Introduction
Chimonobambusa quadrangularis (Fenzi) Makino,
which belongs to Gramineae Bambusoideae Chimonobambusa
(1), predominantly grows in
high-altitude jungles in the Southwest and its shoots are a
natural, high quality food (2).
Square bamboo typically matures in autumn, which is distinct from
other types of bamboo. Square bamboo shoots are nutritionally-rich,
including protein, fat, carbohydrate, cellulose, vitamin C, vitamin
B and other mineral elements such as calcium, phosphorus, iron,
zinc and selenium (3). Fermentation
improves the quality of the food, including its taste and the
proportion of nutrients, whereas fermentation by microbes improves
the physiological functions of dietary fiber in the bamboo shoots
(4).
In modern society, constipation is a common
physiological state, and individuals who suffer from constipation
experience decreased bowel movements (5). Furthermore, the amount of defecation
decreases and difficulty defecating is a result of a lack of water
(6). Due to current working
conditions, ~70% of individuals are in a suboptimal state with
stomach diseases and constipation (7). Foods that may improve these conditions
include health foods (such as green tea, Korea Kimchi) and
functional foods (such as soybean oligosaccharides, dietary fiber)
that aid the return to normal function, and foods that improve
constipation are an important and effective way to improve
intestinal health (8). Fiber in
bamboo shoots has been proven to have a good effect on inhibiting
constipation (9), and it is has been
reported that dietary fiber in the bamboo shoots can be improved
through fermentation, and therefore its effect on constipation may
also improve (10).
Activated carbon is able to reduce intestinal
peristalsis and delay the pass of intestinal contents, which may
lead to constipation when administered under normal conditions
(11). Activated carbon has become
an important experimental method of examining the effects of food
on the inhibition of constipation (12). The present study adopted activated
carbon to induce constipation in mice in order to observe the
preventative effect of fermented bamboo shoots on constipation,
which may provide support for the further development and
utilization of fermented bamboo shoots as a gastrointestinal (GI)
functional food.
Materials and methods
Fermentation of C. quadrangularis
shoot
Cultivated Lactobacillus acidophilus (1.1854)
and Streptococcus thermophilus (1.1855; both China General
Microbiological Culture Collection Center, Beijing, China) were
centrifuged at 3,000 × g for 10 min, after which the
supernatant was discarded, washed with a moderate amount of saline,
and suspended in saline in order to adjust the concentration of the
bacteria liquid to 7.5×109 CFU/ml (L.
acidophilus: S. thermophilus, 1:1). L.
acidophilus and S. thermophilus were subsequently mixed
to achieve C. quadrangularis shoot fermented bacterial
liquid in the proportion of 1:1. Fresh C. quadrangularis
shoot (5 kg) were washed and crumbled, and 500 ml mixed fermented
bacteria liquid was subsequently added at 42°C (fix format) for 16
h for the main fermentation step. Following this, prophase
fermentation was performed at 4°C for 12 h to attain the fermented
C. quadrangularis shoot.
Microscopic evaluation of the fiber in
C. quadrangularis shoots
Fresh C. quadrangularis shoot (CQS) and
fermented C. quadrangularis shoot (FCQS) were smashed using
a grinder and washed for 10 min with saline three times. The liquid
was discarded and 95% alcohol (10X) was added to clean the residue
and the mixture was subsequently centrifuged at 750 × g for
5 min. The supernatant was discarded, the small piece of clean
solid was squashed and 3 drops of 1% ethanol solution of methylene
dye were prepare the slide containing C. quadrangularis
shoot fiber. The slide was observed at ×20 magnification under a
microscope (Y-2A; Nikon Corp., Tokyo, Japan).
Manufacture of C. quadrangularis shoot
feed
Following pasteurization, CQS and FCQS were dried,
smashed and subsequently mixed with the feed at a ratio of 1:9
according to the weight prior to being pressed into a mold. These
CQS and FCQS feeds were then added to the experimental mouse feed
to a total of 10% CQS and FCQS.
Establishment of a mouse model of
constipation
Seven-week-old female imprinting Kun Ming mice
(n=50; weight, 25–30 g) Experimental Animal Center of Chongqing
Medical University, Chongqing, China) were randomly divided into
normal, control, bisacodyl treatment, SCQ and FSCQ groups
(n=10/group). The mice were maintained in a temperature-controlled
facility (temperature 23±1°C, relative humidity 50±5%) with a 12-h
light/dark cycle. During the experiment, mice in the normal group
were not treated. Mice in the control group were not treated during
the first 6 days; however, after this they were administered
activated carbon water by lavage once daily for 3 days. Mice in the
bisacodyl treatment group were administered bisacodyl (100 mg/kg
body weight) once daily, and after 6 days were administered
activated carbon water by lavage once daily without bisacodyl for 3
days. Mice in these three groups were administered water and were
permitted ad libitum access to feed. Mice in the SCQ and
FSCQ groups were administered feed containing 10% SCQ and FSCQ,
respectively, and ad libitum access to water during the
whole experiment, and activated carbon water was administered by
lavage during the final 3 days. All groups were restricted from
consuming food after 9 days for 24 h, and were administered 10%
activated carbon ice water at a concentration of 0.1 ml/10 g by
lavage. Each group was divided into two subgroups. Five mice from
each group were observed to determine the time point at which they
discharged their first black stool defecation, whereas the
remaining five mice were euthanized by cervical vertebra
dislocation 30 min after activated carbon water lavage to observe
the GI transit of activated carbon in the small intestine. GI
transit was calculated as follows: GI (%) = distance traveled by
activated carbon in the small intestine/the total length of small
intestine × 100% (13). These
experiments followed a protocol approved by the Animal Ethics
Committee of Chongqing Medical University (Chongqing, China).
Determination of serum indices
Mouse blood (0.1 ml) was centrifuged at 3,000 ×
g for 10 min. The supernatant, which contained the mouse
serum, was used to determine the indices of motilin (MTL),
endothelin-1 (ET-1), vasoactive intestinal peptide (VIP) and
acetylcholine enzyme (AchE), which were measured according to the
manufacturer protocols outlined in the MTL, ET-1, VIP (Cusabio
Biotech Co., Ltd., Wuhan, China) ELISA kits and AchE kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Pathological observation of the small
intestine
The rats were sacrificed using the cervical vertebra
dislocation method. Following dissection, the small intestine was
placed in 10% formalin solution for 24 h and 95% ethanol to
dehydrate prior to xylene treatment. Transparent blocks were
embedded in melted paraffin, sliced by microtome and stained with
hematoxylin eosin, and observed at ×10 magnification under a Nikon
Y-2A microscope (14).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA (2 µg) was extracted from the small
intestine using RNAzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA) reagent from the small intestine of each group.
