Introduction
Allergic rhinitis (AR) is a chronic inflammatory
disease of the nasal mucosa and is characterized by sneezing, runny
nose, nasal congestion and nasal itching when patients with
allergic diseases come into contact with specific allergens
(1). AR is a common disease
worldwide that affects the quality of peoples' daily lives
(2). Studies have estimated that
patients with AR form ~20% of the global population, and with the
continuing destruction of peoples' living environments, the number
of patients with AR will continue to increase (3–5).
AR is a multifactorial disease, which may involve
local and systemic processes (6). To
date there is no cure for AR, therefore, further research is
required. Recent studies have demonstrated that eosinophils (EOS)
are the primary effector cells in AR, and the localization and
activation of a large number of EOS is an important feature of
allergic diseases (7–10). Due to the signaling link between the
nasal cavity and bone marrow, a large number of EOS are stimulated
by allergens that infiltrate local tissues (11). Bone marrow releases EOS hematopoietic
progenitor cells, namely CD34-positive (CD34+) cells,
which are targeted to various tissues and organs that then
differentiate and develop into mature EOS under the control of
local growth factors (12). Mature
EOS produce, store and rapidly secrete diverse mediators, including
cationic proteins, cytokines, chemokines and growth factors, that
are important in inflammation and immune regulatory responses
(13). Moreover, chemokines are
being increasingly studied due to their association with AR bone
marrow hematopoiesis and hematopoietic cell specification in
situ (14). The majority of
chemokines interact with EOS through binding to the eotaxin
receptor, chemokine receptor 3 (CCR3) (15). CCR3 is a transmembrane G
protein-coupled receptor that is highly expressed in EOS (16). Furthermore, eotaxin belongs to the CC
chemokine family, which activates G-protein-dependent intracellular
signaling cascades that stimulate the migration of EOS (17–19).
Previous studies have indicated that administration
of low molecular weight CCR3 antagonists in mouse models of
allergic airway inflammation could prevent airway
hyperresponsiveness and remodeling (20). Compared with antigen-stimulated
wild-type mice, those treated with CCR3 antagonists exhibited
significantly reduced EOS infiltration into local tissues (21,22) and
higher levels of EOS survival factors [such as interleukin (IL)-5,
granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-3]
in local inflamed tissues, which prolonged EOS survival (23). However, other studies have suggested
that EOS cultured in vitro in the absence of survival
factors can partially survive for 72 h and be recruited to inflamed
tissues, leading to persistent inflammation (24). This process was associated with the
survival of EOS by eotaxin through the CCR3 receptor, which
indicated that CCR3 was closely associated with the apoptosis of
EOS (25,26).
RNA interference (RNAi) can specifically degrade
target RNAs to inhibit and downregulate the expression of specific
genes (27). A previous study
(28) revealed that silencing CCR3
by lentiviral vector-mediated RNAi inhibited the degranulation of
EOS, thereby inhibiting the release of granule proteins and
potentially reducing inflammation in AR. Therefore, it can be
argued that silencing CCR3 by RNAi affects EOS in AR. However, the
mechanisms and processes that underlie this effect have not been
fully elucidated yet (29). In the
present study, lentiviral vectors that express short hairpin RNAs
(shRNAs) to silence the CCR3 gene were transduced into EOS cultured
in vitro in order to observe the effects of CCR3 silencing
(mRNA and protein) on EOS apoptosis. In addition, using an
established AR mouse model, RNAi oligos synthesized in vitro
were used to specifically inhibit the expression of CCR3 in EOS and
block signaling, via the eotaxin/CCR3 pathway, in order to observe
changes in EOS of the bone marrow, peripheral blood and nasal
mucosa. The objective of the present study was to understand the
roles and effects of the CCR3 gene in EOS, and thus develop a
further understanding of the pathogenesis of AR.
Materials and methods
Animals
Male BALB/c mice that were 6–8 weeks old and 20–25 g
(specific pathogen-free grade) were obtained from the Experimental
Animal Center of Nanchang University (Nanchang, China). Mice were
bred in a clean environment at 22–24°C under a 12-h light/dark
cycle and fed with an ovalbumin (OVA)-free diet. The present study
was performed in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. The animal use protocol has been reviewed and
approved by the Institutional Animal Care and Use Committee of
Nanchang University.
Animal grouping and allergization
In total, 24 BALB/c mice were randomly divided into
four groups (n=6 per group): Group I, no-treatment control
(control); group II, PBS treatment control (PBS control); group
III, scramble small interfering RNA (siRNA) treatment (off-target);
and group IV, CCR3 siRNA treatment (pLVX-shRNA-mCCR3).
The mice in groups II to IV were intranasally
administered with 8 µl PBS, control siRNA or CCR3 siRNA,
respectively, twice a day on days 0 and 14. Additionally, these
mice were intraperitoneally injected with an OVA/aluminum hydroxide
[Al(OH)3] mixture [containing 10 µg OVA and 4 mg
Al(OH)3] for allergization twice a day on days 2 and 16
and continuously intranasally administered with 600 µg/ml OVA twice
a day from day 21 to 27 for excitation. At 5 h before excitation,
the mice were administered intranasal treatments as described
above. The control group was administered the same dose of saline.
Samples of the bone marrow, peripheral blood and nasal mucosa were
obtained 24 h after administration of the final treatment.
