Introduction
Infections caused by carbapenem-resistant
Klebsiella pneumoniae (CRKP) have been a serious problem due
to limited therapeutic options all around the world (1). Few optimal therapy strategy are
available for the infections, making its treatments extremely
difficult and leading to poor therapeutic outcomes with the
reported mortality rates ranging from 39–72% (2–4). The
core issue of managing CRKP infection is to find an effective
antibiotic regimen, as carbapenem resistance is often accompanied
by resistance to other families of first-line antibiotics, such as
beta-lactam inhibitors, quinolones and 3rd/4th generation
cephalosporin. Optional antibiotics are usually limited to
polymyxins, aminoglycosides, gentamicin, colistin and tigecycline
(5,6).
Recently, a study reported that treatment with
tigecycline or an aminoglycoside can generate positive outcomes
(7). In addition, there are reports
indicating that the use of carbapenems in combination with other
active agents could contribute to a lower mortality, particularly
when the strains have low levels of in vitro resistance to
those antimicrobials (8).
Furthermore, a number of reports suggest that combinational
therapies are often more effective than monotherapies (9,10).
Therefore, the goal of this retrospective study was to analyze the
effect of combinational therapy of fosfomycin and carbapenemase on
mortality from sepsis due to CRKP.
Patients and methods
Patients
The retrospective study was conducted in a teaching
hospital of Tongji University (Shanghai, China). From January 2012
to November 2014. All CRKP isolates were collected from the
database of the Microbiology Service of the Shanghai Tenth People's
Hospital of Tongji University. The clinical records of patients
with CRKP isolates were reviewed. The enrollment criteria were as
follows: i) Clinical evidence of sepsis, severe sepsis or septic
shock due to CRKP; ii) meropenem or imipenem were used within 72 h
after CRKP cultured from urine, blood, phlegm or drainage liquid;
iii) the administration of meropenem therapy lasted for at least 72
h; and iv) >18 years old.
Admission to intensive care unit (ICU) was defined
as a stay of 24 h before or after diagnosis of the severe sepsis.
Predictors of the primary outcomes included age, sex, underlying
diseases [diabetes mellitus, history of chronic obstructive
pulmonary disease (COPD), heart failure, hepatic failure, renal
failure, gastrointestinal surgery, solid malignancies, hematologic
malignancies], special treatments (central venous catheter,
peripheral catheter, foley catheter, Nasogastric tube, mechanical
ventilation ≥3 days, gastric acidity-lowering agents,
immunosuppressive therapy, total parenteral nutrition), prior use
of antibiotics and bacteremia. Clinical outcome contained length of
ICU stay, mortality or cure, and duration of mechanical
ventilation. The patients with recurrent CRKP isolated from the
blood or urine were defined as microbiological failure at least 7
days after their index culture. Patients who had a negative culture
were defined as treatment success.
Statistical analysis
Quantitative variables are expressed as the mean ±
standard deviation and qualitative variables are depicted as
percentages of the group to which they belonged. Differences
between patients treated with fosfomycin and meropenem and other
antibiotics were analyzed by univariate logistic regression. Risk
factors correlated with clinical outcome and microbiological
failure was analyzed using stepwise multiple logistic regression
analyses. The odds ratio (OR) was calculated with 95% confidence
interval (CI). All statistical tests were two-tailed and utilized a
0.05 significance level. Analyses were performed using SPSS
software, version 20.0 (IBM SPSS, Armonk, NY, USA).
Results
Clinical characteristics of the
patients
A total of 104 unique cases of bacteremia with CRKP
were identified during the experimental period. The demographics
and clinical characteristics of the cases are shown in Table I. The ages of the 104 patients, 79
male and 25 female, ranged from 28 to 95 years, with a median of
67.2 years. The majority of the infections (84; 80.8%) were
hospital acquired. All but one patient had been admitted to the
hospital within a year prior to the episode of bacteremia, with a
majority of them (87; 83.7%) having been admitted to an ICU.
Patients that received ineffective empirical antimicrobial therapy
before the susceptibility results became available are in the
majority and 80 patients (76.9%) received mechanical ventilation.