RNA concentration was adjusted to 1 µg/µl. and 2 µl RNA extracts
were respectively added to 1 µl oligodT18, RNase, dNTP and MLV
enzymes, 5X buffer (GE Healthcare Life Sciences, Chalfont, UK) and
10 µl was used to synthesize cDNA under the conditions of 37°C for
120 min, 99°C for 4 min, and 4°C for 3 min. Expression of c-Kit,
stem cell factor (SCF), glial cell-derived neurotrophic factor
(GDNF), Transient receptor potential cation channel subfamily V
member 1 (TRPV1) and nitric oxide synthase (NOS; Tiangen Biotech
Co., Ltd., Beijing, China) was amplified through RT-PCR and
compared to GAPDH gene expression (Tiangen Biotech Co., Ltd.). cDNA
(2 µl) was mixed with 1 µl of each primer (10 µM) and 16 µl
DNase-free water in a PCR premix tube (AccuPower PCR PreMix;
Bioneer Corporation, Daejeon, Korea), and PCR was performed in an
automatic thermocycler (T100, Bio-Rad, Hercules, CA, USA) for 40
cycles of 94°C for 5 min, 58°C for 30 sec, and 72°C for 90 sec,
followed by a 10 min cycle at 95°C. The primers were as follows:
Forward: 5′-AGA CCG AAC GCA ACT T-3′ and reverse, 5′-GGT GCC ATC
CAC TTC A-3′ for c-Kit; forward, 5′-AAA CTG GTG GCG AAT C-3′;
reverse, 5′-CAC GGG TAG CAA GAA C-3′) for SCF; forward, 5′-TTT TAT
TCA AGC CAC CAT C-3′; reverse, 5′-AGC CCA AAC CCA AGT CA-3′ for
GDNF; forward, 5′-AGC GAG TTC AAA GAC CCA GA-3′ and reverse, 5′-TTC
TCC ACC AAG AGG GTC AC-3′ for TRPV1; and forward, 5′-CCA CAT CTG
GCA GGA TGA GAA-3′ and reverse, 5′-AGG CAC AGA ACT GAG GGT ACA-3′
for NOS (Tiangen Biotech Co., Ltd., Beijing, China); and forward,
5′-CGG AGT CAA CGG ATT TGG TC-3′ and reverse, 5′-AGC CTT CTC CAT
GGT CGT GA-3′ for GAPDH gene expression (Tiangen Biotech Co.,
Ltd.). Subsequently, 2% agarose gels in 1X Tris-acetate-EDTA were
used to assess the amplification of genes by PCR (15).
Western blot analysis
Following the extraction of tissue protein with RIPA
lysate, the total protein concentration of each group was
homogenized with ice-cold radioimmunoprecipitation assay (RIPA;
Easybio, Beijing, China) buffer in ice-cold PBS, then the
extraction was centrifuged at 13,000 × g for 30 min at 4°C.
The protein (30 µg) was determined using an ultraviolet
spectrophotometer, and the concentrations were adjusted to the same
level. Protein samples were separated by 10–12% SDS-PAGE
(Schleicher and Schuell, Keene, NH, USA) for 4 h and transferred to
nitrocellulose membranes. Membranes were sealed for 3 h in sealing
fluid, washed 3 times with Tris-buffered saine (TBS; Easybio,
Beijing, China) and incubated with primary antibodies against c-Kit
(cat no. ab62154, Abcam, Cambridge, MA, USA), SCF (cat no. ab83866;
Abcam), TRPV1 (cat no. ACC-030, Alomone; Beijing, China), GDNF (cat
no. ab18956; Abcam), NOS (cat no. sc-49058; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and β-actin (cat no. ab8226;
Abcam) for 2 h. After washing with PBS containing 0.05% Tween 20
(PBS-T), blots were and then incubated with horseradish
peroxidase-conjugated goat-anti-rabbit IgG secondary antibody (Cell
Signaling Technology, Inc.) for 1 h at room temperature. Following
washing 3 times with TBS, treatment with an ultra-sensitive light
liquid on a light sensitive X-plate (cat no. BE6031, Easybio,
Beijing, China), the nitrocellulose filters were developed and
fixed (16). Protein expression
levels of c-Kit, SCF, TRPV1, GDNF and NOS were determined, with
β-actin as the reference gene.
Statistical analysis
Experimental data are presented as the mean ±
standard deviation. Differences between the mean values for
individual groups were assessed with a one-way analysis of variance
with Duncan's multiple range tests. P<0.05 was considered to
indicate a statistically significant difference. SAS 9.2 (SAS
Institute Inc., Cary, NC, USA, 2009) was used for statistical
analyses.
Results
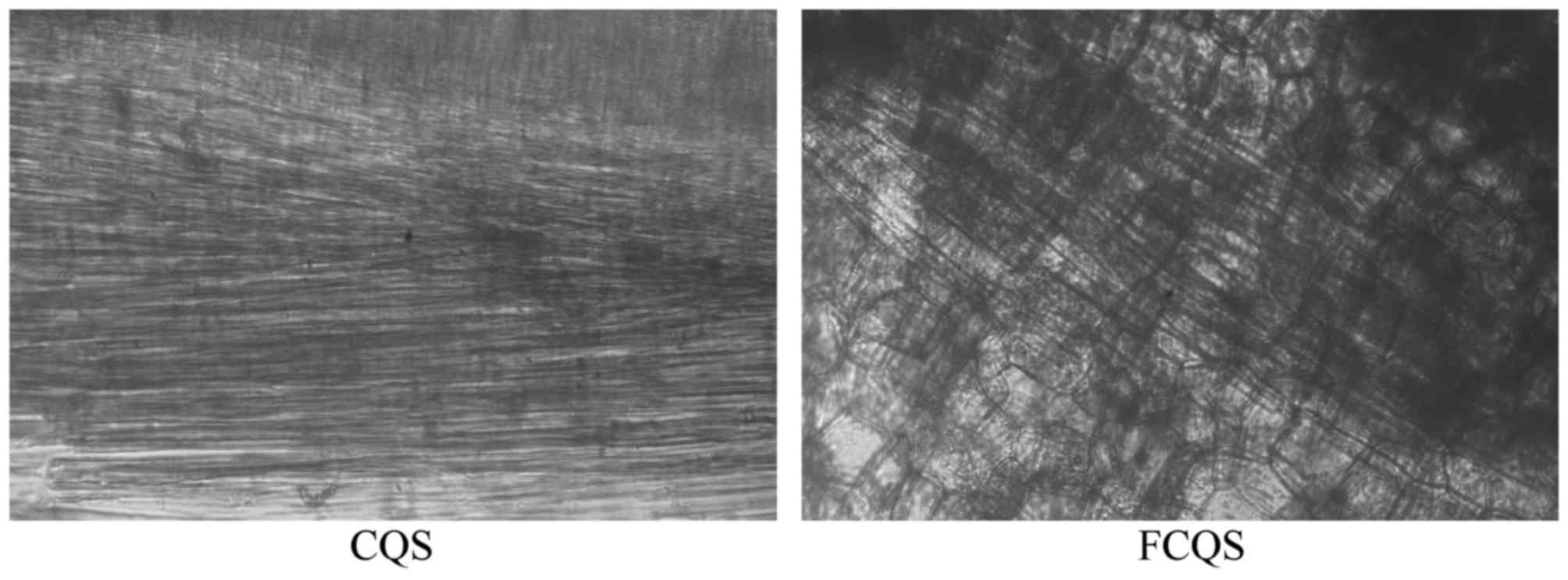
Morphological observation of FCQS and
CQS
As demonstrated in Fig.