Culture and purification of bone
marrow-derived EOS
BALB/c mice were sacrificed and their femurs were
isolated. Femoral marrow was rinsed with RPMI 1640 medium (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA), supplemented with
streptomycin and penicillin (HyClone), 20% fetal bovine serum
(HyClone), Fms-related tyrosine kinase 3 ligand (FLT3-L; PeproTech,
Inc., Rocky Hill, NJ, USA), stem cell factor (SCF; PeproTech, Inc.)
and recombinant mouse IL-5 (rmIL-5; PeproTech, Inc.). A total of
107 cells/ml were incubated at 37°C in 5%
CO2.
Identification of EOS
Untreated cells (1×106) were collected
from culture wells and rinsed with PBS, and the supernatant was
discarded. Anti-IL-5 receptor (alpha) PE antibody (eBioscience,
Inc., San Diego, CA, USA) and anti-CD34 fluorescein isothiocyanate
(FITC) antibodies (eBioscience, Inc.) were added to untreated
cells, single cell suspensions were prepared and
double-immunopositive cells were sorted by flow cytometry.
Lentivirus transduction
Lentivirus transduction was performed on the tenth
day of EOS culture. One day before transduction, EOS were seeded at
a density of 5×105 cells/well into a 12-well culture
plate, which were divided into three groups when performing
transductions. Group I was treated with shRNA-mCCR3 virus mixed
with medium to 100 µl (with MOI=10); group II was treated with the
same amount of control virus; and group III was treated with 0.1%
PBS-medium mixture. Cells were cultured for 48 h after
transduction; subsequently, the medium was aspirated, cells were
washed with 0.1% PBS and then treated according to the following
methods.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of CCR3 expression in
lentivirus-transduced EOS
EOS were resuspended in TRIzol reagent (Takara
Biotechnology Co., Ltd., Dalian, China), and RNA was extracted
according to the manufacturer's instructions. RNA was converted to
cDNA using a reverse transcription kit(Takara Biotechnology Co.,
Ltd.), and primers were designed for CCR3 (IDNM_009914.4) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; ID: NM_017008.4,
as the control, synthesized by Oligo). Reverse
transcription-polymerase chain reaction RT-PCR reactions used to
amplify cDNA were set up in accordance with the appropriate
annealing temperature and cycles. Furthermore, the expression of
mRNA for each group was detected by electrophoresis. Samples of the
bone marrow, peripheral blood and nasal mucosa were tested
according to the procedures described above.
Western blot analysis of CCR3
expression in lentivirus-transduced EOS
EOS were homogenized in radioimmunoprecipitation
assay lysis buffer (Pierce Protein Biology; Thermo Fisher
Scientific, Inc., Rockford, IL, USA). The homogenate was cooled on
ice for 20 min and centrifuged at 4°C and 14,000 rpm to remove the
insoluble material. The supernatant was mixed with 4% SDS sample
buffer, boiled for 5 min, and resolved using 10% SDS-PAGE (Ready
Gel J; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Electrophoresed proteins were transferred to a polyvinylidene
fluoride membrane (EMD Millipore, Billerica, MA, USA), blocked with
5% skimmed milk diluted in 1X Tris-buffered saline with Tween 20
(TBST) buffer for 2 h, and incubated with the primary antibody
(Abcam, Cambridge, UK) overnight at 4°C. The membranes were rinsed
with TBST three times, incubated with the secondary antibody
(Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc., Beijing, China) for 2 h, and washed again with
TBST three times. An anti-GAPDH antibody was used as an internal
reference protein to normalize protein loading (Anbo Biotechnology
Co., Ltd., Nanjing, China) and concentrations of each target
protein were normalized against GAPDH. The membranes were evenly
mixed with chemiluminescence reagent, incubated in the dark at room
temperature for 3–5 min, then exposed and developed using X-ray
films. The intensity levels of bands from the electrophoretic gels
and photographic films were measured using a dedicated image
analysis software (Band Leader 3.0; http://en.bio-soft.net/chip/BandLeader.html).Samples
of the bone marrow, peripheral blood and nasal mucosa were tested
according to the procedures described above.
Apoptosis detection
Cells were treated with lentivirus for 48 h and
control cells were incubated with antibodies provided in an
apoptosis kit (PeproTech, Inc.), and single cell suspensions were
prepared with PBS following the manufacturer's instructions.
Apoptosis was detected with flow cytometry.
Detection of CD34+
cells
Samples of the bone marrow and peripheral blood were
collected, and cells were isolated with lymphocyte isolation liquid
(GE Healthcare Life Sciences) to prepare the single cell
suspension. The cells were labeled with anti-CD34 FITC primary
antibody and detected with flow cytometry following
resuspension.
Statistical analysis
SPSS software, version 18 (SPSS, Inc., Chicago, IL,
USA) was used for analysis of variance in order to determine
intergroup differences, which were expressed as the mean ± standard
deviation. Each experiment was repeated three times. The error bars
indicate the standard error of the samples. P<0.05 was used to
indicate a statistically significant difference.
