Introduction
Multiple sclerosis (MS) is the most common
inflammatory demyelinating disorder of the central nervous system
(CNS) with a predilection for the optic nerves, corpus callosum,
brainstem, spinal cord, cerebellum, and periventricular white
matter (1,2). It is the second most common
neurological disorder within the northern hemisphere in young
adults, and it is characterized by either a relapsing-remitting or
progressive course (3). MS lesions
can be identified by conventional magnetic resonance imaging (MRI);
however, it remains challenging to detect lesions in early stage or
occult MS lesions. Because of this, clinicians are unable to
connect all clinical symptoms with the findings on MRI (4). Advanced MRI techniques, in particular,
diffusion tensor imaging (DTI), have been demonstrated to have the
potential to identify changes in MS lesions at earlier stages,
including in normal appearing white matter. DTI can also be used
for describing biological tissue microstructures, which exploit the
quantification of water diffusion in tissues containing MS lesions
(5). Moreover, DTI is able to
measure the degree of diseased white matter more accurately than
T2-weighted imaging, and may also detect abnormalities earlier than
T2-weighted fast spin-echo imaging (5). DTI parameters, including fractional
anisotropy (FA), mean diffusivity (MD), λ┴ and λ//, may represent
important indicators of neuronal structure and its loss in patients
suffering from MS.
To date, few studies have used DTI to compare
different MS stages or used this technique to compare diseased
tissue with normal appearing white matter (NAWM); the majority of
studies have used a 1.5 T MRI for research. The present study used
3.0 T MR DTI technology to further study the pathophysiology of the
different stages of MS lesions, NAWM and normal appearing deep gray
matter (NADGM), and compared these values to normal controls. The
findings of this study may help advance the early diagnosis,
treatment and prognosis of MS in the future.
Materials and methods
Subjects
The present study was approved by the Ethics
Committee of The First Teaching Hospital of Jilin University
(Changchun, China), and written informed consent was obtained from
all patients.
Ten patients (female, 8; male, 2) and ten sex- and
age-matched, right-handed, healthy controls were enrolled in the
present study between September 2011 to June 2012. Patients were
aged 27–55 years (mean, 37.3 years). All patients were diagnosed
with definite MS according to McDonald criteria (6), and all patients had relapsing-remitting
MS. Eight patients exhibited lesions in both the brain and spinal
cord, whereas the remaining two only had brain lesions.
MRI scans
MRI studies were performed on a Trio Tim 3 T MRI
scanner (Siemens AG, Munich, Germany), using a twelve-channel coil
as a phased-array receiver and a body coil for transmission. Prior
to the DTI scan, all patients were told to minimize eye
movements.
T1-weighted MRIs were obtained with the following
parameters: Repetition time (TR), 440 msec; Echo time (TE), 2.46
msec; TI, 900 msec; slice thickness, 5 mm; matrix size, 256×320;
field of view (FOV), 220×220 mm2; and angle, 130°.
T2-weighted MRIs were acquired with the following parameters: TR,
5,000 msec; TE, 93 msec; slice thickness, 5 mm; matrix size,
320×320; interlayer spacing, 1.5 mm; and FOV, 220×220
mm2. DTI scans were performed using the following
parameters: TR, 5,000 msec; TE, 97 msec; b, 0 and 1,000
sec/mm3; slice thickness, 3 mm; matrix size, 128×128;
scan time, 5 min 42 sec; and diffusion gradient directions number,
64.
DTI measurements
DTIs can be characterized by a tensor ellipsoid,
from which MD and FA values can be calculated. MD is a measure of
the magnitude of molecular diffusion, whereas FA represents the
degree of directionality of diffusion, which indicates the
preference for a single direction of diffusion. When FA=0, this
indicates isotropic diffusion, which is equal in all directions,
and when FA=1, this indicates that diffusion occurs only along one
axis. Notably, when axons or myelin are disrupted, FA values
decrease, indicating that diffusion is not restricted only one
direction. Both MD and FA values can reveal subtle, but definite,
pathological changes that cannot be visualized with conventional
T2-weighted or T1-weighted MRI, particularly in NAWM (7).