The majority of patients had underlying diseases, had undergone
invasive procedures, and had been admitted to ICUs or received
medical services.
 | Table I.Baseline characteristics of 104
patients with severe infection caused by carbapenem-resistant
Klebsiella pneumoniae. Univariate analysis of factors
associated with clinical outcome, N (%). |
Table I.
Baseline characteristics of 104
patients with severe infection caused by carbapenem-resistant
Klebsiella pneumoniae. Univariate analysis of factors
associated with clinical outcome, N (%).
| Demographic
variables | Total (104) | Mortality (26) | Survivors (78) | P-value | OR (95% CI) |
|---|
| Age (mean ± SD) |
67.2±15.7 |
68.4±15.5 |
66.8±15.9 |
0.641 |
|
| Gender |
|
|
|
|
|
| Male | 79
(76.0) | 16
(61.5) | 63
(80.8) |
0.047 | 2.63
(1.00–6.93) |
|
Female | 25
(24.0) | 10
(38.5) | 15
(19.2) |
|
|
| Underlying
disease |
|
|
|
|
|
| Diabetes
mellitus | 43
(41.3) | 13
(50.0) | 30
(38.5) |
0.301 | 0.63 (0.26–1.53) |
| History
of COPD | 29
(27.9) | 12
(46.2) | 17
(21.8) |
0.016 | 0.32
(0.13–0.83) |
| Heart
failure | 24
(23.1) | 8
(30.8) | 16
(20.5) |
0.282 | 0.58 (0.21–1.58) |
| Hepatic
failure | 7
(1.9) | 3
(11.5) | 4
(5.1) |
0.363 | 0.41
(0.09–1.99) |
| Renal
failure | 24
(23.1) | 8
(30.8) | 16
(20.5) |
0.293 | 0.58 (0.21–1.58) |
|
Gastrointestinal surgery | 9
(8.7) | 3
(11.5) | 6
(7.7) |
0.687 | 0.64 (0.15–2.76) |
| Solid
malignancies | 14
(13.5) | 4
(15.4) | 10
(12.8) |
0.746 | 0.81
(0.23–2.84) |
|
Hematologic malignancies | 0 (0) | 0 (0) | 0 (0) |
|
|
| Special treatments
total |
|
|
|
|
|
| Central
venous catheter | 72
(69.2) | 17
(65.4) | 55
(70.5) |
0.624 | 1.27
(0.49–3.25) |
|
Peripheral catheter | 43
(41.3) | 18
(69.2) | 25
(32.1) |
0.001 | 0.21
(0.08–0.55) |
| Foley
catheter | 86
(82.7) | 21
(80.8) | 65
(83.3) |
0.765 | 1.19
(0.38–3.73) |
|
Nasogastric tube | 78
(75.0) | 22
(84.6) | 56
(71.8) |
0.295 | 0.46
(0.14–1.50) |
|
Mechanical ventilation ≥3
days | 70
(68.0) | 19
(73.1) | 51
(66.2) |
0.518 | 0.72
(0.27–1.94) |
| Gastric
acidity-lowering agents | 98
(94.2) | 25
(96.2) | 73
(93.6) | 1.00 | 0.58
(0.07–5.24) |
|
Immunosuppressive therapy | 29
(27.9) | 4
(15.4) | 25
(32.1) |
0.132 | 2.59
(0.81–8.33) |
| Total
parenteral nutrition | 44
(42.3) | 10
(38.5) | 34
(43.6) |
0.647 | 1.24
(0.50–3.06) |
| Prior use of
antibiotics |
|
|
|
|
|
|
β-lactam inhibitors | 52
(50.0) | 17
(65.4) | 35
(44.9) |
0.070 | 0.43
(0.17–1.08) |
|
Quinolones | 42
(40.4) | 14
(53.8) | 28
(35.9) |
0.106 | 0.48
(0.20–1.18) |
| 3rd/4th
Generation cephalosporin | 72
(69.2) | 17
(65.4) | 55
(70.5) |
0.624 | 1.27
(0.49–3.25) |
|
Aminoglycosides | 21
(20.6) | 2
(7.7) | 19