Results
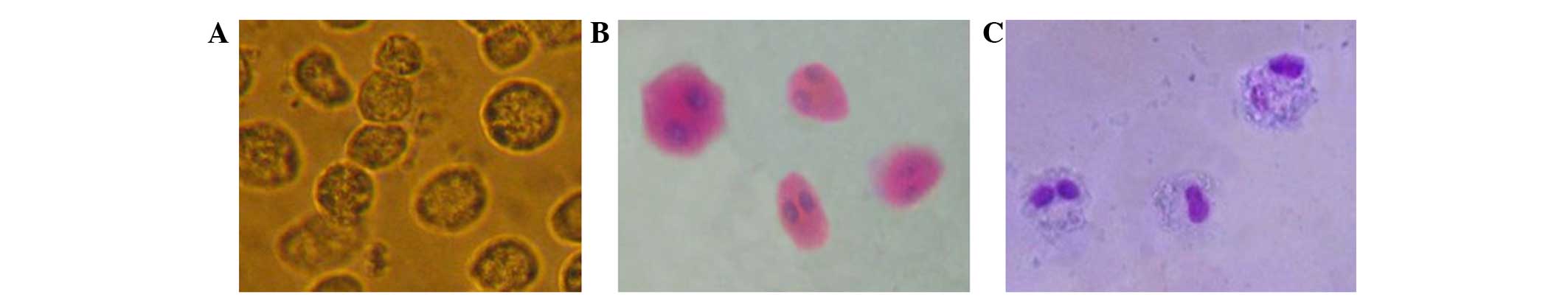
Hematoxylin and eosin and Wright
staining
Under the induction of the growth factors, FLT3-L
and SCF, the majority of the primary bone marrow cells
differentiated into mature EOS. Due to the effect of rmIL-5, EOS
survived and apoptosis was delayed. Moreover, an optical microscope
was used to observe cell morphology after incubation with the
indicated factors. HE and Wright staining of EOS are shown in
Fig. 1.
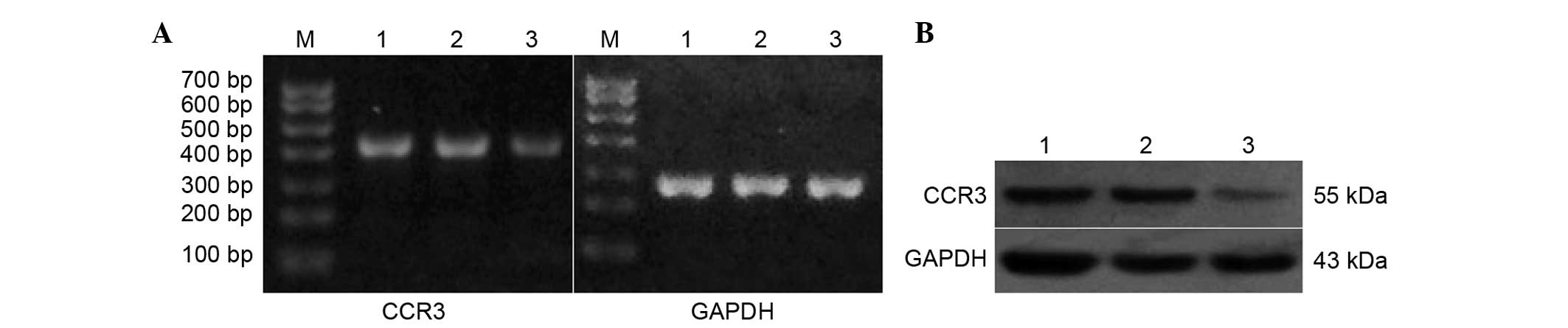
RT-PCR and western blot analyses of
lentivirus-transduced EOS
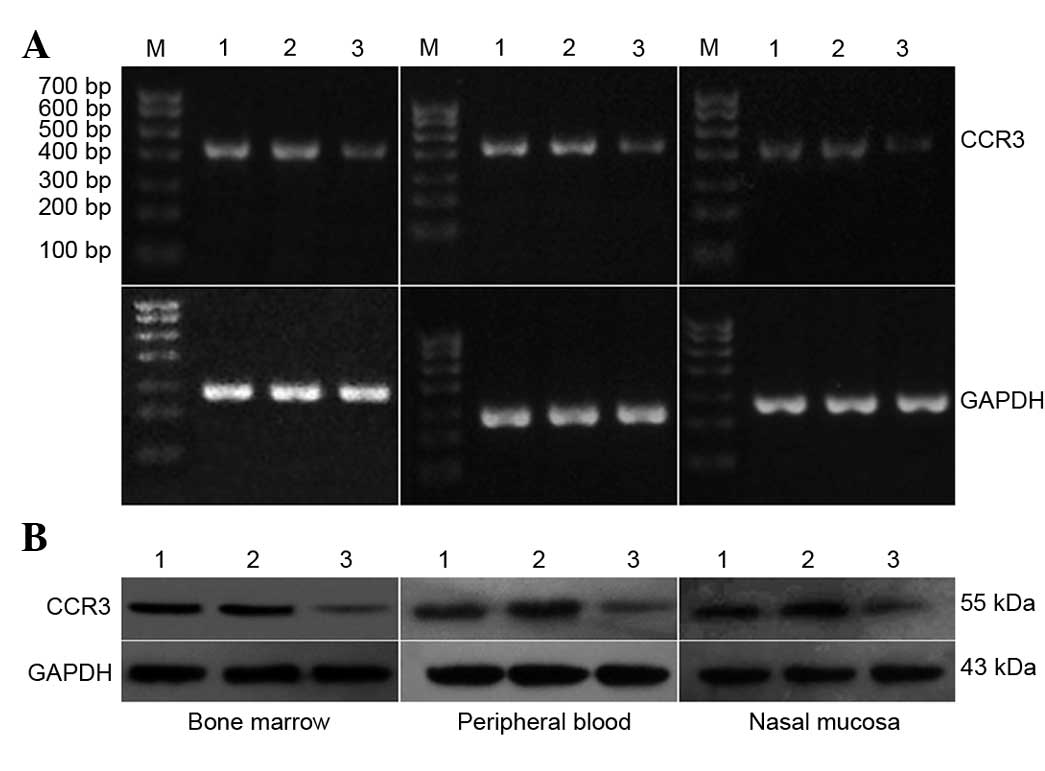
As shown in Fig. 2,
the mRNA expression of CCR3 in the control that did not receive any
treatment and the control siRNA treatment group was not
significantly different (Fig. 2A).