Another important measure analyzed in the present
study was the average diffusion coefficient (ADC), which estimates
total diffusion for each voxel analyzed. The magnitude of axial
(λ1) and radial diffusivity (λ3, λ2) was also determined. ADC
increases if biological tissues are affected, indicating an
increase in radial diffusion due to fewer diffusion hindrances. In
white matter, water diffuses preferentially along axons and nerve
fiber bundles (axial diffusivity, λ1). The diffusion process is
hindered in the perpendicular direction from the fibers by axonal
membranes and is modulated by myelin. This radial diffusivity is
reflected by λ2 & λ3. Usually, λ┴=(λ2+λ3)/2, which reflects
water diffusion process in the perpendicular direction, while
λ//=λ1, and reflects water diffusion in the parallel direction
(5). The directionally-encoded color
(DEC) map directly visualizes the fiber and lesions.
DTI analysis
DTI acquisitions were performed using NEURO
three-dimensional software (Siemens AG). Acquisition parameters
were optimized to provide the best signal-to-noise ratio for the
estimation of diffusion tensors. All data were transferred to an
independent workstation and were analyzed using FUNCTOOL software
(GE Healthcare Life Sciences, Chalfont, UK) provided by the
manufacturer. Qualitative assessment of all images was performed to
evaluate the MS findings. DEC, FA, diffusion-weighted imaging (DWI)
and ADC were measured in multiple MS plaques, regardless of their
size, and in the symmetrical NAWM. For the analysis, a round-shape
of 2.034 mm diameter was placed in the region of interest (ROI).
Four specific regions were measured: i) the center of the MS
lesions located in the silent lesion location; ii) the subacute
phase of the lesion; iii) NAWM (the corpus callosum was selected);
and iv) the deep brain gray matter (DBGM) (the thalamus and caudate
nucleus were selected). In the normal controls, the corpus
callosum, thalamus and caudate nucleus were analyzed. The FA, MD,
λ// and λ┴ values were measured and recorded.
Following the acquisition of DTI data, the data was
processed to analyze the parameters of the diffusion using
diffusion tensor tractography (DTT). For this analysis, a diffusion
tensor matrix was generated for each voxel acquired using DWI. The
RGB (red-green-blue) color-coded scheme attributes a color for each
orientation of the fibers. Fibers crossing from left to right are
visualized in red, fibers crossing anteriorly to posteriorly are
visualized in green, and fibers crossing inferiorly to superiorly
are visualized in blue. All fibers of the MS brain were studied
based on the indicated ROI and computer program analysis.
Statistical analysis
Statistical analysis was performed by employing SPSS
17.0 statistical analysis software (SPSS, Inc., Chicago, IL, USA).
Results are expressed as the mean ± standard deviation. Individual
comparisons of two sample groups were performed by independent
t-test. Comparison of multiple sample groups were performed by
single-factor analysis of variance. Prior to the test, Levene's
test was used to test the homoscedasticity of the sample. Where
necessary, Welch, least significant difference and Tamhane's tests
were also applied. P<0.05 was considered to indicate a
statistically significant difference.
Results
Subjects
Ten patients with MS (female, 8; male; 2) were
enrolled in the present study between September 2011 to June 2012.
McDonald criteria was used for diagnosing the patients with MS, and
all patients had relapsing-remitting MS. Eight of these patients
had lesions in both their brains and spinal cords, whereas the
remaining two only exhibited lesions in their brains. Patient ages
ranged from 27 to 55 years, with a mean age of 37.3 years. Ten sex-
and age-matched healthy volunteers were enrolled as normal
controls.
Comparison of MS lesions, NAWM, and
normal white matter
Significant differences in FA, MD, λ// and λ┴ values
were detected among silent lesions, subacute lesions, NAWM and
white matter of normal controls (P<0.05). In particular, the FA
and λ// values of silent and subacute lesions were lower than those
of NAWM and normal controls (P>0.05); in contrast, the MD and λ┴
values of silent and subacute lesions were higher than those of
NAWM and control group (P>0.05). FA values of NAWM were lower
than that of the normal control (P>0.05); however, MD and λ┴
values were higher than those of the control group (P>0.05).