1, the fiber gaps in FCQS increased and the structure became
more relaxed, whereas the fiber gap of CQS was dense and
tighter.
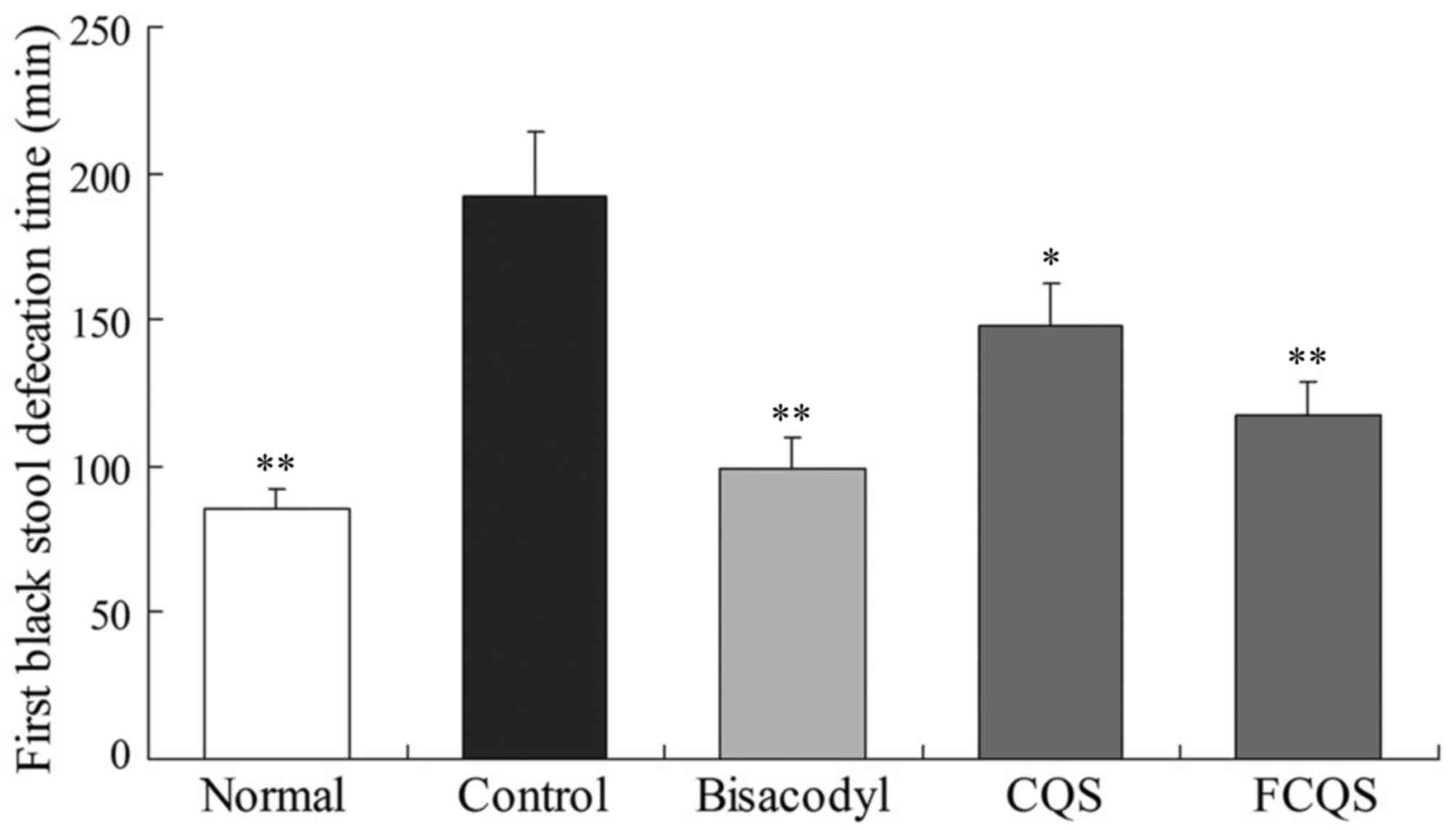
First black stool defecation
Following treatment with activated carbon, the
duration until the first black stool defecation was highest in the
control mice (192 min), as compared with 85 min in the normal mice
(Fig. 2). Treatment with bisacodyl,
which is an anti-constipation drug, decreased the duration between
treatment and first black stool defecation to 99 min. Notably, the
FCQS-fed mice (117 min) exhibited a significantly shorter time than
CQS-fed mice (148 min; P<0.05) and control mice (P<0.01);
however the duration exhibited by FCQS-fed mice remained longer
than bisacodyl-treated and normal mice.