However, the mRNA expression of CCR3 in the CCR3 siRNA treatment
group was significantly lower than that in the other two groups
(P<0.05) (Table I).
 | Table I.CCR3 mRNA expression gray value. |
Table I.
CCR3 mRNA expression gray value.
| Group | Ratio grayscale
value (x±s) |
|---|
| Cell | 0.720±0.078 |
| NCsh | 0.750±0.082 |
| CCR3sh |
0.230±0.053a |
As shown in Fig. 2B,
the protein expression of CCR3 in the control group that did not
receive any treatment and the control siRNA treatment group was not
significantly different, whereas the protein expression of CCR3 in
the CCR3 siRNA treatment group was significantly lower than that in
the other two groups (P<0.05). Furthermore, the experiment was
repeated three times.
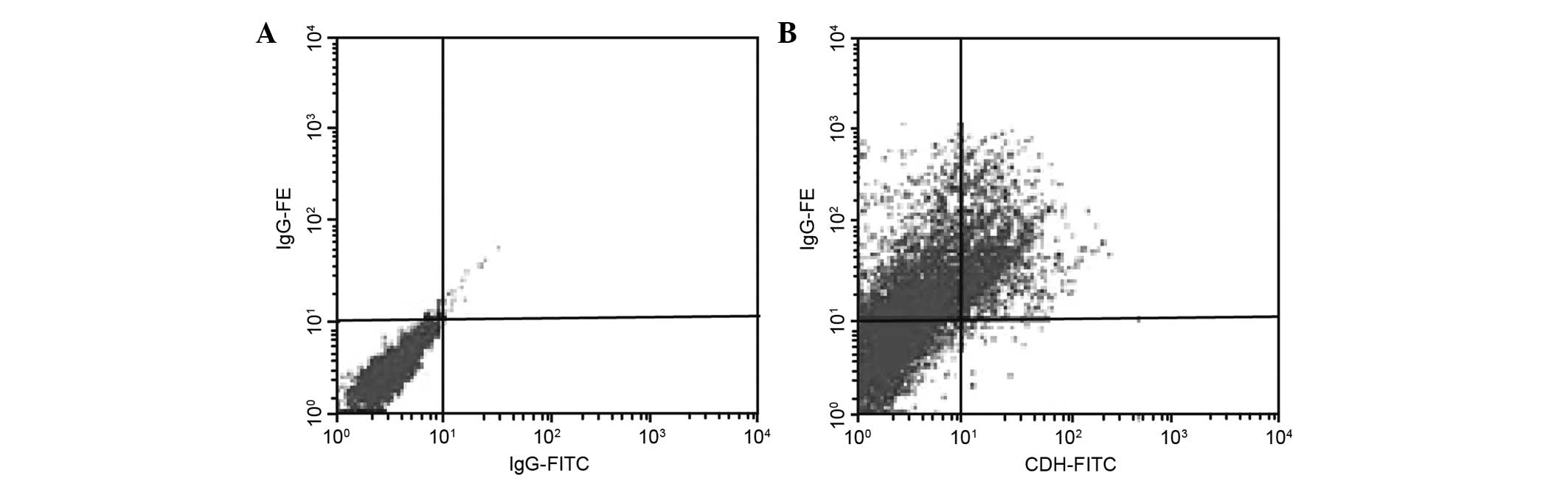
Identification results of EOS
EOS express both CD34 and IL-5; therefore, it is
possible to identify EOS by the specific labeling of these proteins
(Fig. 3) using anti-IL-5 PE and CD34
FITC antibodies. The CD34 epitope could be detected in
early-differentiated EOS, whereas its expression was not detected
significantly in apoptotic EOS. Moreover, the IL-5 epitope could be
detected during both stages of EOS.
Using flow cytometry, the percentage of cultured
cells labeled with the anti-IL-5 PE and CD34 FITC antibodies were
found to be 65.7±3.25 and 17.5±2.27%, respectively, which were also
significantly different (P<0.05). These percentages can be used
to estimate the percentage of EOS in the cultured cells.
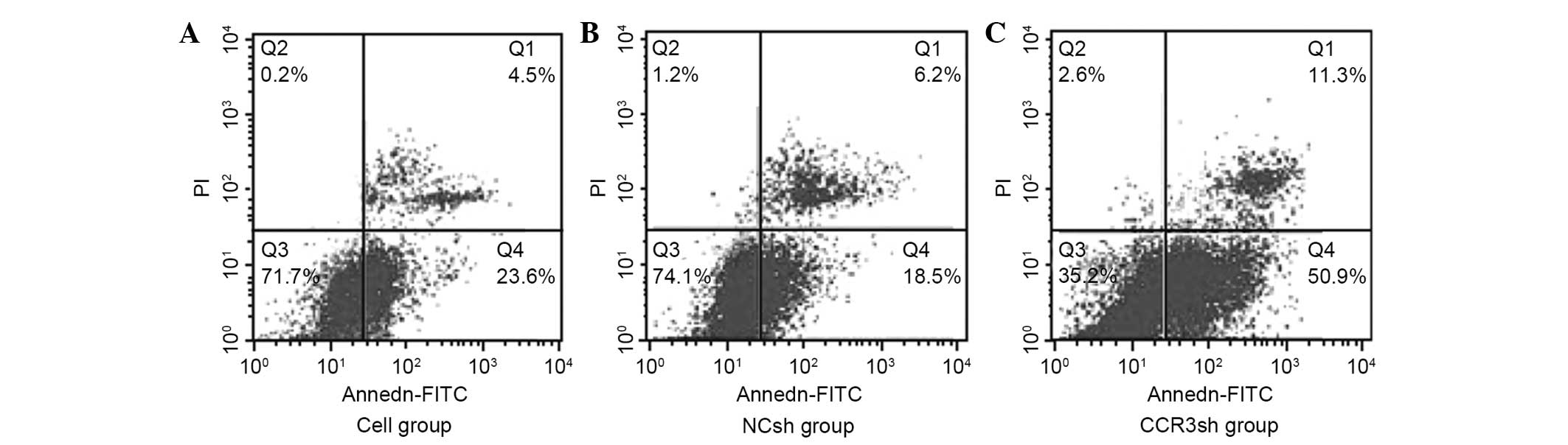
Results of apoptosis detection
The apoptotic rates of EOS were measured for the
following groups: 24.52±4.56% for the control that did not receive
any treatment, 20.7±2.32% for the control siRNA and 67.5±5.88% for
the CCR3 siRNA groups. The apoptotic rate of the CCR3 siRNA group
was significantly higher than the apoptotic rates of the other two
groups (P<0.05). However, there was no significant difference
between the control that did not receive any treatment and the
control siRNA groups (P>0.05; Fig.