(25.0) |
0.060 | 4.0
(0.86–18.53) |
|
Fosfomycin | 6
(5.8) | 0 (0) | 6
(7.7) |
0.333 | 0.74
(0.65–0.83) |
|
Imipenem | 14
(13.5) | 1
(3.8) | 13
(16.7) |
0.097 | 5.00
(0.62–40.25) |
| Type of
infection |
|
|
|
|
|
|
CAP | 28
(26.9) | 6
(23.1) | 22
(28.2) |
0.610 | 1.31
(0.46–3.69) |
|
HAP | 84
(80.8) | 21
(80.8) | 63
(80.8) |
1.000 | 1.00
(0.32–3.08) |
| Urinary
tract infection | 17
(16.3) | 5
(19.2) | 12
(15.4) |
0.646 | 0.76
(0.24–2.42) |
|
Surgical site infection | 11
(10.6) | 2
(7.7) | 9
(11.5) |
0.581 | 1.57
(0.32–7.76) |
|
Intra-abdominal infecton | 4
(3.8) | 1
(3.8) | 3
(3.8) |
1.000 | 1.04
(1.00–1.08) |
| Primary
bacteraemia | 9
(8.7) | 3
(11.5) | 6
(7.7) |
0.546 | 0.64
(0.15–2.76) |
| Central
venous catheterbacteraemia | 0 (0) | 0 (0) | 0 (0) |
|
|
|
Ventilator associated
pneumonia | 1
(1.0) | 0 (0) | 1
(1.3) |
1.000 | 0.75
(067–0.83) |
| Targeted
treatment |
|
|
|
|
|
|
Monotherapy | 32
(30.8) | 11
(42.3) | 21
(26.9) |
0.141 | 0.50
(0.20–1.27) |
|
Combination therapy | 72
(69.2) | 15
(57.7) | 57
(73.1) |
|
|
|
Fosfomycin combination | 24
(23.1) | 2
(7.7) | 22
(28.2) |
0.034 | 4.71
(1.03–21.65) |
| Other
treatment regimens | 65
(61.9) | 16
(24.6) | 49
(75.4) |
| Length of ICU
stays |
|
15.2±10.5 |
17.6±12.2 |
0.355 |
|
| Duration of
mechanical ventilation |
|
10.7±10.6 |
10.9±10.9 |
0.958 |
|
Antimicrobial susceptibility
Colistin exerted the highest susceptibility rate
with 93.3% (Table II). Tigecycline
and minocyline were active against 68 (65.4%) and 79 (76.5%)
isolates, respectively. With regard to fosfomycin, 40 isolates
(38.5%) were susceptible, 54 (51.9%) showed intermediate
susceptibility and 10 (9.6%) were resistant. A less marked
susceptibility to amikacin (28, 26.9%) and gentamicin (14, 13.5%)
was observed.
 | Table II.Antimicrobial susceptibility test
result of 104 patients with severe infection caused by
carbapenem-resistant Klebsiella pneumoniae. |
Table II.
Antimicrobial susceptibility test
result of 104 patients with severe infection caused by
carbapenem-resistant Klebsiella pneumoniae.
| Drug | Sensitive | Intermediary | Resistance |
|---|
| Tigecycline | 68 (65.4) | 1 (1.0) | 35
(33.7) |
| Minocyline | 79 (76.0) | 17 (16.3) | 8
(7.7) |
| Colistin | 97 (93.3) | 0 (0) | 7
(6.7) |
| Gentamicin | 14 (13.5) | 0 (0) | 91
(86.5) |
| Amikacin | 28 (26.9) | 0 (0) | 76
(73.1) |
| Meropenem | 1 (1.00 | 0 (0) | 103 (99.0) |
| Imipenem | 1 (1.00 | 0 (0) | 103 (99.0) |
| Ertapenem | 1 (1.00 | 0 (0) | 103 (99.0) |
| Cefepime | 9 (8.7) | 0 (0) | 95
(91.3) |
| Fosfomycin | 40 (38.5) | 54 (51.9) | 10 (9.6) |
Antibiotic treatment
All patients received targeted antibiotic treatment.
