Introduction
In past two decades, the survival and discharge
rates following cardiac arrest (CA) with neurological sequelae
remain a clinical problem; for instance, the survival rate
following hospital admission was reported to be 25%, and was
<15% in the 5 years following resuscitation (1,2).
Mild therapeutic hypothermia (TH) is an effective
treatment approach for organ protection following CA (3,4).
However, this does not provide complete neuroprotection (5) and is still underutilized in CA
treatment (6), which necessitates
investigation into additional agents to improve clinical outcomes
when combined with TH. Increasing evidence suggests that TH
combined with other treatments may represent a helpful strategy to
provide better protection for the brain and other organs subsequent
to the return of spontaneous circulation (ROSC) (5,7–13). Fries et al (12) reported that during induction of mild
TH, even a short exposure to xenon provided significant
improvements in functional recovery and ameliorated myocardial
dysfunction in a pig model of CA. Meybohm et al (13) reported that a combination of TH and
sevoflurane postconditioning reduced myocardial and cerebral
damages following cardiopulmonary resuscitation (CPR). Therefore,
combined therapy of TH and other treatments appears to be a more
effective approach.
Emulsified isoflurane (EIso), the emulsified
preparation of isoflurane, in a recent study by the present
authors, was revealed to have neuroprotective effects on CA
(14). In this previous study, EIso
(4 ml/kg) improved survival rate, neural deficit score (NDS) and
memory function, and ameliorated hippocampal CA1 region cell death
and apoptosis at 7 days after ROSC. Furthermore, a phase I clinical
trial of Eiso was recently performed in West China Hospital
(Sichuan University, Chengdu, China), and the results demonstrated
that EIso had marked anesthetic potency and may be used safely in
patients (15). In addition, EIso
has many potential therapeutic applications for multiple-organ
protection in the heart (16–19),
kidneys (20), liver and lungs
(21) when induced by
ischemia/reperfusion (I/R) injury. Therefore, it was hypothesized
in our previous study (14) that
EIso combined with TH may produce additive efficacy compared with
either treatment alone following CPR. The present study was
designed to evaluate whether a combination of EIso and TH achieves
better survival and neurological outcomes compared with either
treatment alone.
Materials and methods
Animal and drug preparations
The present study was approved by the Institutional
Animal Care and Use Committee of Sichuan University. All animals
were maintained in compliance with the Guide for the Care and Use
of Laboratory Animals. A total of 65 adult male Sprague-Dawley rats
(age, 3 months) weighing 250–350 g were used (West China Animal
Breeding Centre of Sichuan University, China). These were
maintained at a room temperature of 27±2°C, with a 12 h light-dark
cycle. In accordance with a previous protocol (15), EIso was prepared in our laboratory.
Briefly, 18.4 ml 30% intralipid (Sino-Swed Pharmaceutical Corp.
Ltd., Beijing, China) and 1.6 ml liquid isoflurane (Abbott
Pharmaceutical Co., Ltd., Lake Bluff, IL, USA) were mixed in a
20-ml glass ampoule, which was sealed using an alcohol blowtorch.
The concentration of EIso (8%) was rechecked by gas chromatography
(4890 D; Agilent Technologies, Inc., Santa Clara, CA, USA) at the
beginning of experiments.
CA model
All animals were fasted overnight, but had free
access to water. All procedures were performed under sterilized
conditions. Rats were anesthetized with 10% chloral hydrate
(Sigma-Aldrich; Merck KGaA; 30 mg/100 g) intraperitoneally,
intubated with a 16-gauge cannula (B. Braun Melsungen AG,
Melsungen, Germany) and mechanically ventilated with a tidal volume
of 10 ml/kg, a respiratory rate of 60 breaths/min, and a fraction
of inspired oxygen (FiO2) of 0.21 for 10 min. The left
femoral artery and vein were cannulated with 24-gauge and 22-gauge
catheters, respectively. The mean arterial pressure (MAP) and heart
rate were monitored continuously by a BL-420E biological and
functional experiment recorder (Chengdu Technology Co., Ltd.,
Chengdu, China. The rat core temperature was maintained at 37±0.5°C
with a rectal temperature probe. Vecuronium (Yichang Humanwell
Pharmaceutical Co. Ltd., Yichang, China) (1 mg/kg/h, intravenously)
was used for immobilization and was administered prior to asphyxia.
After 6.0 min of asphyxiation (approximately 3.5 min of CA) without
ventilation, which was achieved by clamping the tracheal tube,
mechanical ventilation was restarted with 100% oxygen, at a rate of
80 breaths/min and a tidal volume of 10 ml/kg. Epinephrine (0.02
mg/kg; Yichang Humanwell Pharmaceutical Co., Ltd.) and sodium
bicarbonate (1 mg/kg; Yichang Humanwell Pharmaceutical Co., Ltd.)
were administered intravenously, followed by rapid manual chest
compressions until ROSC was achieved.