Further findings identified that there were no significant
differences in FA, MD and λ┴ values between silent and subacute
lesions (P>0.05). Furthermore, there was no significant
difference in λ// values among silent lesions, NAWM and the normal
controls (P>0.05), and there were no significant differences in
MD values between subacute lesions, NAWM and normal controls
(P>0.05; Table I). An example
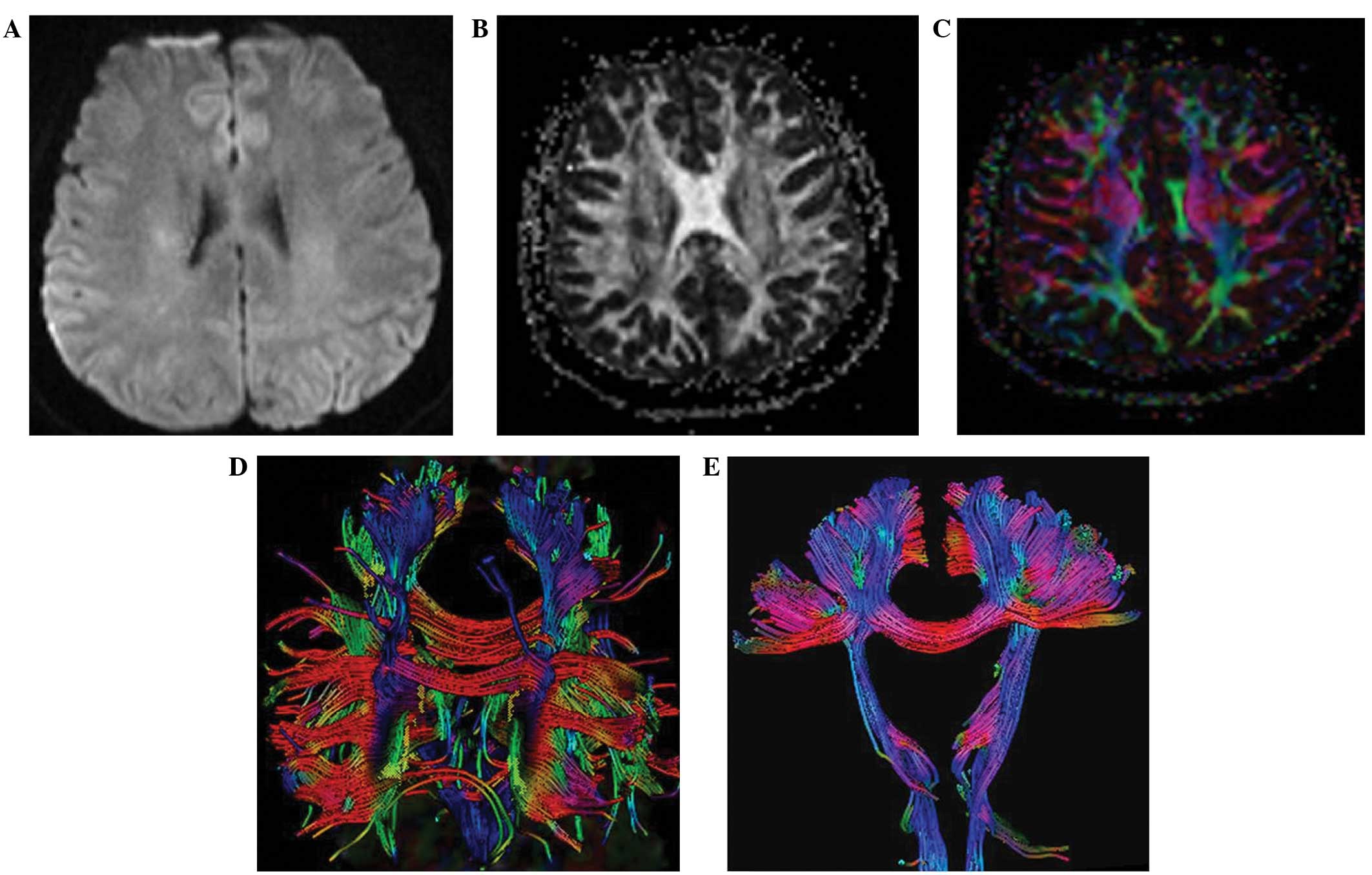
from a 27-year-old patient who was admitted with slurred speech
that lasted for one week is shown in Fig. 1. DWI showed that the right corona
radiated an abnormal signal (Fig.