Targeted treatment was optimal in 78 patients (75.0%). The targeted
antibiotics used were shown in Table
III. A total of 10 patients were infected with a
fosfomycin-resistant CRKP strain. This combination treatment group
consisted of 24 patients (23.1%) that were administered a dose of
12 mg/24 h and were not administered fosfomycin as monotherapy. In
the fosfomycin combinational therapy group, 16 patients were
administered meropenem (1 g every 8 h) following with fosfomycin
with the other 8 patients received fosfomycin, meropenem,
tigecycline or (and) minocyline. Those patients received fosfomycin
combination were less likely to fail therapy (OR: 4.71, 95% CI:
1.03–21.65, P=0.030). However, no difference between length of ICU
stays and duration of mechanical ventilation was observed in these
two groups (Table I). The primary
characteristics of the patients who received fosfomycin
combinational therapy are shown in Table IV. According to the result, the
number of patients that underwent prior use of minoglycosides and
carbapenems differed between the two groups. Compared with other
regimens (10.2±11.0 vs. 13.0±11.2), fosfomycin combination group
tends to have shorter duration of mechanical ventilation, however
the difference did not display statistical significance. An
interesting thing is that the age of fosfomycin combinational
therapy group is older than other treatments group (68.7±14.6 vs.
62.0±18.3, P=0.065), which might give us a hint that fosfomycin
combinational therapy is safer and more suitable to be applicable
to aged people. The fosfomycin combinational therapy group has an
overall mortality of 8.3% with one case died of pneumonia and
another died of the infection of the central nervous system
(Table V).
 | Table III.Target antibiotics used in 104
patients with severe infection caused by carbapenem-resistant
Klebsiella pneumoniae, N (%). |
Table III.
Target antibiotics used in 104
patients with severe infection caused by carbapenem-resistant
Klebsiella pneumoniae, N (%).
| Target
antibiotic | Total | Mortality | Survivors |
|---|
| Target
monotherapy | 32 (30.8) | 11 (42.3) | 21 (26.9) |
|
Pipercillin/sulbactam | 4
(12.5) | 1 (9.1) | 3
(14.3) |
|
Aminoglycosides | 5
(15.6) | 1 (9.1) | 4
(19.0) |
| 3rd/4th
Generation cephalosporin | 13 (40.6) | 6
(54.5) | 7
(33.3) |
|
Quinolones | 4
(12.5) | 0 (0) | 0 (0) |
|
Meropenem | 7
(21.9) | 3
(27.3) | 4
(19.0) |
|
Imipenem | 1 (3.1) | 0 (0) | 1 (4.8) |
|
Tigecycline | 1 (3.1) | 0 (0) | 1 (4.8) |
|
Minocyline | 1 (3.1) | 0 (0) | 1 (4.8) |
| Target
combination therapy | 72 (69.2) | 15 (57.7) | 57 (73.1) |
|
Fosfomycin+meropenem | 16 (19.4) | 1 (6.7) | 15 (24.6) |
|
Fosfomycin+meropenem+Tigecycline/minocyline/amikacin | 8
(11.1) | 1 (6.7) | 7
(12.2) |
|
Tigecycline+meropenem | 4 (5.6) | 1 (5.3) | 3 (1.2) |
|
Tigecycline+meropenem+amikacin | 6 (8.3) | 1 (5.3) | 5 (8.8) |
|
Tigecycline+minocyline | 3 (4.2) | 0 (0) | 3 (5.3) |
|
Tigecycline+minocyline+meropenem | 1 (1.4) | 0 (0) | 1 (1.8) |
|
Minocyline+carbapenems | 4 (5.6) | 2
(13.3) | 2 (3.5) |
|
Minocyline+pipercillin/sulbactam | 1 (1.4) | 1 (5.3) | 0 (0) |
|
Gentamicin+meropenem | 2 (2.8) | 0 (0) | 2 (3.5) |
|
Amikacin+meropenem | 3 (4.2) | 0 (0) | 3 (5.3) |
|
Amikacin+cefepime | 3 (4.2) | 0 (0) | 3 (5.3) |
|
Amikacin+pipercillin/sulbactam | 1 (1.4) | 0 (0) | 1 (1.8) |
|
Cefepime+amoxicillin/sulbactam | 1 (1.4) | 0 (0) | 1 (1.8) |
|
Cefepime+pipercillin/sulbactam | 1 (1.4) | 0 (0) | 1 (1.8) |
|
Cefepime+sulfamethoxazole | 2 (2.8) | 2
(13.3) | 0 (0) |
|
Cefepime+aztreonam | 1 (1.4) | 0 (0) | 1 (1.8) |
|
Cefoperazone/sulbactam+carbapenems | 4 (5.6) | 0 (0) | 4 (7.0) |
|
Cefoperazone/sulbactam+quinolones | 2 (2.8) | 1 (5.3) | 1 (1.8) |
|
Carbapenems+quinolones | 5 (6.9) | 3
(20.0) | 2 (3.5) |
|
Pipercillin/sulbactam+carbapenems | 4 (5.6) | 2
(13.3) | 2 (3.5) |
 | Table IV.Analysis of clinical variables in 24
patients that received fosfomycin combination therapy, N (%). |
Table IV.