4).
RT-PCR and western blot analyses of
animal specimens
The mRNA expression levels of CCR3 in the PBS and
control siRNA groups were not significantly different, whereas the
mRNA expression of CCR3 in the CCR3 siRNA group was significantly
lower than that in the other two groups (P<0.05; Fig. 5A). In addition, the experiment was
repeated three times.
Moreover, the protein expression of CCR3 in the PBS
and the control siRNA group was not significantly different,
whereas the protein expression of CCR3 in the CCR3 siRNA group was
significantly lower than that in the other two groups (P<0.05)
(Table II and Fig. 5B). The experiment was repeated three
times.
 | Table II.CCR3 protein expression value. |
Table II.
CCR3 protein expression value.
| Groups | Ratio grayscale
value (x±s) |
|---|
| Cell | 0.950±0.158 |
| NCsh | 0.930±0.087 |
| CCR3sh |
0.250±0.042a |
Histological staining of the nasal
mucosa
Edema, inflammatory cell infiltration, epithelial
shedding and quantity of exudates and EOS in the nasal mucosa were
worse in the PBS and control siRNA groups compared with the control
group, indicating that the histological results of these animal
models met the pathological characteristics of AR. Moreover, in the
CCR3 siRNA group IV, the number of EOS was decreased and mucosal
edema was alleviated, indicating that silencing of CCR3 reduced the
pathology of AR (Fig. 6).
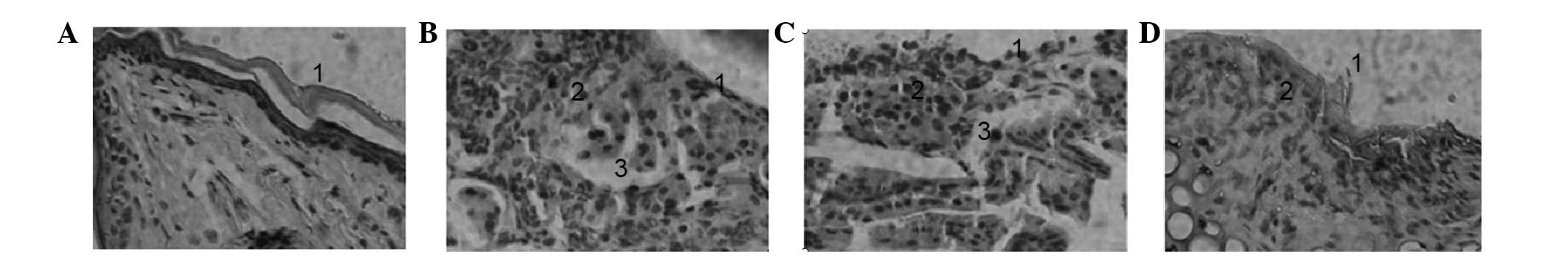 | Figure 6.Hematoxylin and eosin staining of
nasal mucosa. (A) Group I, nasal mucosal epithelium was intact,
without inflammatory cell infiltration (magnification, ×200); (B)
Group II, mucosa swelled, epithelium shed, there existed submucosal
edema, vascular dilatation and congestion, as well as the
infiltration of a large number of plasma cells, lymphocytes and EOS
(magnification, ×200); (C) Group III, submucosal vessels dilated,
with mucosal edema and EOS infiltration (magnification, ×200); (D)
Group IV, nasal mucociliary layer on mucosal surface was more
complete, without thickening of mucous layer, while submucosal
vasocongestion and edema were not obvious (magnification, ×200).