1A). Using FA, the MS lesions appeared as a region of
relatively reduced hyperdensity, as compared with the hyperdensity
signal of surrounding NAWM (Fig.
1B). Notably, the lesion was not noticeable when the DWI method
was used, whereas the lesion can be visualized clearly using FA
(Fig. 1A and B). DEC revealed a
reduced color signal in the lesion fiber (Fig. 1C), and DTT fiber tractography
indicated that the fiber was broken in MS plaques, which is known
as the ‘plaque avoid’ phenomenon (8)
(Fig. 1D), indicating that there
were fewer fibers downstream of the lesion compared with the
contralateral side (Fig. 1E).
 | Table I.FA, MD, λ// and λ┴ values in all
regions of interest (unitx10−3 mm2/s). |
Table I.
FA, MD, λ// and λ┴ values in all
regions of interest (unitx10−3 mm2/s).
|
| FA | MD | λ// | λ┴ |
|---|
| 1 | 0.245±0.015 | 1.214±0.341 | 1.517±0.334 | 1.058±0.377 |
| 2 | 0.227±0.093 | 0.922±0.279 | 1.131±0.272 | 0.817±0.284 |
| 3 | 0.779±0.098 | 0.789±0.085 | 1.718±0.168 | 0.324±0.121 |
| 4 | 0.863±0.059 | 0.705±0.048 | 1.699±0.153 | 0.208±0.069 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
| P1-2 | 0.999 |
0.123 | 0.029 | 0.322 |
| P1-3 | <0.001 | <0.001 | 0.053 | <0.001 |
| P1-4 | <0.001 | <0.001 | 0.094 | <0.001 |
| P2-3 | <0.001 | 0.726 | 0.002 | 0.005 |
| P2-4 | <0.001 | 0.259 | 0.002 | 0.001 |
| P3-4 | <0.001 | <0.001 | 0.988 | <0.001 |
DBGM of patients with MS and deep gray
matter of normal controls
FA, MD, λ// and λ┴ values in the thalamus were
significantly increased in patients with MS as compared with those
of normal patients (P<0.05). In the caudate nucleus and deep
brain gray matter, the FA values were lower in patients with MS as
compared with those of normal controls (P>0.05); whereas MD, λ//
and λ┴ values were higher. Furthermore, the FA and λ┴ values were
significantly different in the caudate nucleus between patients
with MS and normal controls (P<0.05). There was a significant
increase in λ// and λ┴ values in the MS deep gray matter, as
compared with normal gray matter (P<0.05; Table II).
 | Table II.FA, MD, λ// and λ┴ values in different
regions of interest in MS and normal brains (unitx10−3
mm2/s). |
Table II.
FA, MD, λ// and λ┴ values in different
regions of interest in MS and normal brains (unitx10−3
mm2/s).