Analysis of clinical variables in 24
patients that received fosfomycin combination therapy, N (%).
| Demographic
variable | Total | Fosfomycin
combination (n=24) | Other regimens
(n=80) | P | OR (95% CI) |
|---|
| Age (mean ±
SD) | 67.2±15.7 | 68.7±14.6 | 62.0±18.3 | 0.065 |
|
| Gender |
|
|
|
|
|
|
Male | 79 (76.0) | 20 (83.3) | 59 (73.8) | 0.422 | 1.78
(0.55–5.81) |
|
Female | 25 (24.0) | 4
(16.7) | 21 (26.2) |
|
|
| Underlying
disease |
|
|
|
|
|
|
Diabetes mellitus | 43 (41.3) | 6
(25.0) | 37 (46.2) | 0.064 | 0.39
(0.14–1.08) |
| History
of COPD | 29 (27.9) | 4
(16.7) | 25 (31.2) | 0.201 | 0.44
(0.14–1.42) |
| Heart
failure | 24 (23.1) | 2 (8.3) | 22 (27.5) | 0.057 | 0.24
(0.05–1.11) |
| Hepatic
failure | 7 (6.0) | 0 (0) | 7 (8.8) | 0.197 | 0.75
(0.67–0.84) |
| Renal
failure | 24 (23.1) | 3
(12.5) | 21 (26.2) | 0.268 | 0.40
(0.11–1.49) |
|
Gastrointestinal surgery | 9 (8.7) | 1 (4.2) | 8
(10.0) | 0.681 | 0.39
(0.05–3.30) |
| Solid
malignancies | 14 (13.5) | 2 (8.3) | 12 (15.0) | 0.513 | 0.52
(0.11–2.48) |
|
Hematologic malignancies | 0 (0) | 0 (0) | 0 (0) |
|
|
| Special treatments
total |
|
|
|
|
|
| Central
venous catheter | 72 (69.2) | 17 (70.8) | 55 (68.8) | 0.846 | 1.10
(0.41–3.00) |
|
Peripheral catheter | 43 (41.3) | 9
(37.5) | 34 (42.5) | 0.663 | 0.81
(0.32–2.07) |
| Foley
catheter | 86 (82.7) | 18 (75.0) | 68 (85.0) | 0.256 | 0.53
(0.18–1.61) |
|
Nasogastric tube | 78 (75.0) | 17 (70.8) | 61 (76.2) | 0.591 | 0.76
(0.27–2.10) |
|
Mechanical ventilation ≥3
days | 70 (68.0) | 15 (62.5) | 55 (68.0) | 0.513 | 0.73
(0.28–1.90) |
| Gastric
acidity-lowering agents | 98 (94.2) | 23 (95.8) | 75 (93.8) | 1.00 | 1.53
(0.17–13.80) |
|
Immunosuppressive therapy | 29 (27.9) | 10 (41.7) | 19 (43.7) | 0.086 | 2.29
(0.88–6.00) |
| Total
parenteral nutrition | 44 (42.3) | 13 (54.2) | 31 (38.8) | 0.139 | 1.80
(0.74–4.69) |
| Prior use of
antibiotics |
|
|
|
|
|
|
β-lactam inhibitors | 52 (50.0) | 15 (62.5) | 37 (46.2) | 0.163 | 1.94
(0.76–4.94) |
|
Quinolones | 42 (40.4) | 7
(29.2) | 35 (43.8) | 0.202 | 0.53
(0.20–1.42) |
| 3rd/4th
Generation cephalosporin | 58 (55.2) | 23 (57.5) | 35 (53.8) | 0.715 | 1.16
(0.52–2.57) |
|
Aminoglycosides | 21
(20.6) | 10
(45.5) | 11
(13.8) | 0.001 | 5.23
(1.82–15.00) |
|
Fosfomycin | 6 (5.8) | 3