EOS, eosinophils. |
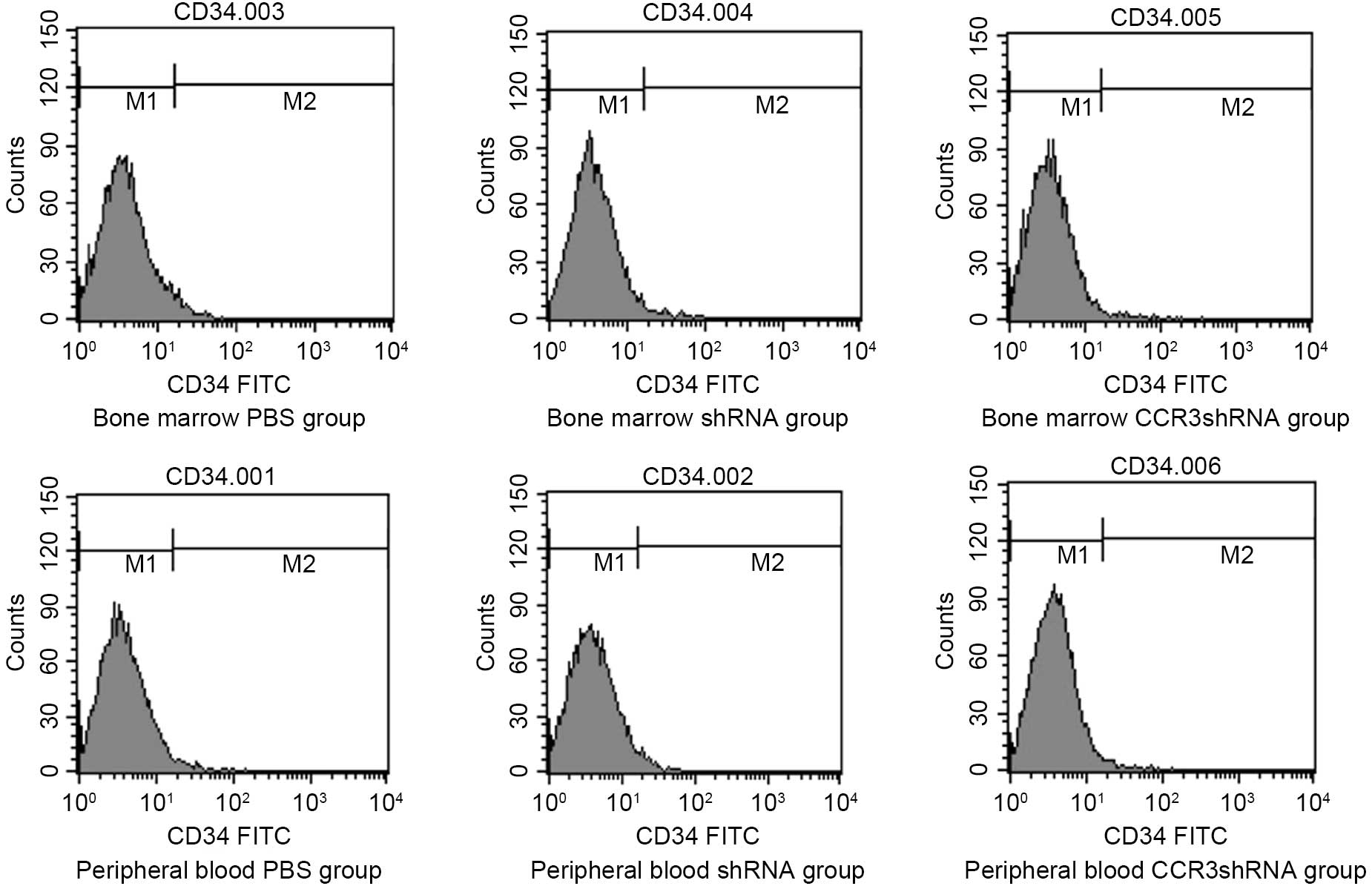
Detection of CD34+
cells
No significant difference was detected in the number
of CD34+ cells from the bone marrow, peripheral blood
and CCR3 siRNA groups (P>0.05; Fig.
7). Moreover, the experiment was repeated three times.
Discussion
EOS are important effector cells in the induction of
inflammation. The recruitment and activation of a large number of
EOS in local tissues are characteristic of allergic diseases and T
Helper 2 (Th2)-type immune responses (30). EOS release cytotoxic granules that
contain biomolecules, including major allergy-related basic
proteins, eosinophil peroxidase, eosinophil-derived neurotoxin and
eosinophil cationic proteins through degranulation or cytolysis in
local tissues (31). Conditioned
media of degranulated EOS can reproduce pathological features of
allergic diseases, including tissue damage, vascular leakage, mucus
secretion and airway contraction (32,33).
As important processes in the development of
allergic diseases, promoting the apoptosis of EOS and reducing the
infiltration of EOS into local tissues could potentially relieve
local inflammation in allergic diseases (7). IL-3, IL-5 and GM-CSF can promote the
survival of EOS (23). Furthermore,
IL-5 receptors quickly activate tyrosine kinase cascades that are
transduced through the Janus kinase 2/signal transducer and
transcription activating protein pathway (34). However, previous results have
demonstrated that the treatment of patients with allergic diseases
with anti-IL-5 monoclonal antibodies could only partially reduce
the number of infiltrating EOS in airways, while EOS numbers in the
peripheral circulation and bone marrow remained at a high level
(22).
CCR3 is a transmembrane G protein-coupled receptor,
and its ligand eotaxin belongs to the CC chemokine family (16). Furthermore, the binding of eotaxin to
CCR3 induces EOS to migrate to specific tissues. CCR3 receptors are
primarily expressed in EOS, while Th2 cells, basophils and mast
cells may also constitutively or temporarily express CCR3 (35). In addition, the downregulation of
CCR3 inhibits the delay of EOS apoptosis by IL-5, thus inducing EOS
apoptosis and reducing local tissue invasion by EOS (23).
EOS is derived from CD34+ hematopoietic
progenitor cells under the stimulation of GM-CSF, IL-3 and IL-5 and
other cytokines in the bone marrow (36). Ben et al (37) has performed external intervention
with anti-CCR3 monoclonal antibody in an allergic mouse model.
Furthermore, anti-CCR3 monoclonal antibodies downregulated CCR3,
inhibited chemokine-mediated migration of bone marrow
CD34+ progenitor cells and inhibited the IL-5/eotaxin
pathway thus significantly reducing allergen-induced infiltration
of EOS and CD34+ progenitor cells. As a result, airway
hyperresponsiveness was maintained at a lower level and the
production of mucus was reduced (38).