|
| FA | MD | λ// | λ┴ |
|---|
| 1 | 0.346±0.091 | 0.743±0.076 | 1.068±0.241 | 0.601±0.094 |
| 2 | 0.164±0.043 | 0.729±0.145 | 0.873±0.080 | 0.693±0.081 |
| 3 | 0.329±0.037 | 0.696±0.069 | 0.932±0.079 | 0.576±0.068 |
| 4 | 0.194±0.045 | 0.702±0.067 | 0.840±0.081 | 0.632±0.069 |
| 5 | 0.255±0.116 | 0.737±0.115 | 0.971±0.204 | 0.647±0.098 |
| 6 | 0.262±0.080 | 0.699±0.067 | 0.886±0.092 | 0.604±0.074 |
| t1-5 | 0.751 | 2.066 | 2.397 | 0.959 |
| P1-3 | 0.459 | 0.046 | 0.022 | 0.343 |
| t2-4 | −2.117 | 0.769 | 1.298 | 2.596 |
| P2-4 | 0.041 | 0.446 | 0.202 | 0.013 |
| t5-6 | −0.292 | 1.786 | 2.400 | 2.226 |
| P5-6 | 0.771 | 0.078 | 0.019 | 0.029 |
Discussion
MS, which is a demyelinating disease of the central
nervous system, is the most common cause of non-traumatic
neurological disability in young adults. Conventional MRI has
limitations in the identification of MS lesions; however, novel
neuroradiologic techniques and measurements are emerging that may
offer a improved estimation of disease status and the amplitude of
injuries. One of these new methods, magnetic resonance DTI, has
been demonstrated to improve understanding of the cerebral white
matter configuration and the different pathologies that may
influence it (5). In the present
study, measurements from DTI, including MD, FA, λ//, and λ┴ values,
were used to study MS lesions, NAWM and the white matter of normal
controls. Significant differences were detected in the FA, MD, λ//
values among silent lesions, subacute lesions, NAWM and normal
white matter. Specifically, the FA and λ// values in silent lesion
and subacute lesions were lower, whereas MD and λ┴ values in silent
lesions and subacute lesions were higher than those of NAWM and
normal controls. Theoretically, FA represents the degree of
directionality of diffusion in the studied tissue, and MD indicates
the degree of diffusion of water molecules. The λ// and λ┴ values
indicate the diffusion parallel to the nerve fibers and vertical
direction to the nerve fibers, respectively. The reduction of FA
values correlates with axonal injury and the destruction of myelin
integrity. Increasing MD values are associated with infection and
tissue edema (9). MS pathological
changes reduce the resistance of water molecules, thereby
increasing the degree of dispersion, which correlates to an
increase in the MD, λ// and λ┴ values and a decrease in the FA
values within MS plaques (5). These
findings are consistent with pathological study results from MS
autopsy and biopsy cases as published by Barkhof et al
(1), Lucchineeti et al
(10), Storch et al (11).
Previous studies have suggested that λ// values are
associated with axonal degeneration (12,13),
whereas λ┴ values are related to demyelination, edema, and
proliferation of neuroglial cells (12,13).
Using a mouse model of MS, it was demonstrated that an increased λ┴
value reflected demyelination of the corpus callosum (14). Furthermore, in an animal model of
retinal ischemia, λ// value reduction correlated with axon damage
(14). These data suggests that the
decrease in the FA value of NAWM and the increase in MD, λ// and λ┴
values as compared to normal controls in the present study
indicates the presence of occult damage to the NAWM area. The
present findings are also consistent with in vivo data from
Trip et al (15), who found
that mean MD was elevated and mean FA was reduced in patients with
optic neuritis, as compared with normal controls. This suggests
that the pathological change in optic neuritis is similar to MS, as
indicated by the present findings. Notably, these findings suggest
that DTI measurements provide an indication of the structural
integrity of axons.
The present study found that λ// values in silent
lesions are similar to that of NAWM and normal controls, whereas
the MD values of subacute lesions are close to that of NAWM and
normal controls. This phenomenon may be associated with
remyelination. MS lesions are composed of remyelination, astrocytes
and oligodendrocytes regeneration (16–20),
which act as a barrier for water molecule movement, resulting in a
decrease in the degree of dispersion and the reduction of the MD
and λ// values. Pulizzi et al (21) found that T1WI hypodensity of MS
lesions can be changed into isodensity during the evolvement of
lesions, which may also be related to remyelination. It has been
proposed that damaged tissue and surrounding astrocytes are able to
release molecules that mediate a rapid microglial response towards
injury (16–18,22). The
increased FA values observed in the present study are relevant to
the response of astrocytes to local injury of MS lesions, and
supports the hypothesis that remyelination occurs in MS
lesions.
The corpus callosum is the largest white matter
tract in the brain, connecting the two brain hemispheres. It has an
important role in movement, and the sensory and cognitive function
between two hemispheres. It is also important in the behavioral and
cognitive functions of patients with MS. The corpus callosum was
selected as the area of normal white matter as it has a strong
anisotropy with a high FA value. The present findings indicated
that when white matter is involved in MS lesions, there is a
reduction of hyperdensity of MS lesions on MRI, which has sharp
contrast with surrounding white matter.