(12.5) | 3 (3.8) | 0.134 | 3.67
(0.6–19.51) |
|
Carbapenems | 14
(13.5) | 8
(33.3) | 6 (7.5) | 0.001 | 6.17
(1.88–20.24) |
| Type of
infection |
|
|
|
|
|
|
CAP | 28 (25.7) | 6
(22.5) | 22 (27.7) | 0.809 | 0.88
(0.31–2.50) |
|
HAP | 84 (80.8) | 19 (79.2) | 65 (81.2) | 0.820 | 0.88
(0.28–2.73) |
| Primary
bacteraemia | 9 (8.7) | 4
(16.7) | 5 (6.2) | 0.206 | 3.00
(0.74–12.22) |
| Central
venous catheter bacteraemia | 0 (0) | 0 (0) | 0 (0) |
|
|
|
Ventilator associated
pneumonia | 1 (1.0) | 1 (4.2) | 0 (0) | 0.381 | 1.03
(0.98–1.08) |
| Urinary
tract infection | 17 (16.3) | 3
(12.5) | 14 (17.5) | 0.756 | 0.67
(0.18–2.57) |
|
Surgical site infection | 11 (10.6) | 4
(16.7) | 7 (8.8) | 0.273 | 2.09
(0.56–7.84) |
|
Intra-abdominal infecton | 4 (3.8) | 1 (4.2) | 3 (3.8) | 1.000 | 1.12
(0.11–11.25) |
| Length of ICU
stays |
| 17.4±11.1 | 15.6±14.2 | 0.515 |
|
| Duration of
mechanical ventilation |
| 10.2±11.0 | 13.0±11.2 | 0.264 |
|
 | Table V.Characteristics of patients with
severe infection caused by carbapenem-resistant Klebsiella
pneumoniae treated with targeted fosfomycin combination;
N=24. |
Table V.
Characteristics of patients with
severe infection caused by carbapenem-resistant Klebsiella
pneumoniae treated with targeted fosfomycin combination;
N=24.
| Characteristic | N (%) |
|---|
| Overall
mortality | 2/24 (8.3) |
| Mortality by
infection site |
|
|
Pneumonia | 1/24 |
| Urinary
tract infection | 0/24 |
|
Surgical wound infection | 0/24 |
|
Intra-abdominal infection | 0/24 |
|
Catheter-related
bacteraemia | 0/24 |
|
Infection of the CNS | 1/24 (4.2) |
| Treatment
regimens |
|
|
Fosfomycin+meropenem | 16/24 (66.7) |
|
Fosfomycin+meropenem+tigecycline/minocyline/amikacin | 8/24
(33.3) |
Taking the difference in prior use of
aminoglycosides into consideration, the effect of fosfomycin in
combination with meropenem and with other regimens treatment was
compared (Table VI). This
combinational therapy group has 16 patients. Compared with other
therapy, a significant difference was observed (Table VI, P=0.010) when compared with
treatment by using 3rd/4th generation (monotherapy or combinational
therapy).
 | Table VI.Comparison of patients treated with
fosfomycin combination therapy against other treatment regimens, N
(%). |
Table VI.