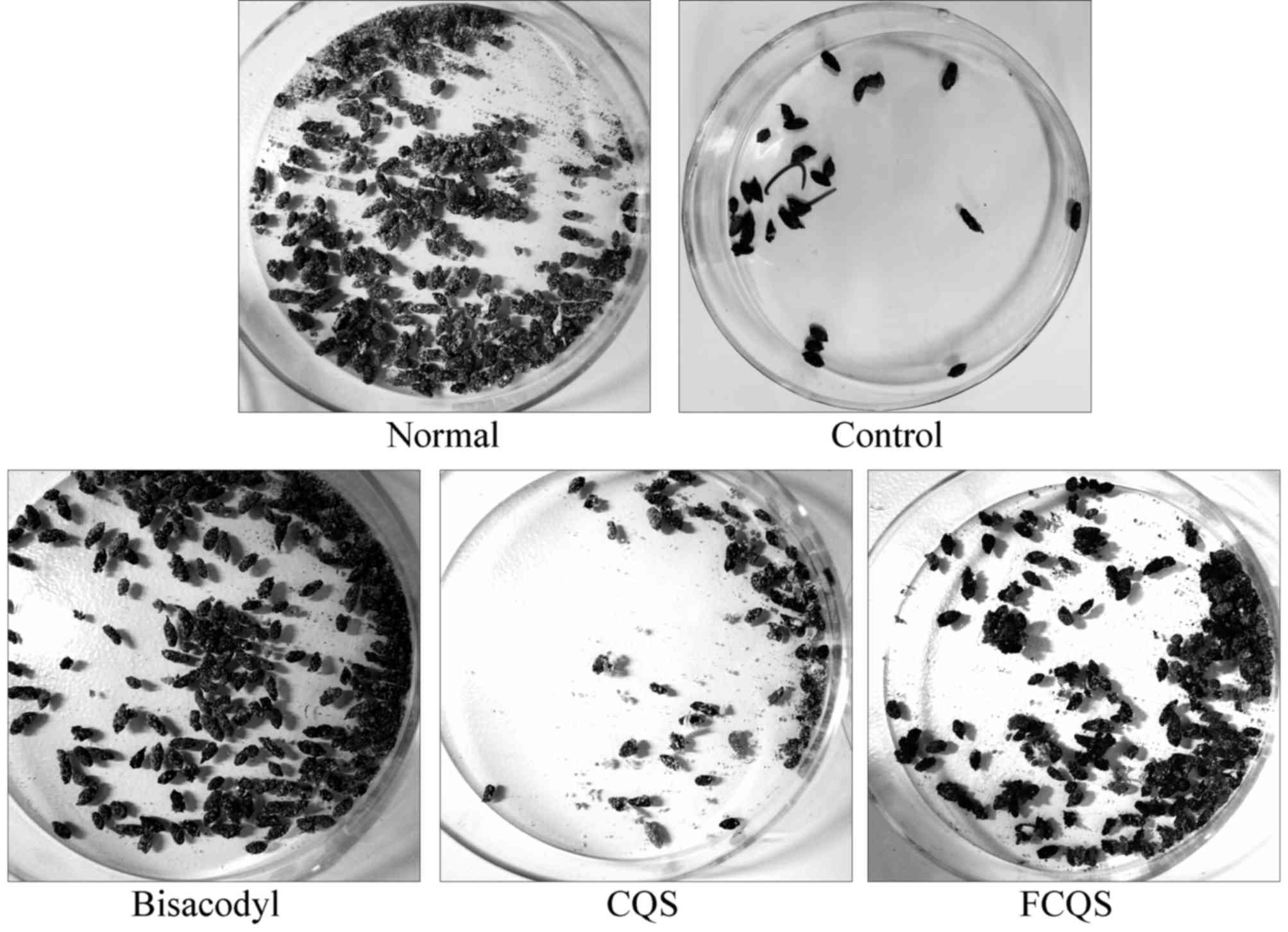
Defecation status
After inducing constipation, the defecation status
of the mice in the different groups altered. The defecation count
was highest in the normal mice, whereas the control group mice
exhibited the lowest defecation count (Fig. 3). The defecation count of the FCQS
group was higher than the CQS group, but remained lower than the
bisacodyl group. The stool of the control mice was very dry, and
CQS-treated mice also exhibited dry stool. Mice in the normal,
bisacodyl and FCQS groups exhibited normal moist stool.
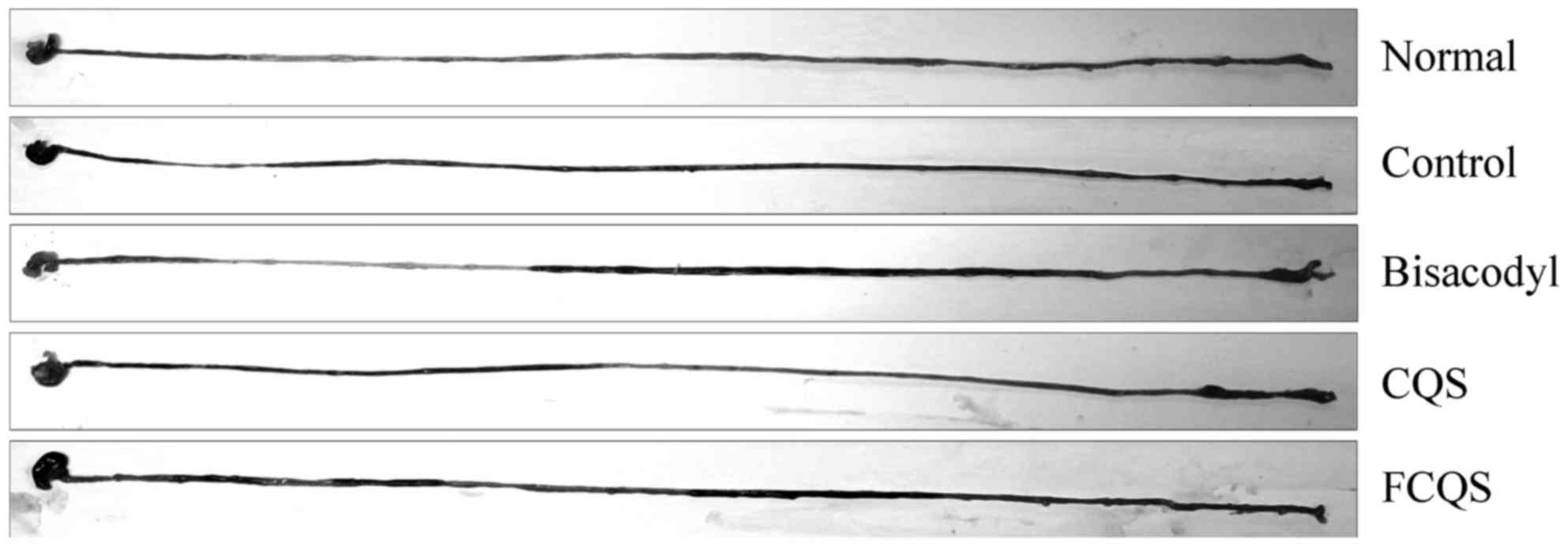
GI transit
On the final day of experimentation, all mice were
treated with activated carbon by lavage. All the activated carbon
was able to pass through the small intestine in normal mice 30 min
post-treatment and this was set as 100% (Fig. 4; Table
I). Activated carbon passed through the small intestine with
difficulty in the control mice. The control mice exhibited a GI
transit value of 37.9%. Bisacodyl-treated mice exhibited a high GI
transit value at 88.3%. FCQS and CQS-treated mice also exhibited
increased GI transit compared to the control mice, and FCQS-treated
mice (73.8%) exhibited increased GI transit, as compared with CQS
(61.7%; P<0.01).