RNAi robustly inhibits expression, has high in
vitro transfection efficiency and has high target specificity
(39). Moreover, RNAi can be
potentially used for long-term treatments by silencing target genes
in specific tissues or cells using specific tissue or cell
promoters, which would potentially prevent damage in other tissues
or cells and reduce complications by other factors. In addition,
siRNA plasmids can be administered by liposomes and directly
through the mouse mucosa, which makes it feasible to directly treat
and improve AR with the transnasal mucosal administration of RNAi
(40).
In the present study, EOS from mice were cultured
and purified in vitro for transduction by CCR3
siRNA-recombinant lentiviral vectors. By measuring the expression
of CCR3 at the mRNA and protein levels and measuring the apoptosis
rates of EOS, the present study revealed that lentiviral vectors
were effective in silencing and inhibiting the effects of CCR3,
which could significantly promote the apoptosis of EOS.
Furthermore, CCR3 siRNA lentiviral vectors were used as an
intervention in mice in vivo. The measurement of the
expression of CCR3 at the mRNA and protein levels and measurement
of EOS infiltration in local tissues revealed that direct
transnasal administration could effectively silence the expression
of CCR3 and could ameliorate pathological changes of the nasal
mucosa in mice, including reductions in the migration and invasion
of EOS and in nasal inflammation in mice with AR. Moreover, in the
present study RNAi was effective in silencing the expression of the
CCR3 gene in EOS, thus providing new directions to discover
effective targets for the treatment of AR through gene therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81060084), Jiangxi
Provincial Natural Science Foundation (grant no. 2010GZY0251) and
the Supportive Project of Jiangxi Provincial Science and Technology
Department (grant no. 20133BBG70071).
References
|
1
|
Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY,
Ye YM, Shin YS and Park HS: Detection of Allergen specific
antibodies from nasal secretion of allergic rhinitis patients.
Allergy Asthma Immunol Res. 8:329–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franzke N, Schäfer I, Jost K, Blome C,
Rustenbach SJ, Reich K, Reusch M, Maurer M and Augustin M: A new
instrument for the assessment of patient-defined benefit in the
treatment of allergic rhinitis. Allergy. 66:665–670. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu CH, Tsai WC, Lee TJ, Huang CC, Chang PH
and Su Pang JH: Simvastatin Inhibits IL-5-Induced Chemotaxis and
CCR3 Expression of HL-60-Derived and Human Primary Eosinophils.
PLoS One. 11:e01571862016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pawankar R, Bunnag C, Khaltaev N and
Bousquet J: Allergic rhinitis and its impact on asthma in asia
pacific and the ARIA update 2008. World Allergy Organ J. 5:(Suppl
3). S212–S217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y and Zhang L: Prevalence of
allergic rhinitis in China. Allergy Asthma Immunol Res. 6:105–113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han DH, Ahn JC, Mun SJ, Park SK, Oh SY and
Rhee CS: Novel Risk Factors for Allergic Rhinitis in Korean
Elementary School Children: ARCO-kids Phase II in a Community.
Allergy Asthma Immunol Res. 7:234–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu X, Wang J, Xia Y, Simayi M, Ikramullah
S, He Y, Cui S, Li S and Wushouer Q: Resveratrol induces cell cycle
arrest and apoptosis in human eosinophils from asthmatic
individuals. Mol Med Rep. 2016.
|
|
8
|
Fulkerson PC and Rothenberg ME: Targeting
eosinophils in allergy, inflammation and beyond. Nat Rev Drug
Discov. 2:117–129. 2013. View
Article : Google Scholar
|
|
9
|
Muir AB, Markowitz JE and Liacouras CA: 45
– Allergic and Eosinophilic Gastrointestinal Disease. Pediatric
Allergy Principles & Practice. 399–408. 2016. View Article : Google Scholar
|
|
10
|
Khorasanizadeh M, Eskian M, Assa'ad AH,
Camargo CA Jr and Rezaei N: Efficacy and Safety of Benralizumab, a
Monoclonal Antibody against IL-5Rα, in Uncontrolled Eosinophilic
Asthma. Int Rev Immunol. 35:294–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahajan L, Madan T, Sarma P Usha and
Kishore U: Human lung surfactant protein, SP-D, modulates
eosinophil activation and survival and enhances phagocytosis of
apoptotic bosinophils. US patent, US8883730. 2014
|
|
12
|
Cruz FF, Borg ZD, Goodwin M, Coffey AL,
Wagner DE, Rocco PR and Weiss DJ: CD11b+ and Sca-1+ Cells Exert the
Main Beneficial Effects of Systemically Administered Bone
Marrow-Derived Mononuclear Cells in a Murine Model of Mixed
Th2/Th17 Allergic Airway Inflammation. Stem Cells Transl Med.
5:488–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long H, Liao W, Wang L and Lu Q: A Player
and Coordinator: The Versatile Roles of Eosinophils in the Immune
System. Transfus Med Hemother. 43:96–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith H, Whittall C, Weksler B and
Middleton J: Chemokines stimulate bidirectional migration of human
mesenchymal stem cells across bone marrow endothelial cells. Stem
Cells Dev. 21:476–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Millard CJ, Ludeman JP, Canals M,
Bridgford JL, Hinds MG, Clayton DJ, Christopoulos A, Payne RJ and
Stone MJ: Structural basis of receptor sulfotyrosine recognition by
a CC chemokine: The N-terminal region of CCR3 bound to
CCL11/eotaxin-1. Structure. 22:1571–1581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rupprecht KM, Daugherty B, Mudgett J and
Parsons WH: Chapter 14. CCR3 antagonists for the treatment of
respiratory diseases. Annu Rep Med Chem. 38:131–140. 2003.
