
disappeared compared with the surrounding normal skin. After 6–8
months, pigmentation was stably restored, and the skin color was
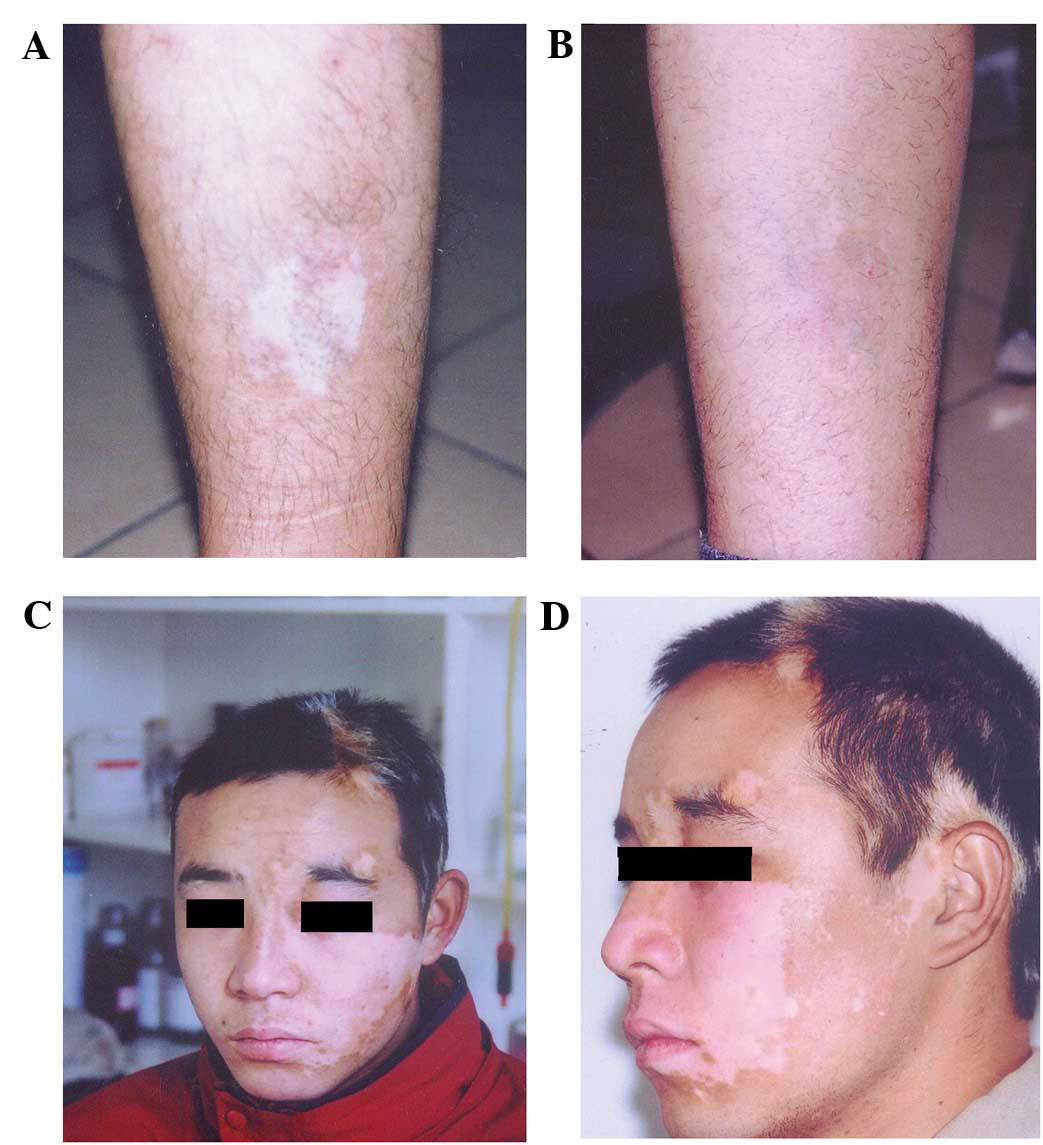
similar to that of the adjacent normal skin (Fig. 5A-D). The normal skin and
transplantation area did not have white borders and scar as well as
other side effects. At two months after transplantation, pigment
was restored to near normal levels in certain cases. A total of 16
patients had 28 skin whitened areas, including seven males and nine
females, aged 19–40 years.