 | Table I.Effects of samples on gastrointestinal
(GI) transit in mouse model of activated carbon-induced
constipation. |
Table I.
Effects of samples on gastrointestinal
(GI) transit in mouse model of activated carbon-induced
constipation.
| Variable | Normal | Control | Bisacodyl | CQS | FCQS |
|---|
| Small intestine
length (cm) |
48.3±2.2a | 47.8±3.2a | 47.9±1.9a | 47.5±2.5a | 47.7±2.6a |
| Distance traveled
(cm) |
48.3±2.2a | 18.1±3.9e | 42.3±2.1b | 29.3±2.2d | 35.2±1.8c |
| GI transit (%) |
100.0±0.0a | 37.9±5.3e | 88.3±3.1b | 61.7±4.0d | 73.8±3.3c |
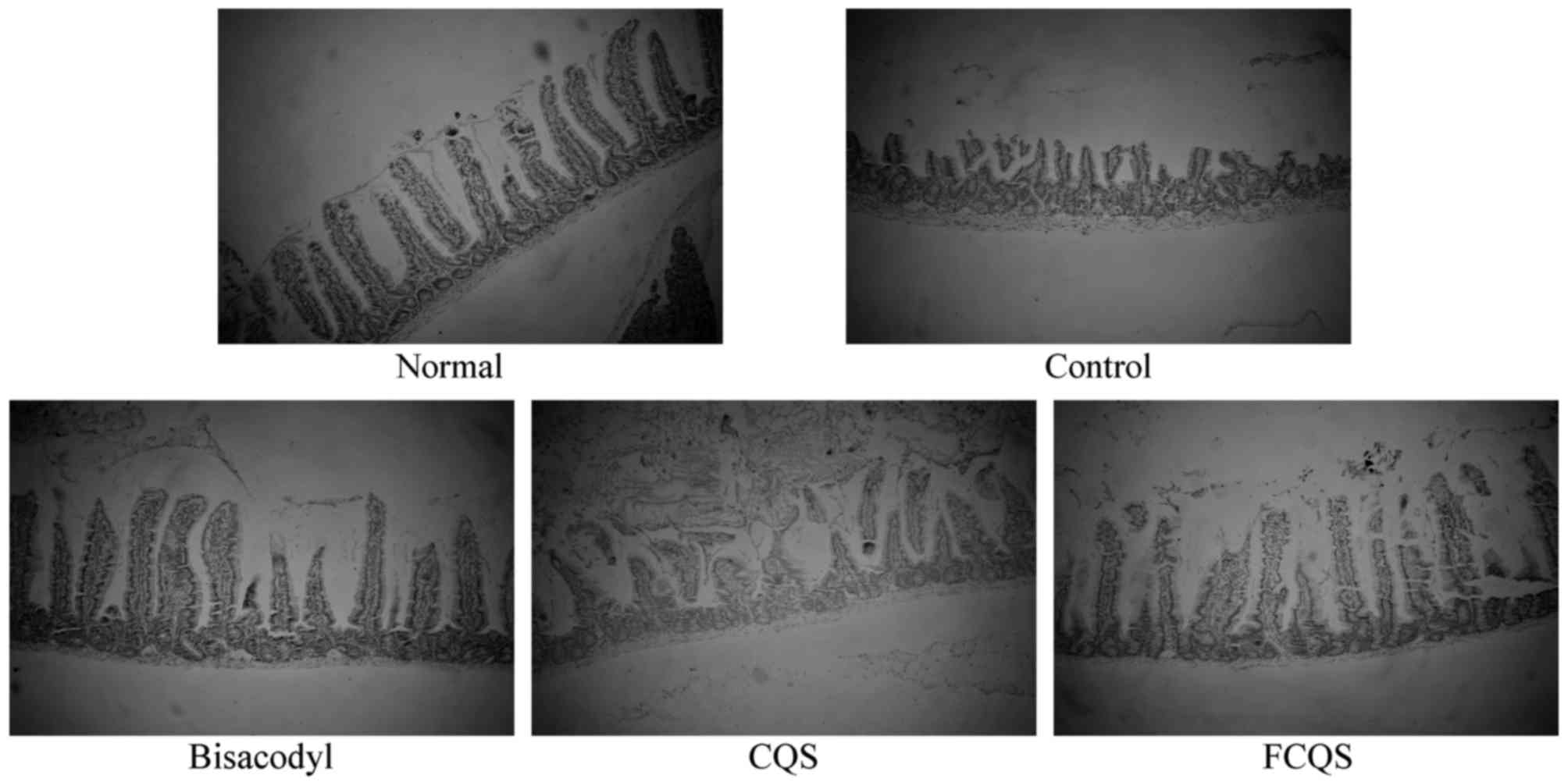
Morphological observation of the small
intestine
In normal mice, the small intestinal villi were
orderly, and the thicknesses of the small intestinal wall was
homogeneous (Fig. 5). The villi of
control mice were injured and broken, and the thickness of the
small intestinal wall was heterogeneous. The small intestinal villi
of bisacodyl-treated mice exhibited minimal damage and the villi of
CQS and FCQS-treated mice exhibited more severe injury than
bisacodyl-treated mice. CQS-treated mice exhibited more severe
intestinal villi damage than FCQS-treated mice.
Serum MTL, ET-1, VIP and AchE
levels
MTL, ET-1, VIP and AchE levels in the serum were
highest in the normal mice (Table
II), whereas the levels were lowest in control mice
(P<0.05). Serum levels from bisacodyl-treated mice were similar
to those in normal mice and were higher than CQS and FCQS-fed mice.