Experimental protocol
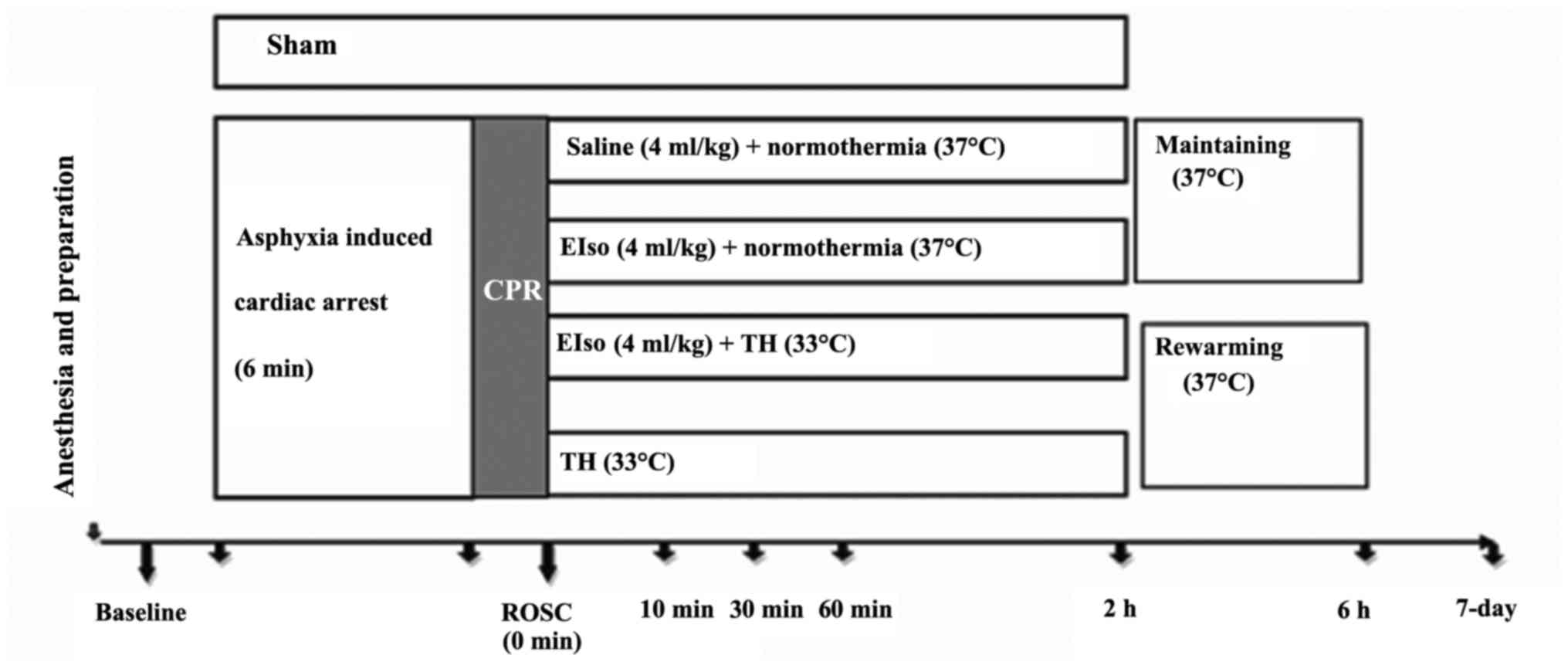
A total of 5 min after achieving ROSC (Fig. 1), rats were randomly assigned to five
groups, as follows: i) Sham group (sham-operated group; animals
were not subjected to ischemia/reperfusion, and no additional
medication was administered except Ringer's lactate solution); ii)
CPR, under normothermia (37°C); iii) TH (33°C for 2 h); iv) EIso
(intravenous 4 ml/kg EIso for 30 min under normothermia); and v)
EIso plus TH groups (intravenous 4 ml/kg EIso for 30 min under 33°C
for 2 h).
In animals assigned to the TH group, body cooling
was initiated 5 min after ROSC. Rectal temperature was reduced to
33°C within 10 min with the aid of ice packs and fans. Once
reached, the target temperature was maintained for 2 h and then
returned to 37°C at a rate at 1°C/h, similarly to a previous study
(22). Following a recovery period,
rats were weaned from the ventilator, all vascular catheters and
tracheal tubes were removed and surgical wounds were sutured.
Subsequent to extubation, rats were observed in FiO2 of
1.0 for 30 min, then returned to a chamber to breathe normal air
for 30 min. A cardiac rhythm with a MAP of >60 mmHg for a
minimum of 5 min was defined as successful ROSC. CPR was continued
unless the animal was either successfully resuscitated or
pronounced dead after a total of 15 min CPR.
The survival rate study used 65 rats; 9 of these
were excluded from subsequent analyses (4 rats did not achieve
successful ROSC; CA was not induced due to a technical failure in 4
rats; and an excessive EIso dose was administered in 1 rat).
Randomization occurred following ROSC. The 7-day survival rate and
a survival curve were evaluated for all five groups. All
evaluations were processed at 1 day prior to the operation
(baseline), and at 1 and 7 days after ROSC by one investigator, who
was blinded to the experimental groups.
NDS
NDS was determined using a scale from a previous
study (23). The neurological
function system consists of five components: Baseline metrics (body
weight, heart rate, body temperature), cranial nerve function,
motor function, sensory function, and coordination. A normal rat
has an NDS of 500, and a score of 0 is attained at mortality.
Cognitive function
The fear potentiated startle reflex test was used to
evaluate rats' cognitive function at 1 day before CPR (baseline), 1
day and 7 days (all measured in the same rats) after ROSC, in
accordance with a previous study (24). After a 5-min acclimation period,
noise bursts (startle stimuli) were applied at either 100 or 110
dB, lasting 50 msec each. These were imposed either alone or after
2.5 sec of light emittance, with the light continuing to 3 sec.
This provided a total of 9 trials. The extent to which each rat
jumped in response to the noise in the presence and absence of
light was measured by the average acceleration generated by the
jump over the 100 msec after initiation of the noise; cognitive
function was considered to be decreased when the average
acceleration of the startle was less than control group. Following
this test, rats were trained for potentiated startle. After 5-min
acclimation period, rats received 0.7 mA shocks to their feet,
delivered during the last 0.5 sec of a 3-sec light presentation.
The average light interval duplicated that of the above protocol.
Following this training, rats were tested for potentiated startle.
No shocks were given during testing. After a 5-min acclimation
period, rats received a startle stimulus (noise burst) alone or in
conjunction with a light presentation as in the matching protocol.
A greater response to noise plus light indicated potentiating,
i.e., that the rat had learned the association between the light
and a shock.
Histopathological analysis
At 7 days after ROSC, rats were perfused with
phosphate-buffered saline under anesthesia with 10% chloral
hydrate, followed by 4% paraformaldehyde. Subsequent to perfusion,
rats were decapitated; brains were removed carefully and postfixed
in formalin for 24–48 h. Following this, dehydration was performed
in graded concentrations of ethanol and butanol and
paraffin-embedded coronal sections (10-µm thick) were made at the
hippocampal level (approximately at the bregma, −3.0 mm). For the
detection of DNA fragmentation, terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) staining was
used as previously described (25).
At a magnification of 400x, the hippocampal CA-1 sector was
analyzed thoroughly by counting all TUNEL-positive cells (Vivien v.
1.2.2; CAST, Toronto, ON, Canada). For evaluations of the
proportion of viable neurons in the hippocampal CA-1 sector, brain
sections were stained with toluidine blue to mark Nissl bodies at
10-µm sections at the same level as the hippocampus. These
examinations were made by a pathologist who was blinded to the
experimental groups.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Homogeneity of variance was evaluated using Levene's test.
Physiological variables, duration of CA and the viable neuron count
were analyzed by one-way analysis of variance with least
significant difference correction for post hoc comparisons between
multiple experimental groups. Survival rate was compared by the
Kaplan-Meier method with a log-rank test. Statistical analysis was
performed using SPSS software 18.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to represent a statistically significant
difference.