Four cases at legs, seven cases at the forehead and
face (two cases at the eyelid), four cases at the neck, one case at
the shoulder and ears, with an area of 2–200 cm2. The
repigmentation area accounted for >80, 50–80 and <50% of the
transplant area in nine, five and two cases, respectively. Patients
with a repigmentation area of >50% accounted for 87.5% among all
patients with transplantation. The lower limbs, torso and face were
colored evenly and the effect was promising (repigmentation area
accounted for >80%). Neck, eyelids, lips and other sites were
sometimes unevenly colored; however, reservoirs of pigmentation
were formed. In exposed parts of the transplanted area, it was
colored faster. The transplantation area was observed at five years
following transplantation, and no recurrence was observed.
Discussion
Vitiligo is characterized by the disappearance of
pigment cells. Due to unclear factors, melanocytes of skin lesions
disappear, and the incidence showed an increasing trend in recent
years (1). The aim of the present
study was to investigate the pathogenesis of vitiligo, which is
important for developing novel clinical treatments. Lymphocyte
infiltration was observed at the edge of vitiligo lesion area, and
anti-melanocyte antibody was detected in the blood, which indicated
that abnormal autoimmunity was one of the underlying causes of
vitiligo (2). In addition, the
present results showed an increased percentage and high levels of
anti-melanocyte antibodies in the serum of patients with vitiligo
in the development phase, suggesting that humoral immunological
mechanisms played an important role in the occurrence and
development of vitiligo. The amplification of autologous
melanocytes cultured in vitro was performed to obtain
sufficient numbers of pure melanocytes, providing the necessary
conditions for the establishment of immunofluorescent antibody
detection. The immunofluorescence of melanocyte antibody was
established, was simple to perform and easy to observe, laying the
foundation for the development of clinical testing. Fluorescence
detection was used for antibody located in the cytoplasm of
melanocytes, indicating that melanocyte autoantigens were located
in the cytoplasm. Melanocyte-specific protein tyrosinase,
TRP-1,TRP-2 and melanin-concentrating hormone receptor 1, which may
be autoantigen components (14,15).
Drugs and physiotherapy do not show good efficacy
for vitiligo patients (7). The
source of allogeneic melanocytes is more convenient; however, there
may be a rejection reaction. There are only individual exploratory
reports, with poor clinical results (8). Transplantation of autologous
melanocytes is an effective therapeutic method. There are several
approaches for transplantation of autologous melanocytes: Direct
transplantation of autologous skin (6), autologous epidermal cell suspension
(16) and autologous melanoma cells
are cultured in vitro. Melanocytes only account for 2–3% of
skin cells. As melanocytes are too few in autologous epidermal cell
suspension, they are not suitable for large-scale migration
(12). By amplification of
autologous melanocytes cultured in vitro, sufficient numbers
of pure melanocytes may be harvested for transplantation, as
determined by the present study. Previous studies observed no
rejection reaction after transplantation, with stable effects. When
melanocytes were cultured, fibroblasts and keratinocytes account
for a high proportion and had fast growth. Thus, it was necessary
to inhibit fibroblasts and keratinocyte cells and make selective
growth of melanocytes (17,18). In order to continuously subculture
melanocytes in vitro, tissue plasminogen activator (TPA),
CT, IMBX and bovine pituitary extract have been added in early
medium to promote cell division or growth agents (19). TPA is a potent mitotic accelerator,
and the carcinogenic risk is possible, CT is toxic and bovine
pituitary extract has the risk of spreading diseases like cerebral
cavernous malformation. (11) For
these reasons, the above ingredients were used to culture
melanocytes which were not suitable for clinical treatment in the
present study. The medium was used to inhibit the growth of
fibroblasts and keratinocytes. Melanocytes had no contamination by
these two cells for 4–5 passages. Furthermore, 5 ng/ml bFGF
functioned as a melanocyte growth promoter, which is the normal
growth factor in the body and is able to eliminate or minimize the
risk factors (11). The medium was
used to culture melanocytes in vitro for 20 passages, and
the cells maintained good growth. A total of 28 lesions among 16
patients were treated by the transplantation of melanocytes. The
legs, trunk and face were evenly colored, and the effect was
better. The neck, eyelids, lips and other sites were unevenly
colored. This result may be related to the site which was in active
stage against uniform growth of transplanted cells. In exposed
parts of the transplanted area colored faster, it was indicated
that sunlight can promote pigment-production of transplanted cells.
The stable stage and development stage of positive and negative
patients were detected by immunofluorescence testing of melanocyte
antibody, which was important for preventing and reducing
recurrence after transplantation of melanocytes.