Notably, respective FCQS and CQS treatment increased these levels
compared with the control mice (P<0.05), and fermentation
further increased the levels of MTL, ET-1, VIP and AchE compared
with the CQS-fed mice.
 | Table II.Effect of various samples on serum
MTL, ET-1, VIP and AchE levels in a mouse model of activated
carbon-induced constipation. |
Table II.
Effect of various samples on serum
MTL, ET-1, VIP and AchE levels in a mouse model of activated
carbon-induced constipation.
| Level (pg/ml) | Normal | Control | Bisacodyl | CQS | FCQS |
|---|
| MTL |
189.3±23.6a |
89.3±10.7e |
162.4±17.9b |
125.2±14.6d |
149.8±15.2c |
| ET-1 |
20.1±1.1a |
8.2±0.9e |
18.2±0.5b |
11.0±0.7d |
15.2±0.8c |
| VIP |
61.2±4.2a |
26.7±2.8e |
51.2±1.9b |
38.9±2.7d |
44.3±1.3c |
| AchE |
37.8±1.5a |
13.5±0.4e |
30.6±0.8b |
19.3±1.2d |
26.4±1.1c |
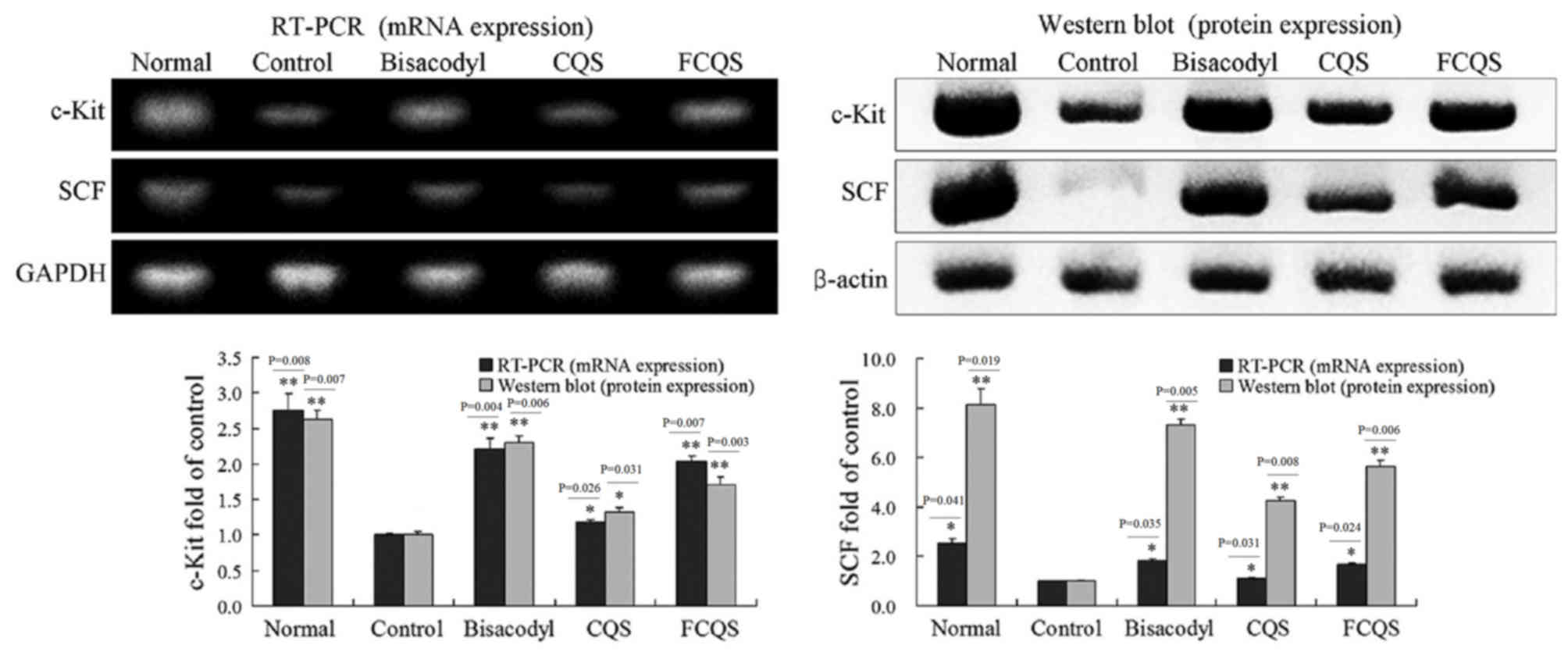
Expression levels of c-Kit and
SCF
c-Kit and SCF expression levels in the small
intestine of mice were determined by RT-PCR and western blotting
(Fig. 6). c-Kit and SCF mRNA and
protein expression levels were highest in the normal mice, whereas
the control mice exhibited the lowest levels of c-Kit and SCF.
Expression levels of bisacodyl-treated mice were higher than all
other groups, with the exception of the normal group. FCQS-treated
mice exhibited increased expression levels, as compared with the
control (P<0.01) and CQS-treated mice (P<0.05).
Expression levels of TRPV1, GDNF and
NOS
TRPV1, GDNF and NOS are enteric nerve-related genes
(16). FCQS was able to increase
GDNF mRNA and protein levels, and decrease TRPV1 and NOS levels, as
compared with the control mice (P<0.01) and CQS-treated mice
(P<0.05; Fig. 7). TRPV1, GDNF and
NOS mRNA and protein expression levels in FCQS-treated mice were
similar to those exhibited by normal and bisacodyl-treated
mice.
Discussion
Constipation can damage the intestinal wall,
including shortening and fracturing of the villi, affecting normal
absorption and defecation (11). As
a result, constipation has a notable influence on the intestine. It
has previously been demonstrated that the increased fiber gap of
FCQS facilitates the improved penetration of water to the fibers
and increases its absorbency and water-holding capacity, which
increases the volume of excrement that stimulates intestinal
peristalsis (17). Furthermore,
increased water content in excrement may prevent dry excrement,
which increases bowel movements(18). Previous research has demonstrated
that fermentation can significantly improve the water-holding
ability of bamboo (10), which was
confirmed in the present study. Therefore, the influence of
fermentation on FCQS fiber may promote defecation and effectively
relieve constipation.