Results
There were no differences amongst the 5 experimental
groups with regard to hemodynamics and arterial blood gases, as
reported in Table I. Times taken for
the CPR process, including CA, asphyxia and CPR time were not
significantly different (Table
II).
 | Table I.Physiological variables at the
baseline, 30, 60 and 120 min after the return of spontaneous
circulation. |
Table I.
Physiological variables at the
baseline, 30, 60 and 120 min after the return of spontaneous
circulation.
| Group | Time, mins | MAP, mmHg | HR, bpm | pH | PaCO2,
mmHg | LAC, mmol/l | BE, mmol/l | HCO3-,
mmol/l |
|---|
| CPR | Baseline | 95.6±19.6 | 367±59 | 7.34±0.49 | 35±8 | 1.1±0.5 | −6.9±2.7 | 18.9±2.0 |
|
| 30 | 72.9±14.7 | 351±46 | 7.29±0.07 | 41±12 | 3.1±0.7 | −10.4±2.4 | 16.0±2.3 |
|
| 60 | 73.2±16.5 | 365±31 | 7.28±0.06 | 46±7 | 2.5±0.3 | −6.0±2.5 | 19.1±2.2 |
|
| 120 | 72.3±17.2 | 351±48 | 7.32±0.07 | 42±13 | 2.4±0.6 | −5.8± 2.7 | 18.8±3.5 |
| EIso | Baseline | 97.9±17.8 | 362±72 | 7.33±0.03 | 34±3 | 1.7±0.6 | −7.5±2.6 | 18.6±1.7 |
|
| 30 | 71.3±14.4 | 359±43 | 7.24±0.08 | 35±10 | 3.7±1.4 | −11.5±3.6 | 15.3±2.3 |
|
| 60 | 75.4±16.9 | 364±49 | 7.23±0.09 | 47±13 | 2.5±1.0 | −7.6±2.5 | 17.6±2.0 |
|
| 120 | 73.8±13.8 | 351±39 | 7.32±0.12 | 46±11 | 2.3±0.4 | −5.6±3.4 | 17.8±3.1 |
| EIso+TH | Baseline | 101.6±14.2 | 341±56 | 7.35±0.04 | 34±6 | 1.0±0.3 | −6.1±2.2 | 19.6±1.9 |
|
| 30 | 72.6±13.6 | 323±30 | 7.33±0.09 | 43±17 | 3.2±1.5 | −9.7±2.3 | 16.0±1.7 |
|
| 60 | 78.3±14.3 | 366±42 | 7.25±0.06 | 47±8 | 2.6±0.4 | −6.1±2.7 | 18.4±1.9 |
|
| 120 | 74.2±12.7 | 361±32 | 7.28±0.11 | 44±12 | 2.3±0.4 | −5.7±2.5 | 18.0±2.7 |
| TH | Baseline | 104.9±15.6 | 371±58 | 7.31±0.08 | 32±4 | 1.2±0.3 | −6.2±3.8 | 19.1±3.0 |
|
| 30 | 74.5±13.9 | 342±35 | 7.27±0.09 | 36±12 | 4.1±1.5 | −12.5±3.5 | 15.2±2.4 |
|
| 60 | 75.6±11.2 | 359±33 | 7.28±0.06 | 48±16 | 2.5±0.2 | −7.5±2.1 | 17.7±1.8 |
|
| 120 | 75.2±13.5 | 341±43 | 7.26±0.10 | 45±12 | 2.3±0.6 | −5.5±3.6 | 17.3±4.5 |
 | Table II.Time taken for CA, asphyxia and ROSC,
measured in sec. |
Table II.
Time taken for CA, asphyxia and ROSC,
measured in sec.
| Group | CA | Asphyxia | ROSC |
|---|
| CPR | 243±46 | 117±42 | 76±27 |
| EIso | 238±40 | 125±34 | 81±36 |
| EIso+TH | 225±35 | 132±41 | 74±40 |
| TH | 228±31 | 130±38 | 75±24 |
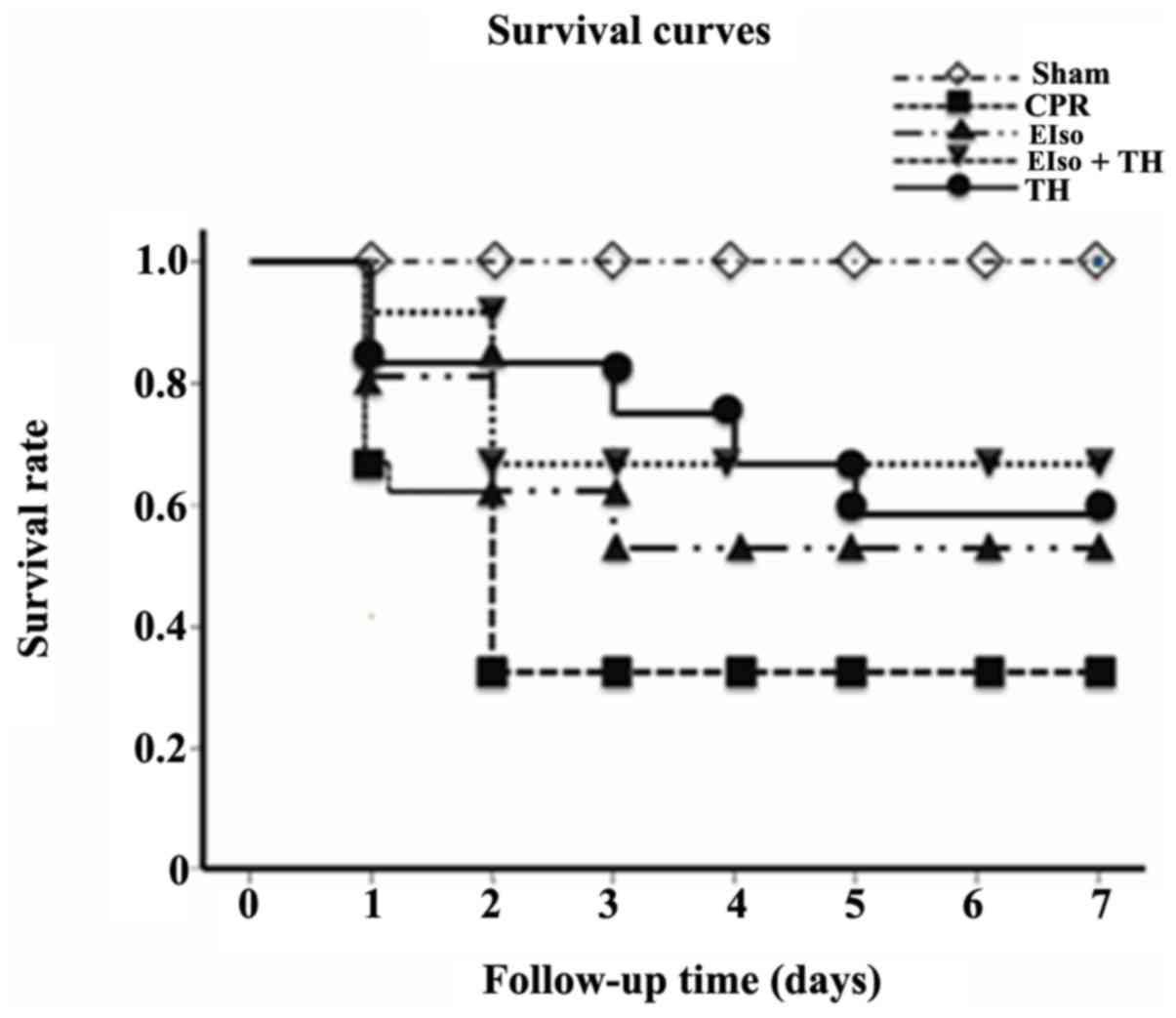
The survival rate at 7 days after ROSC was 4 of 13
rats (30.7%) in the control group, 6 of 10 rats (60%) in the EIso
group, 7 of 11 rats (63.6%) in the TH group and, 8 of 11 rats
(72.7%) in the EIso plus TH group (P<0.05 vs. control group;
Fig. 2).