View Article : Google Scholar
|
|
17
|
Proudfoot AE: Chemokine receptors:
Multifaceted therapeutic targets. Nat Rev Immunol. 2:106–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JH, Jang AS, Park SW, Kim DJ and Park
CS: Gene-Gene Interaction Between CCR3 and Eotaxin Genes: The
Relationship With Blood Eosinophilia in Asthma. Allergy Asthma
Immunol Res. 6:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Jang AS, Park SW, Kim DJ and Park
CS: Gene-Gene Interaction Between CCR3 and Eotaxin Genes: The
Relationship With Blood Eosinophilia in Asthma. Allergy Asthma
Immunol Res. 6:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rose CE Jr, Lannigan JA, Kim P, Lee JJ, Fu
SM and Sung SS: Murine lung eosinophil activation and chemokine
production in allergic airway inflammation. Cell Mol Immunol.
7:361–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gauvreau GM and Denburg JA: Hemopoietic
progenitors: The role of eosinophil/basophil progenitors in
allergic airway inflammation. Expert Rev Clin Immunol. 1:87–101.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rådinger M and Lötvall J: Eosinophil
progenitors in allergy and asthma - do they matter? Pharmacol Ther.
121:174–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waseda K, Hagiya H, Hanayama Y, Terasaka
T, Kimura K, Tsuzuki T, Hasegawa K, Nada T, Nakamura E, Murakami K,
et al: Complication of chronic eosinophilic pneumonia in an elderly
patient with Sjögren syndrome. Acta Med Okayama. 69:123–127.
2015.PubMed/NCBI
|
|
24
|
Sakai-Kashiwabara M, Abe S and Asano K:
Suppressive activity of quercetin on the production of eosinophil
chemoattractants from eosinophils in vitro. In Vivo. 28:515–522.
2014.PubMed/NCBI
|
|
25
|
Ryu SH, Na HY, Sohn M, Han SM, Choi W, In
H, Hong S, Jeon H, Seo JY, Ahn J, et al: Reduced expression of
granule proteins during extended survival of eosinophils in
splenocyte culture with GM-CSF. Immunol Lett. 173:7–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burnham ME, Koziol-White CJ, Esnault S,
Bates ME, Evans MD, Bertics PJ and Denlinger LC: Human airway
eosinophils exhibit preferential reduction in STAT signaling
capacity and increased CISH expression. J Immunol. 191:2900–2906.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lioy DT and Klein M: Compositions and
Methods for Regulating Gene Expression. US patent, US20090082297.
2009.
|
|
28
|
Zhu XH, Liao B, Liu K and Liu YH: Effect
of RNA interference therapy on the mice eosinophils CCR3 gene and
granule protein in the murine model of allergic rhinitis. Asian Pac
J Trop Med. 7:226–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohr SE, Smith JA, Shamu CE, Neumüller RA
and Perrimon N: RNAi screening comes of age: Improved techniques
and complementary approaches. Nat Rev Mol Cell Biol. 15:591–600.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong TW, Doyle AD, Lee JJ and Jelinek DF:
Eosinophils regulate peripheral B cell numbers in both mice and
humans. J Immunol. 192:3548–3558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang K-C, Lo C-W, Fan TC, Chang MD, Shu
CW, Chang CH, Chung CT, Fang SL, Chao CC, Tsai JJ, et al: TNF-α
mediates eosinophil cationic protein-induced apoptosis in BEAS-2B
cells. BMC Cell Biol. 11:62010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Curran CS: Human eosinophil adhesion and
receptor expression. Methods Mol Biol. 1178:129–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehta P and Furuta GT: Eosinophils in
Gastrointestinal Disorders: Eosinophilic Gastrointestinal Diseases,
Celiac Disease, Inflammatory Bowel Diseases, and Parasitic
Infections. Immunol Allergy Clin North Am. 35:413–437. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bao L, Zhang H and Chan LS: The
involvement of the JAK-STAT signaling pathway in chronic
inflammatory skin disease atopic dermatitis. JAK-STAT.
2:e241372013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Forssmann U, Elsner J, Escher S and
Sposberg N: Method of inhibiting the emigration of cells from the
intravascular compartment into tissues. US patent, US7741292.
2010
|
|
36
|
Zhu Y, Tuerxun A, Hui Y and Abliz P:
Effects of propranolol and isoproterenol on infantile hemangioma
endothelial cells in vitro. Exp Ther Med. 8:647–651.
2014.PubMed/NCBI
|
|
37
|
Ben S, Li X, Xu F, Xu W, Li W, Wu Z, Huang
H, Shi H and Shen H: Treatment with anti-CC chemokine receptor 3
monoclonal antibody or dexamethasone inhibits the migration and
differentiation of bone marrow CD34 progenitor cells in an allergic
mouse model. Allergy. 63:1164–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gelfand EW and Dakhama A: Method for
reducing allergen-induced airway hyperresponsiveness. Patent,
EP1265626. 2005
|
|
39
|
Lee CC and Chiang BL: RNA interference:
New therapeutics in allergic diseases. Curr Gene Ther. 8:236–246.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Cristofaro P, Silbermann R, Pusch
O, Boden D, Konkin T, Hovanesian V, Monfils PR, Resnick M and Moss
SF: Engineering mucosal RNA interference in vivo. Mol Ther.
14:336–342. 2006. View Article : Google Scholar : PubMed/NCBI
|