Patients with constipation have difficulty
defecating, and their frequency is lower than that of individuals
with normal GI function and transit. A mouse constipation model may
be used to simulate human constipation, and how long until mice can
discharge black excrement produced by activated carbon in the state
of constipation is used as the standard to measure the degree of
constipation (17). The results of
the present study demonstrated that the duration between treatment
and the first black stool defecation in the FCQS group was
significantly shorter than that of the CQS group, indicating that
FCQS may be more effective at promoting intestinal health and
countering constipation.
The most characteristic representation of
constipation is difficulty defecating with low volumes and dry
grains due to lack of water (7).
Observation of the excrement of experimental mice in the present
study demonstrated that, after constipation induced by activated
carbon, the amount of stools passed by the control group was
markedly decreased, whereas the effects of bisacodyl, CQS and FCQS
were preferable, compared with normal square bamboo. These findings
demonstrated that CQS is a suitable and effective food for
relieving constipation, and fermentation may improve its
efficacy.
Distance traveled and GI transit time of activated
carbon are key indicators used to measure intestinal function,
which affects the degree of constipation. Increased distance and GI
transit time indicate lower degrees of constipation (19). The mouse constipation model used in
the present study indicated that the intestinal activity varied and
the GI transit time of activated carbon in the normal and control
groups differed significantly. CQS was able to significantly
relieve constipation, markedly improving the GI transit time of
activated carbon in the intestine. Notably, FCQS had an superior
effect on easing constipation, as compared with CQS. Furthermore,
the results of the present study indicated that the effect of FCQS
on constipation is similar to that of bisacodyl, which was
demonstrated to be superior to the effect of fresh CQS.
MTL is a type of GI hormone that stimulates the
secretion of pepsin and promotes intestinal peristalsis, which is
required for normal physiological intestinal activity and
defecation (20). Endothelin ensures
the tension of vessels, maintaining a normal cardiovascular system
and avoiding other diseases (cardiovascular disease, hypertension)
caused by constipation (21). ET-1
is a key factor that regulates cardiovascular function, promoting
the normal contraction and dilation of blood vessels and relieving
the abnormal contraction of blood vessels caused by constipation
(22). VIP relaxes smooth muscles,
promoting the dilation of blood vessels, increasing the production
of intestinal secretions, and stimulating intestinal peristalsis. A
previous study has demonstrated that a decrease in the secretion of
VIP may directly lead to constipation (23). AchE modulates intestinal contraction,
promotes the secretion of mucus, and promotes defecation by
enhancing intestinal contraction and the secretion of mucus, which
may help to avoid constipation (24). It has been demonstrated that the
levels of MTL, ET-1, VIP and AchE in mouse serum decrease with the
degree of constipation (19). In the
present study, compared with the control group, FCQS was able to
significantly increase the levels of MTL, ET-1, VIP and AchE, and
the effect was greater than that of the fresh CQS group.
A previous study demonstrated that c-Kit is
expressed in the smooth muscles of mice intestines; therefore,
blocking the function of c-Kit may lead to severe intestinal
dysfunction and intestinal obstruction. c-Kit and SCF are
interdependent, thus they are able to promote each other, therefore
any decline in the expression levels of c-Kit and SCF in the
intestine may cause constipation (25).
After binding with its ligand, TRPV1 opens cell
passages and releases a large number of neuropeptides, excitatory
amino acids and GI peptides, which causes increased intestinal
sensitivity and movement disorders, leading to constipation
(26). GDNF regulates the growth and
development of nerve cells, ensuring the protection and repair of
damaged nerves. Exogenous GDNF significantly improves intestinal
function and relieves constipation (27). NOS has an important role in the
intestinal tract. For example, NOS and AchE maintain the balance of
intestinal movements. NOS inhibits the release of AchE, and NOS and
VIP exhibit a close morphological relationship that is directly
associated with constipation. Excessive NOS may lead to
constipation and a decrease of other factors that are beneficial to
the intestine (28).
The aim of the present study was to investigate
whether C. quadrangularis shoot has a preventative effect
against activated carbon-induced constipation and whether
fermentation was able to increase this effect in mice. FCQS
exhibits a higher quality of fiber compared with CQS. FCQS-fed mice
exhibited an increased duration between treatment and the first
black stool defecation, as compared with bisacodyl-treated and
normal mice; however FCQS-fed mice exhibited a shorter duration
than CQS-fed and control mice. The dejection states of FCQS-fed
mice were also similar to the bisacodyl-treated and normal mice. GI
transit time was increased in FCQS-fed mice, as compared with
CQS-fed and control mice; however, FCQS-fed mice exhibited a GI
transit time that was similar to the bisacodyl-treated and normal
mice.
Numerous small intestinal injuries were detected in
the control mice. Although CQS was able to decrease these injuries,
FCQS exhibited a superior recovery than CQS, as determined by
morphological observation. Serum MTL, ET-1, VIP and AchE levels of
FCQS-fed mice were increased, as compared with CQS-fed and control
mice. FCQS-fed mice exhibited increased mRNA expression levels of
c-Kit, SCF and GDNF in the small intestine, as compared with
CQS-fed and control mice; whereas TRPV1 and NOS expression levels
in the small intestine of FCQS-fed mice were decreased, as compared
with CQS-fed and control mice.
In conclusion, these findings suggest that FCQS
exhibits a superior preventive effect on activated carbon-induced
constipation in mice, as compared with CQS. Further research is
required to fully elucidate the protective effects and dosage
requirements for FCQS as a therapeutic strategy for the treatment
of constipation.
Acknowledgements
The present research was supported by the Scientific
and Technological Research Program of Chongqing Municipal Education
Commission (grant no. KJ1401411), the Studio of Chongqing Urban and
Rural Teacher's Overall-planning Education Research Center (grant
no. JDGZS201507), Chongqing Application Development Projects (grant
no. cstc2014yykfB80017), the Construction Program of Chongqing
Engineering Research Center (grant no. cstc2015yfpt_gcjsyjzx0027)
and the Program for Innovation Team Building at Institutions of
Higher Education in Chongqing (grant no. CXTDX201601040).