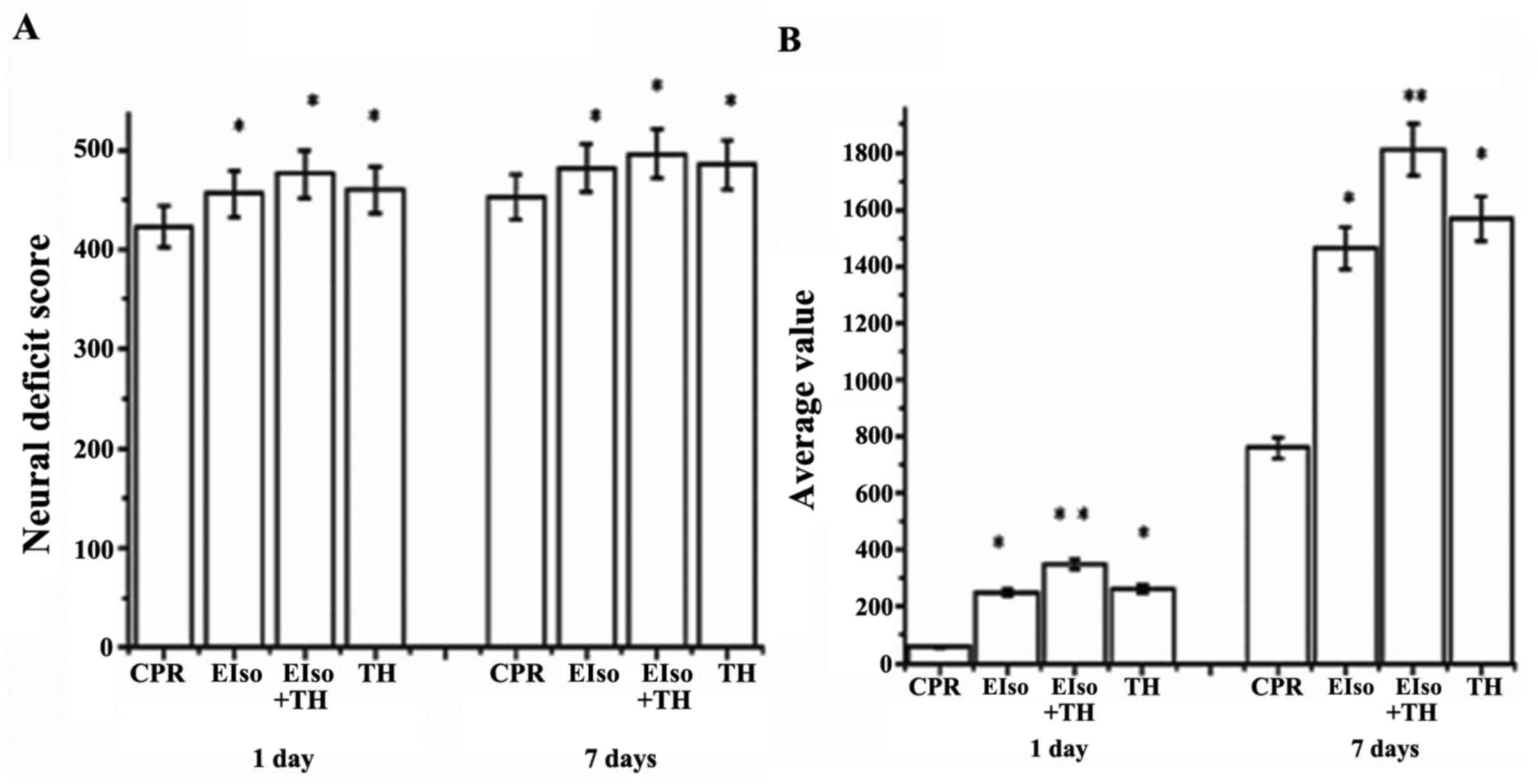
The NDS was evaluated at 1 and 7 days after ROSC.
NDS values at 1 and 7 days after ROSC were significantly higher in
the EIso (448±24, 469±34; P=0.014), TH (452±36, 482±32; P=0.035),
and EIso plus TH groups (467±40, 492±15; P=0.021) than in the CPR
group (414±32, 445±40) (Fig. 3A),
and the scores in the EIso plus TH group were significantly higher
than in the TH or EIso groups. Improved neurological outcomes after
EIso treatment at 1 and 7 days were consistent with our unpublished
work.
Rats were also subjected to a fear potentiated
startle reflex test to assess cognitive function at 1 and 7 days
after ROSC. The EIso, TH, and EIso plus TH groups had improved
cognitive function (528.2±316.2, P=0.004; 378.2±132, P=0.043; and
315.4±134, P=0.017, respectively) at 1 day and (1,834.5±432.2,
P=0.002; 1,638.2±332.4, P=0.025; and 1,416.4±234.7, P=0.031,
respectively) at 7 days after ROSC, compared with the CPR group
(84.5±32.2, 728.2±326.3), whereas these values in the EIso plus TH
group were higher than in the TH or EIso group (Fig. 3B).