DEC uses a RGB color-coded scheme and attributes a
color for each orientation of the fibers, such that fibers crossing
from left to right are visualized in red, fibers crossing
anteriorly to posteriorly are visualized in green, and fibers
crossing inferiorly to superiorly are visualized in blue. In
addition, FA allows for the visualization of lesions more clearly
and larger than with DWI. The application of DTT may aid in the
identification of broken fibers and the diminishment of distal
fibers in MS lesions, which are associated with vasogenic edema,
demyelination, axonal degenetation and other pathological changes
related to disintegrity of the nerve fibers (13,14). No
obvious abnormality of NAWM was detected by FA, DEC or DTT in the
present study. This may be due to the pathological alterations in
NAWM not being significant enough to be detected. Therefore, for
optimal pathological diagnosis and study of patients with MS, we
propose that FA, MD, λ// and λ┴ values should be utilized in
combination.
To date, few studies have focused on the role of DTI
in deep brain gray matter in patients with MS, and the results
remain controversial (23–25). The present DTI study found that FA,
MD, λ//, λ┴ values in the thalamus were increased in patients with
MS, compared with those of normal patients. Furthermore, the λ//,
λ┴ and MD values were increased and the FA values were decreased in
the caudate nucleus and deep brain gray matter of patients with MS,
as compared with those of normal controls. The λ// and λ┴ values
are also increased in the lesions of patients with MS, as compared
with those of deep gray matter. The present finding of increased FA
and MD values in the thalamus are consistent with previous studies,
including a report by Tovar-Moll et al (26), which suggested that an increase in
the FA values in the thalamus correlates with thalamus reactive
reconstruction (26–28).
Future studies addressing the relationship between
DTI and different subtypes of MS (10,11,19,20),
particularly with progressive MS, and a comparison to
histopathology, are necessary to elucidate the exact pathological
correlation between white matter to gray matter and its involvement
in patients with MS.
Acknowledgements
The authors would like to thank Hongwei Zhou (The
First Teaching Hospital of the Jilin University) for technical
advice on the neuroradiological images.
References
|
1
|
Balcer LJ: Clinical practice. Optic
neuritis. N Engl J Med. 354:1273–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shams PN and Plant GT: Optic neuritis: A
review. Int MS J. 16:82–89. 2009.PubMed/NCBI
|
|
3
|
Heesen C, Kasper J, Segal J, Köpke S and
Mühlhauser I: Decisional role preferences, risk knowledge and
information interests in patients with multiple sclerosis. Mult
Scler. 10:643–650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barkhof F: The clinico-radiological
paradox in multiple sclerosis revisited. Curr Opin Neurol.
15:239–245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beaulieu C: The basis of anisotropic water
diffusion in the nervous system-a technical review. NMR Biomed.
15:435–455. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamout B, Alroughani R, Al-Jumah M, Khoury
S, Abouzeid N, Dahdaleh M, Alsharoqi I, Inshasi J, Hashem S,
Zakaria M, et al: Consensus guidelines for the diagnosis and
treatment of multiple sclerosis. Curr Med Res Opin. 29:611–621.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szeszko PR, Vogel J, Ashtari M, Malhotra
AK, Bates J, Kane JM, Bilder RM, Frevert T and Lim K: Sex
differences in frontal lobe white matter microstructure: A DTI
study. Neuroreport. 14:2469–2473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kealey SM, Kim Y, Whiting WL, Madden DJ
and Provenzale JM: Determination of multiple sclerosis plaque size
with diffusion-tensor MR imaging: Comparison study with healthy
volunteers. Radiology. 236:615–620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciccarelli O, Werring DJ, Barker GJ,
Griffin CM, Wheeler-Kingshott CA, Miller DH and Thompson AJ: A
study of the mechanisms of normal appearing white matter damage in
multiple sclerosis using diffusion tensor imaging-evidence of
Wallerian degeneration. J Neuro1. 250:287–292. 2003.
|
|
10
|
Lucchinetti CF, Brück W and Noseworthy J:
Multiple sclerosis: Recent developments in neuropathology,
pathogenesis, magnetic resonance imaging studies and treatment.