References
|
1
|
Li R, Wu LR and Zhou CP: A study on the
mineral nutrition composition of Chimonobambusa quadrangularis
bamboo shoots. J Bamboo Res. 26:37–39. 2007.
|
|
2
|
Feng HY, Li M and Ma YH: Technology of
harvesting processing on Chimonobambusa utilis (Keng) Keng f. World
Bamboo Rattan. 14:27–28. 2006.
|
|
3
|
Zhang RG, Li M, Wang XQ and Liu LC:
Preliminary study of the forming of special quality of
Chimonobambusa utilis bamboo shoot in Nanchuan, Chongqing. World
Bamboo Rattan. 8:30–33. 2010.
|
|
4
|
Li AP, Xie BX, Tian YF and Gu ML: Effect
of fermentation on anti-nutritional factor contents and adsorption
of dietary fiber for Ca2+, Mg2+,
Pb2+. Food Sci Technol (Campinas). 35:96–99. 2010.
|
|
5
|
Hsu HH, Leung WH and Hu GC: Treatment of
irritable bowel syndrome with a novel colonic irrigation system: A
pilot study. Tech Coloproctol. 20:551–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christer R, Robinson L and Bird C:
Constipation: Causes and cures. Nurs Times. 99:26–27.
2003.PubMed/NCBI
|
|
7
|
Liu W, Liu XH, Fang XC, Zhou LK, Yang XL,
Ke MY, Zhao HC, Zhang M, Xie PY, Hao JY, et al: A multicenter
epidemiological investigation on outpatients with chronic
constipation in Beijing area. Clin J Gastroenterol. 15:95–98.
2010.
|
|
8
|
Zhou HJ, Zhang QS, Shi L, Li H, Xu JY,
Huang CY, Chen G and Li M: Effect of direct vat set Lactobacillus
on intestinal function in mice with constipation. Food Ferment
Technol. 49:26–29. 2003.
|
|
9
|
Wang Y, Cai DL, Yuan JY, Zhang YZ, Zhou JH
and Li HQ: Effect of dietary fibers on patient with functional
constipation. Wu Jing Yi Xue. 25:13–15. 2014.(In Chinese).
|
|
10
|
Jiang NQ, Xie BX, He G, Li AP and Xie T:
Studies on intestinal peristalsis induced by fermenting
bamboo-shoots dietary fiber. Acta Nutr Sinica. 24:439–440.
2002.
|
|
11
|
Qian Y, Zhao X and Kan J: Preventive
effect of resistant starch on activated carbon-induced constipation
in mice. Exp Ther Med. 6:228–232. 2013.PubMed/NCBI
|
|
12
|
Li G, Wang Q, Qian Y, Zhou Y, Wang R and
Zhao X: Component analysis of Pu-erh and its anti-constipation
effects. Mol Med Rep. 9:2003–2009. 2014.PubMed/NCBI
|
|
13
|
Li GJ, Qian Y, Sun P, Feng X, Zhu K and
Zhao X: Preventive effect of polysaccharide of Larimichthys Crocea
swimming bladder on activated carbon-induced constipation in mice.
J Korean Soc Appl Biol Chem. 57:167–172. 2014. View Article : Google Scholar
|
|
14
|
Chen LH, Song JL, Qian Y, Zhao X, Suo HY
and Li J: Increased preventive effect on colon carcinogenesis by
use of resistant starch (RS3) as the carrier for polysaccharide of
Larimichthys crocea swimming bladder. Int J Mol Sci. 15:817–829.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Wang Q, Li GJ, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese Bowl tea. J Funct Food.
7:590–598. 2014. View Article : Google Scholar
|
|
16
|
Zhao X, Suo HY, Qian Y, Li GJ, Liu ZH and
Li J: Therapeutic effects of Lactobacillus casei Qian treatment in
activated carbon-induced constipated mice. Mol Med Rep.
12:3191–3199. 2015.PubMed/NCBI
|
|
17
|
Wu LR, Gao GB, Bai RH, Shao Q and Li R:
Research and application of dietary fiber from bamboo shoots. J
Bamboo Res. 29:1–5+33. 2010.
|
|
18
|
Whitehead WE, Palsson OS and Simrén M:
Biomarkers to distinguish functional constipation from irritable
bowel syndrome with constipation. Neurogastroenterol Motil.
28:783–792. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian Y, Suo H, Du M, Zhao X, Li J, Li GJ,
Song JL and Liu Z: Preventive effect of Lactobacillus fermentum Lee
on activated carbon-induced constipation in mice. Exp Ther Med.
9:272–278. 2015.PubMed/NCBI
|
|
20
|
Liu Y, Zhao XR, Wang R, Qiu GQ and Zhang
M: Effect of Zhizhuwan on gastrointestinal peptide concentrations
in plasma of diabetic gastroenteropathy with constipation patients.
Zhongguo Zhong Yao Za Zhi. 33:2966–2968. 2008.(In Chinese).
PubMed/NCBI
|
|
21
|
Zhao X, Qian Y, Suo HY, Du MY, Li GJ, Liu
ZH, et al: Preventive effect of Lactobacillus fermentum Zhao on
activated carbon-induced constipation in mice. J Nutr Sci
Vitaminol. 61:131–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song HL, Tian FS, Qiao P and Duan HB:
Effects of Sanhuang tablet on plasma ET and NO levels in patients
with diabetic constipation. Chinese J Tradit Med Sci Technol.
17:285–286. 2010.
|
|
23
|
King SK, Sutcliffe JR, Ong SY, Lee M, Koh
TL, Wong SQ, Farmer PJ, Peck CJ, Stanton MP, Keck J, et al:
Substance P and vasoactive intestinal peptide are reduced in right
transverse colon in pediatric slow-transit constipation.
Neurogastroenterol Motil. 22:883–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suo H, Zhao X, Qian Y, Li G, Liu Z, Xie J
and Li J: Therapeutic effect of activated carbon-induced
constipation mice with Lactobacillus fermentum Suo on treatment.
Int J Mol Sci. 15:21875–21895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brading AF and McCloskey KD: Mechanisms of
disease: Specialized interstitial cells of the urinary tract-An
assessment of current knowledge. Nat Clin Pract Urol. 2:546–554.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geppetti P and Trevisani M: Activation and
sensitisation of the vanilloid receptor: Role in gastrointestinal
inflammation and function. Br J Pharmacol. 141:1313–1320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu GP, Fan YH and Lv B: Advances in
understanding the role of neurotrophins in physiological and
pathological processes in the intestinal tract. World Chinese J
Digestol. 18:2884–2888. 2010.
|
|
28
|
Tomita R, Igarashi S, Fujisaki S and
Tanjoh K: The effects of neurotensin in the colon of patients with
slow transit constipation. Hepatogastroenterology. 54:1662–1666.
2007.PubMed/NCBI
|