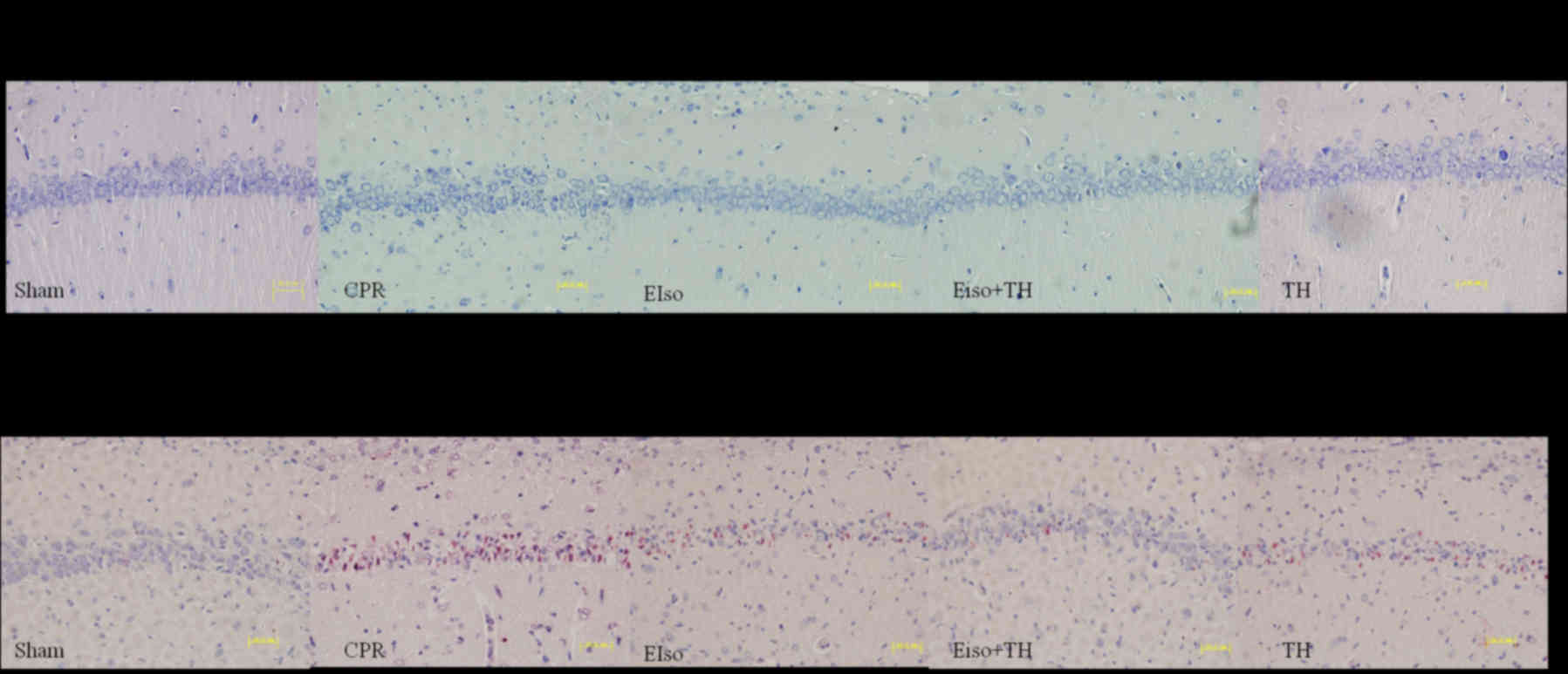
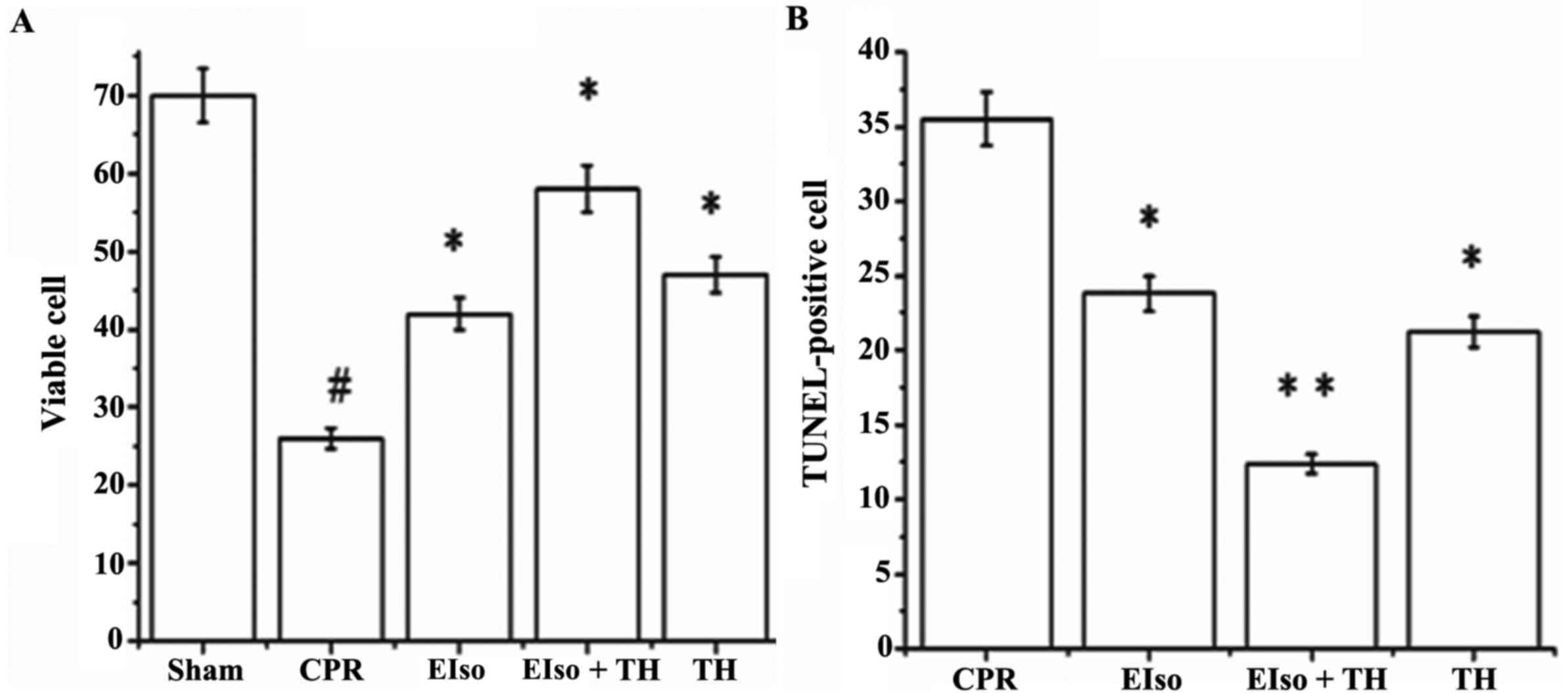
A transient duration of global cerebral
ischemia/reperfusion during CPR and following CA is associated with
delayed neuronal death in selectively vulnerable areas such as the
hippocampus (26). The percentage of
viable hippocampal CA-1 pyramid neurons (stained Nissl bodies) in
the CPR group was significantly less than in response to the three
treatment groups at 7 days after ROSC (P=0.016), whereas the
percentage of normal neurons in EIso plus TH was enhanced compared
with the EIso or TH groups (Figs. 4
and 5A). As reported in Figs. 4 and 5B, the number of TUNEL-positive cells in
the hippocampal CA-1 region was significantly lower in the EIso,
TH, and EIso plus TH groups compared with the CPR group (P=0.041
for EIso and TH; P=0.003 for EIso plus TH vs. control).