Curr Opin Neurol. 14:259–269. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Storch MK, Piddlesden S, Haltia M,
Iivanainen M, Morgan P and Lassmann H: Multiple sclerosis: In situ
evidence for antibody- and complement-mediated demyelination. Ann
Neurol. 43:465–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kolbe S, Bajraszewski C, Chapman C, Nguyen
T, Mitchell P, Paine M, Butzkueven H, Johnston L, Kilpatrick T and
Egan G: Diffusion tensor imaging of the optic radiations after
optic neuritis. Hum Brain Mapp. 33:2047–2061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Jones M, DeBoy CA, Reich DS,
Farrell JA, Hoffman PN, Griffin JW, Sheikh KA, Miller MI, Mori S
and Calabresi PA: Diffusion tensor magnetic resonance imaging of
Wallerian degeneration in rat spinal cord after dorsal root
axotomy. J Neurosci. 29:3160–3171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH
and Neufeld AH: Diffusion tensor imaging detects and differentiates
axon and myelin degeneration in mouse optic nerve after retinal
ischemia. Neuroimage. 20:1714–1722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trip SA, Wheeler-Kingshott C, Jones SJ, Li
WY, Barker GJ, Thompson AJ, Plant GT and Miller DH: Optic nerve
diffusion tensor imaging in optic neuritis. Neuroimage. 30:498–505.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lucchinetti CF, Brück W, Parisi J,
Scheithauer B, Rodriguez M and Lassmann H: Heterogeneity of
multiple sclerosis lesions: Implications for the pathogenesis of
demyelination. Ann Neurol. 47:707–717. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lucchinetti CF, Bruck W and Lassmann H:
Evidence for pathogenic heterogeneity in multiple sclerosis. Ann
Neurol. 56:3082004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barnett MH and Prineas JW: Relapsing and
remitting multiple sclerosis: Pathology of the newly forming
lesion. Ann Neurol. 55:458–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weinshenker BG: The natural history of
multiple sclerosis. Neurol Clin. 13:119–146. 1995.PubMed/NCBI
|
|
20
|
Lucchinetti CF, Bruck W and Lassmann H:
Pathology and pathogenesis of multiple sclerosis. 2nd. Elsevier
Science; USA: 2003
|
|
21
|
Pulizzi A, Rovaris M, Judica E, Sormani
MP, Martinelli V, Comi G and Filippi M: Determinants of disability
in multiple sclerosis at various disease stages: A muitiparametric
magnetic resonance study. Arch Neurol. 64:1163–1168. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davalos D, Grutzendler J, Yang G, Kim JV,
Zuo Y, Jung S, Littman DR, Dustin ML and Gan WB: ATP mediates rapid
microglial response to local brain injury in vivo. Nat Neurosci.
8:752–758. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griffin CM, Chard DT, Ciccarelli O, Kapoor
B, Barker GJ, Thompson AI and Miller DH: Diffusion tensor imaging
in early relapsing-remitting multiple sclerosis. Mult Scler.
7:290–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filippi M, Bozzali M and Comi G:
Magnetization transfer and diffusion tensor MR imaging of basal
ganglia from patients with multiple sclerosis. J Neurol Sci.
183:69–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steenwijk MD, Daams M, Pouwels PJ, Balk
LJ, Tewarie PK, Killestein J, Uitdehaag BM, Geurts JJ, Barkhof F
and Vrenken H: What explains gray matter atrophy in long-standing
multiple sclerosis? Radiology. 272:832–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tovar-Moll F, Evangelou IE, Chiu AW,
Richert ND, Ostuni JL, Ohayon JM, Auh S, Ehrmantraut M, Talagala
SL, McFarland HF and Bagnato F: Thalamic involvement and its impact
on clinical disability in patients with multiple sclerosis: A
diffusion tensor imaging study at 3T. AJNR Am J Neuroradiol.
30:1380–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kipp M, Wagenknecht N, Beyer C, Samer S,
Wuerfel J and Nikoubashman O: Thalamus pathology in multiple
sclerosis: From biology to clinical application. Cell Mol Life Sci.
72:1127–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sorgun MH, Yucesan C and Tegin C: Is
malnutrition a problem for multiple sclerosis patients? J Clin
Neurosci. 21:1603–1605. 2014. View Article : Google Scholar : PubMed/NCBI
|