Acknowledgements
The current study would like to thank Ms. Wang Hong
for her assistance in the manuscript.
References
|
1
|
Lu T, Gao TT, Wang AH, Li Q, Li CY, Zhao
XD and Sun DJ: The prevalence of vitiligo in Shaanxi Province of
China. Zhong Hua Pi Fu Ke Za Zhi. 37:406–407. 2004.(In
Chinese).
|
|
2
|
Namazi MR: Neurogenic dysregulation,
oxdative stress, autoimmunity and melanocytorrhagy in vitiligo: Can
they be interconnected? Pigment Cell Res. 20:360–363. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui J, Arita Y and Bystryn JC: Cytolytic
antibodies to melanocytes in vitiligo. J Invest Dermatol.
100:812–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruiz-Argüelles A, Brito GJ,
Reyes-Izquierdo P, Pérez-Romano B and Sánchez-Sosa S: Apoptosis of
melanocytes in vitiligo results from antibody penetration. J
Autoimmun. 29:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandoval-Cruz M, García-Carrasco M,
Sánchez-Porras R, Mendoza-Pinto C, Jiménez-Hernández M,
Munguía-Realpozo P and Ruiz-Argüelles A: Immunopathogenesis of
vitiligo. Autoimmun Rev. 10:762–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horikawa T, Mishima Y, Nishino K and
Ichihashi M: Horizontal and vertical pigment spread into
surrounding piebald epidermis and hair follicles after suction
blister epidermal grafting. Pigment Cell Res. 12:175–180. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boersma BR, Westerhof W and Bos JD:
Repigmentation in vitiligo vulgaris by autologous minigrafting:
Results in nineteen patients. J Am Acad Dermatol. 33:990–995. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu T, Gao TW, Liu YF, Li CY and Sun LC:
The explore of allogenic transplantation of melanocytes to treat
vitiligo. Zhong Guo Pi Pu Xing Bing Xue Za Zhi. 15:240–242.
2001.(In Chinese).
|
|
9
|
Mulekar SV: Melanocyte-keratinocyte cell
transplantation for stable vitiligo. Int J Dermatol. 42:132–136.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YF, Chang JS, Yang PY, Hung CM, Huang
MH and Hu DN: Transplant of cultured autologous pure melanocytes
after laser-abrasion for the treatment of segmental vitiligo. J
Dermatol. 27:434–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olsson MJ and Juhlin L: Long-term
follow-up of leucoderma patients treated with transplants of
autologous cultured melanocytes, ultrathin epidermal sheets and
basal cell layer suspension. Br J Dermatol. 147:893–904. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olsson MJ and Juhlin L: Leucoderma treated
by transplantation of a basal cell layer enriched suspension. Br J
Dermatol. 138:644–648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beck AJ, Phillips J, Smith-Thomas L, Short
RD and MacNeil S: Development of a plasma-polymerized surface
suitable for the transplantation of keratinocyte-melanocyte
cocultures for patients with vitiligo. Tissue Eng. 9:1123–1131.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le-Poole IC and Luiten RM: Autoimmune
etiology of generalized vitiligo. Curr Dir Autoimmun. 10:227–243.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kemp EH, Waterman EA, Hawes BE, O'Neill K,
Gottumukkala RV, Gawkrodger DJ, Weetman AP and Watson PF: The
melanin-concentrating hormone receptor 1, a novel target of
autoantibody responses in vitiligo. J Clin Invest. 109:923–930.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kao CH, Ko WC, Ko SS and Tsai RY: A
comparative study on autologous graft for segmental type vitiligo.
Dermatol Sinica. 13:65–74. 1995.
|
|
17
|
Halaban R and Alfano FD: Selective
elimination of fibroblasts from cultures of normal human
melanocytes. In Vitro. 20:447–450. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips J, Gawkrodger DJ, Caddy CM,
Hedley S, Dawson RA, Smith-Thomas L, Freedlander E and Mac Neil S:
Keratinocytes suppress TRP-1 expression and reduce cell number of
co-cultured melanocytes: Implications for grafting of patients with
vitiligo. Pigment Cell Res. 14:116–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisinger M and Mark O: Selective
proliferation of normal human melanocytes in the presence of
phorbol ester and cholera toxin. Proc Natl Acad Sci USA.
79:2018–2022. 1982. View Article : Google Scholar : PubMed/NCBI
|