Furthermore, the number of TUNEL-positive cells was significantly
lower in the EIso plus TH group compared with the EIso group
(P=0.039), and TH group (P<0.05) but this was not significantly
different between EIso group and TH group (P>0.05). Combined
therapy of EIso and TH was therefore concluded to be superior to
EIso or TH alone.
Discussion
In the present study, a combination of EIso and TH
treatment had an additive effect in improving both survival and
neurological outcomes subsequent to ROSC in a rat model, and was
more effective than EIso or TH alone.
Neurological dysfunction resulting from CA
contributes to morbidity and mortality following initially
successful CPR (27,28). To date, TH is the only intervention
confirmed to improve neurological recovery (27). Despite this, the mortality rate of
patients admitted to critical care units after CA remain high, and
severe neurological sequelae are common among those who survive
(29,30). Previous studies have reported that TH
combined with other treatments is a helpful strategy to improve
survival rate and neurological outcomes subsequent to ROSC
(27). In the present study, the
combination of EIso and TH similarly demonstrated an additive
effect in improving survival and neurological outcomes following
ROSC.
The survival rate (72.7%) in the EIso plus TH group
at 7 days after ROSC was higher than that of the TH (63.6%) or EIso
(60%) groups alone, although this was significantly different. This
result was consistent with previous studies using a rat model of
6-minute CA (11,31). Ma et al (31) demonstrated that 4 of 10 animals
survived >72 h in a cannabinoid receptor agonist WIN55, 212-2
hypothermia group, and Hayashida et al (11) reported 77% (10/13) survival in an
H2 plus TH group after 72 h. Furthermore, the
experimental NDS and average value in the fear potentiated startle
reflex test at 1 and 7 days after ROSC in the EIso plus TH group
was also higher than that of the TH and EIso groups. As mentioned
above, average value is to reflect cognitive capability of rats,
and higher average value demonstrates better cognitive function.
Based on this theory, combined therapy of EIso plus TH was superior
to TH or EIso alone in improving neurological function and
cognitive function at 1 and 7 days after ROSC.
Neuronal degeneration in the hippocampal CA-1 region
was suppressed by both TH alone and EIso alone, and the combined
therapy of EIso plus TH was most effective. Brain histopathological
analysis revealed that the percentage of viable Nissl neurons at 7
days after ROSC in the EIso plus TH group was significantly
preserved in comparison to that in the TH and EIso groups, and
fewer TUNEL-stained apoptotic neurons were reported in the EIso
plus TH group than that in the in the TH and EIso groups.
Therefore, based on these data, a combination treatment of EIso and
TH demonstrates advantages in response to cerebral
ischemia/reperfusion injury compared with TH or EIso treatment
alone in a rat model of CA. These results were consistent with
previous studies in a rat of CA; at 7 days after ROSC, combined
therapy reduced the proportion of apoptotic cells and protected the
living neurons compared with other treatments (5,9–11,31). In
the current study, it was confirmed that the animals treated with
EIso after CPR retained significantly better brain functions than
the control group; this demonstrated that the combination of EIso
and TH showed an additive effect in improving both survival and
neurologic outcomes after ROSC. Combined therapy of TH with other
treatments appears the most effective approach. Furthermore, if the
protective effect of intravenous EIso combined with TH is confirmed
by additional, larger clinical trials, the combined therapy of EIso
and TH may represent a promising approach for induction of mild
hypothermia in or following CA.
The survival and neurologic outcomes after ROSC in
the EIso plus TH group were superior than in the TH alone and EIso
group alone. The additive effect suggested that both EIso and
hypothermia may share common mechanisms of neuroprotection,
eliminating reactive oxygen species generation and inhibiting the
expression of caspase-3, leading to cellular apoptosis and massive
autophagy in rats experiencing ischemia/reperfusion injury
(14,32–34).
In the present study and our previous unpublished
work, EIso was demonstrated to significantly ameliorate cellular
apoptosis and improve neuroprotection. There were a number of
reasons for no significant differences among three groups: First,
in the current model, EIso (4 ml/kg) was only administered for a
short, 0.5-h period but yielded an effect nonetheless. In our
previous unpublished work, although 4 ml/kg EIso was more effective
in terms of survival rate and neurological outcomes than 2 ml/kg
EIso, the dose-effect relationship between EIso and cerebral
ischemic protection requires additional exploration. Second, the
duration of this 3.5 min CA may be shorter than that experienced by
patients. However, it was hypothesized that lower brain neuron
injury would be observed under this short duration of CA, and that
EIso plus TH may still achieve an addictive effect. A potential
limitation is that a longer duration may have significant effects
(35), although the brain morphology
demonstrated significant differences between treatment and control
groups following a 3.5-min CA.
Previous experimental studies have suggested that
the benefit afforded by hypothermia is closely linked to the timing
of TH, specifically the rapidity in body temperature decrease
following resuscitation and rewarming rate (9–11). In
present study, TH at a target temperature of 33°C within 15 min
following CA and a rewarming rate at 1°C/h were typically reached
in this rat model (5,9–11,31). Lu
et al (22) demonstrated that
a rewarming rate at 0.5–1°C/h reduced the severity of myocardial
and cerebral injuries, and inflammatory reaction after CPR; a rapid
rewarming rate at 2°C/h abolished these beneficial effects. The
present findings included significantly attenuated short- and
long-term brain injury, and improved NDS and cognitive function
compared with the control group, which is consistent with previous
studies (9–11,22).
There is little doubt that hypothermia improves the outcomes of
CPR. It is also hypothesized that if the optimal strategies of
cooling and rewarming rate were used for this present study, an
increased benefit of EIso combined with TH may have been
achieved.
Patients receive TH treatment in hospital following
ROSC, and fentanyl and midazolam are used for prevention of pain,
vasoconstriction and shivering (14). However, mild hypothermia alters the
pharmacokinetics of these drugs and reduces the systemic clearance
of fentanyl and midazolam in rats subsequent to CA (36). Therefore, for CA patients who are
undergoing TH, midazolam may be administered as an overdose, which
is accompanied with delaying in awakening, prolonging mechanical
ventilation and other subsequent complications (14). Analgesic and sedative drugs combined
with TH should therefore have short half-life, which is more
beneficial and controllable. (14)
EIso was one such agent. A previous study has revealed that EIso
treatment enabled more easily controlled depth of anesthesia than
isoflurane inhalation for rapid anesthetic induction, and recovery
of anesthesia following intravenous EIso administration was faster
than following treatment with propofol (37). From the perspective of of TH
sedation, EIso may achieve improved results during TH in hospital
(in the intensive care unit or elsewhere) subsequent to CA.
Additional studies are required evaluate the clinical effects of
combined therapy.
It is believed that the current findings are notable
for three reasons. First, the present study demonstrates the
synergistic effects of these two interventions in a clinically
relevant animal model of cerebral ischemia/reperfusion injury using
CA and subsequent CPR. Secondly, for inhaled anesthetics, the need
for a vaporizer and associated equipment may limit its use in
out-of-hospital CA resuscitation. Furthermore, by circumventing the
anesthesia circuitry and the lung's functional residual capacity,
intravenous administration of volatile anesthetics may accelerate
the induction of anesthesia (38).
Intravenous EIso, may offset these limitations and provide rapid
anesthetic induction and recovery. Finally, beyond its
neuroprotective effects, EIso offers multiple-organ protection,
especially in the hemorrhagic shock model. For the synergistic
effects of these two interventions, EIso makes TH more
applicable.
There are several limitations of the present study.
Firstly, there are different metabolic and rheological properties
between the small rodent brain and the complex human brain.
Secondly, the results were achieved in healthy animals, which
preclude a direct translation to humans, many of whom have
comorbidities. Findings analogous to those from the rat CA model
are yet to be reported in larger animal and clinical studies.
In conclusion, the present results demonstrated that
in a clinically relevant, rat model of CA and CPR, a combination of
EIso and TH had an additive effect in improving both survival and
neurological outcomes following ROSC compared with EIso or TH
alone. In contrast to inhaled anesthetics, EIso provided more
benefits to utilize the advantages of TH therapy for CA
patients.
Acknowledgements
The present study was supported by a research grant
from the National Natural Science Foundation of China (no.
81101403).
References
|
1
|
Fishman GI, Chugh SS, Dimarco JP, Albert
CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X,
et al: Sudden cardiac death prediction and prevention: Report from
a national heart, lung and blood institute and Heart Rhythm Society
Workshop. Circulation. 122:2335–2348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu K and LaManna JC: The loss of hypoxic
ventilatory responses following resuscitation after cardiac arrest
in rats is associated with failure of long-term survival. Brain
Res. 1258:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernard SA, Gray TW, Buist MD, Jones BM,
Silvester W, Gutteridge G and Smith K: Treatment of comatose
survivors of out-of-hospital cardiac arrest with induced
hypothermia. N Engl J Med. 346:557–563. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hypothermia after Cardiac Arrest Study
Group, . Mild therapeutic hypothermia to improve the neurologic
outcome after cardiac arrest. N Engl J Med. 346:549–556. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang AY, Gonzalez FF, Sheldon RA and
Ferriero DM: Effects of combination therapy using hypothermia and
erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr
Res. 73:12–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merchant RM, Soar J, Skrifvars MB,
Silfvast T, Edelson DP, Ahmad F, Huang KN, Khan M, Hoek TL Vanden,
Becker LB and Abella BS: Therapeutic hypothermia utilization among
physicians after resuscitation from cardiac arrest. Crit Care Med.
34:1935–1940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batista LM, Lima FO, Januzzi JL Jr,
Donahue V, Snydeman C and Greer DM: Feasibility and safety of
combined percutaneous coronary intervention and therapeutic
hypothermia following cardiac arrest. Resuscitation. 81:398–403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolfrum S, Pierau C, Radke PW, Schunkert H
and Kurowski V: Mild therapeutic hypothermia in patients after
out-of-hospital cardiac arrest due to acute ST-segment elevation
myocardial infarction undergoing immediate percutaneous coronary
intervention. Crit Care Med. 36:1780–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai MS, Huang CH, Tsai CY, Chen HW, Cheng
HJ, Hsu CY, Chang WT and Chen WJ: Combination of intravenous
ascorbic acid administration and hypothermia after resuscitation
improves myocardial function and survival in a ventricular
fibrillation cardiac arrest model in the rat. Acad Emerg Med.
21:257–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Ohta S, Fukuda K and Hori S: Hydrogen inhalation during
normoxic resuscitation improves neurological outcome in a rat model
of cardiac arrest independently of targeted temperature management.
Circulation. 130:2173–2180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Maekawa Y, Kawamura A, Abe T, Ohta S, Fukuda K and Hori
S: H(2) gas improves functional outcome after cardiac arrest to an
extent comparable to therapeutic hypothermia in a rat model. J Am
Heart Assoc. 1:e0034592012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fries M, Brücken A, Çizen A, Westerkamp M,
Löwer C, Deike-Glindemann J, Schnorrenberger NK, Rex S, Coburn M,
Nolte KW, et al: Combining xenon and mild therapeutic hypothermia
preserves neurological function after prolonged cardiac arrest in
pigs. Crit Care Med. 40:1297–1303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meybohm P, Gruenewald M, Albrecht M,
Zacharowski KD, Lucius R, Zitta K, Koch A, Tran N, Scholz J and
Bein B: Hypothermia and postconditioning after cardiopulmonary
resuscitation reduce cardiac dysfunction by modulating
inflammation, apoptosis and remodeling. PLoS One. 4:e75882009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YJ, Wu MJ, Li Y and Yu H:
Cardiocerebral protection by emulsified isoflurane during
cardiopulmonary resuscitation. Med Hypotheses. 84:20–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang H, Li R, Liu J, Zhang W, Liao T and
Yi X: A phase I, dose-escalation trial evaluating the safety and
efficacy of emulsified isoflurane in healthy human volunteers.
Anesthesiology. 120:614–625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiari PC, Pagel PS, Tanaka K, Krolikowski
JG, Ludwig LM, Trillo RA Jr, Puri N, Kersten JR and Warltier DC:
Intravenous emulsified halogenated anesthetics produce acute and
delayed preconditioning against myocardial infarction in rabbits.
Anesthesiology. 101:1160–1166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao Y, Wang YL, Zhang WS and Liu J:
Emulsified isoflurane produces cardiac protection after
ischemia-reperfusion injury in rabbits. Anesth Analg.
106:1353–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu ZY, Luo NF and Liu J: The protective
effects of emulsifled isoflurane on myocardial ischemia and
reperfusion injury in rats. Can J Anaesth. 56:115–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Guo QL, Zhang Z, Long L and Yang Y:
Effect of emulsified isoflurane on apoptosis of
anoxia-reoxygenation neonatal rat cardiomyocytes. Asian Pac J Trop
Med. 6:977–981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin Z, Lv E, Zhan L, Xing X, Jiang J and
Zhang M: Intravenous pretreatment with emulsified isoflurane
preconditioning protects kidneys against ischemia/reperfusion
injury in rats. BMC Anesthesiol. 14:282014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Luo N, Liu J, Duan Z, Du G, Cheng
J, Lin H and Li Z: Emulsified isoflurane preconditioning protects
against liver and lung injury in rat model of hemorrhagic shock. J
Surg Res. 171:783–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu X, Ma L, Sun S, Xu J, Zhu C and Tang W:
The effects of the rate of postresuscitation rewarming following
hypothermia on outcomes of cardiopulmonary resuscitation in a rat
model. Crit Care Med. 42:e106–e113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Che D, Li L, Kopil CM, Liu Z, Guo W and
Neumar RW: Impact of therapeutic hypothermia onset, and duration on
survival, neurologic function and neurodegeneration after cardiac
arrest. Crit Care Med. 39:1423–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kandel L, Chortkoff BS, Sonner J, Laster
MJ and Eger EI II: Nonanesthetics can suppress learning. Anesth
Analg. 82:321–326. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan H, Chen D, Liu B, Xie X, Zhang J and
Yang G: Effects of sodium hydrosulfide on intestinal mucosal injury
in a rat model of cardiac arrest and cardiopulmonary resuscitation.
Life Sci. 93:24–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou C, Huang H, Liu J, Wang X, Chen X and
Zhang W: Emulsified isoflurance increases convulsive thresholds of
lidocaine and produces neural protection after convulsion in rats.
Anesth Analg. 118:310–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oddo M, Ribordy V, Feihl F, Rossetti AO,
Schaller MD, Chioléro R and Liaudet L: Early predictors of outcome
in comatose survivors of ventricular fibrillation and
non-ventricular fibrillation cardiac arrest treated with
hypothermia: A prospective study. Crit Care Med. 36:2296–2301.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peberdy MA, Callaway CW, Neumar RW,
Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM,
Zaritsky AL, Merchant R, et al: Part 9: Post-cardiac arrest care:
2010 American Heart Association Guidelines for cardiopulmonary
resuscitation and emergency cardiovascular care. Circulation.
122(18): Suppl 3. S768–S786. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arrich J, Holzer M, Havel C, Müllner M and
Herkner H: Hypothermia for neuroprotection in adults after
cardiopulmonary resuscitation. Cochrane Database Syst Rev.
2:CD0041282016.PubMed/NCBI
|
|
30
|
Nielsen N, Friberg H, Gluud C, Herlitz J
and Wetterslev J: Hypothermia after cardiac arrest should be
further evaluated-a systematic review of randomised trials with
meta-analysis and trial sequential analysis. Int J Cardiol.
151:333–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma L, Lu X, Xu J, Sun S and Tang W:
Improved cardiac and neurological outcomes with postresuscitation
infusion of cannabinoid receptor agonist WIN55, 212-2 depend on
hypothermia in a rat model of cardiac arrest. Crit Care Med.
42:e42–e48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu ZY and Liu J: Effects of emulsified
isoflurane on haemodynamics and cardiomyocyte apoptosis in rats
with myocardial ischaemia. Clin Exp Pharmacol Physiol. 36:776–783.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu J, Shen Y, Qian HY, Liu LJ, Zhou BC,
Xiao Y, Mao JN, An GY, Rui MZ, Wang T and Zhu CL: Effects of mild
hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of
hippocampus nerve cells in rats after cardiopulmonary
resuscitation. World J Emerg Med. 5:298–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soleimanpour H, Rahmani F, Safari S and
Golzari SE: Hypothermia after cardiac arrest as a novel approach to
increase survival in cardiopulmonary cerebral resuscitation: A
review. Iran Red Crescent Med J. 16:e174972014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Idris AH, Becker LB, Ornato JP, Hedges JR,
Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR,
et al: Utstein-style guidelines for uniform reporting of laboratory
CPR research. A statement for healthcare professionals from a task
force of the American Heart Association, the American College of
Emergency Physicians, the American College of Cardiology, the
European Resuscitation Council, the Heart and Stroke Foundation of
Canada, the Institute of Critical Care Medicine, the Safar Center
for Resuscitation Research and the Society for Academic Emergency
Medicine. Circulation. 94:2324–2336. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Empey PE, Miller TM, Philbrick AH, Melick
JA, Kochanek PM and Poloyac SM: Mild hypothermia decreases fentanyl
and midazolam steady-state clearance in a rat model of cardiac
arrest. Crit Care Med. 40:1221–1228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou JX, Luo NF, Liang XM and Liu J: The
efficacy and safety of intravenous emulsified isoflurane in rats.
Anesth Analg. 102:129–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu ZY, Abbott GW, Fang YD, Huang YS and
Liu J: Emulsified isoflurane postconditioning produces
cardioprotection against myocardial ischemia-reperfusion injury in
rats. J Physiol Sci. 63:251–261. 2013. View Article : Google Scholar : PubMed/NCBI
|