Comparison of patients treated with
fosfomycin combination therapy against other treatment regimens, N
(%).
| Treatment | Total (104) | Mortality (26) | Survival (78) | P | OR (95% CI) |
|---|
|
Fosfomycin+meropenem | 16 (15.4) | 1 (3.8) | 22 (28.2) |
|
|
| Monotherpy and in
combination |
|
|
|
|
|
|
Tigecycline/minocyline | 21 (20.2) | 2 (7.7) | 19 (24.4) | 0.371a | 0.26
(0.04–1.50) |
|
Aminoglycosides | 12 (11.5) | 1 (3.8) | 11 (14.1) | 1.000a | 0.73
(0.04–13.05) |
|
β-lactam inhibitors | 8 (7.7) | 2 (7.7) | 6 (7.7) | 0.249a | 0.14
(0.03–0.75) |
|
Quinolones | 8 (7.7) | 2 (7.7) | 6 (7.7) | 0.249a | 0.20
(0.02–2.64) |
| 3rd/4th
Generation | 21 (20.2) | 10 (38.5) | 11 (14.1) |
0.010a,b | 0.07
(0.01–0.66) |
Risk factors for mortality
Differences between cases of mortality and survival
was observed using univariate analysis (Table I). Peripheral catheter and history of
COPD have a negative influence on survival rates (P=0.01 and
P=0.016). Meanwhile, compared with female patients, male patients
have a higher mortality. Fosfomycin combinational therapy (7.7 vs.
24.6%) was associated with lower mortality (Table I). Compared with other targeted
therapy, target treatment with fosfomycin exerted preponderance
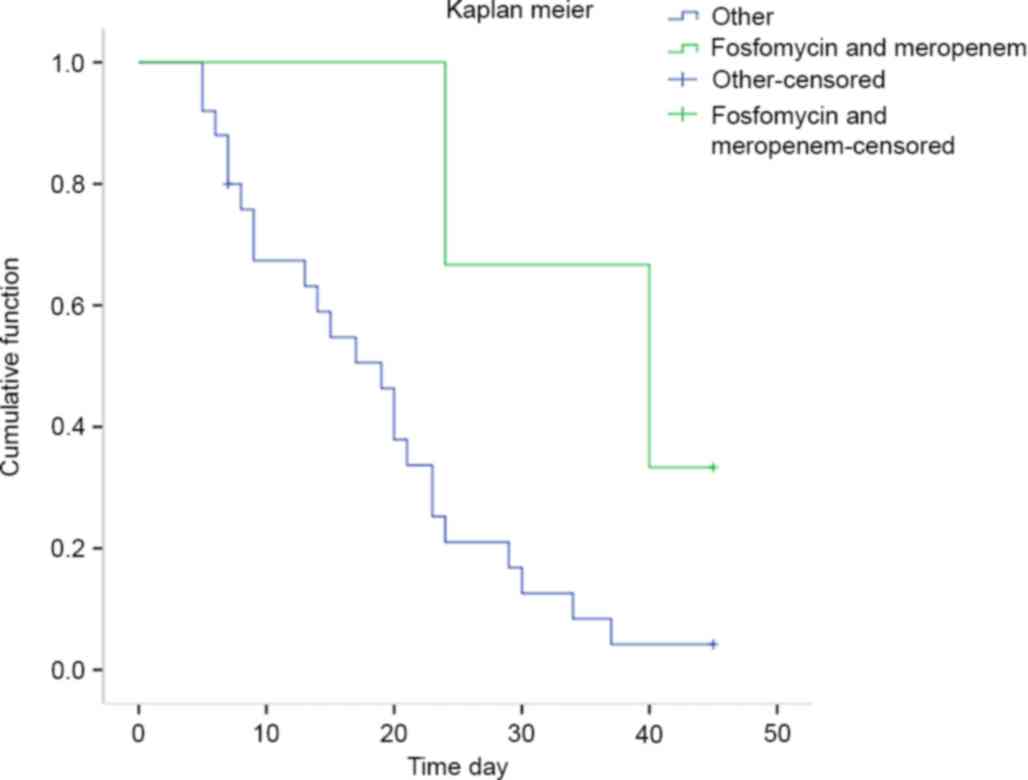
(P=0.034, OR: 4.71 [1.03–21.65]). The survival analysis performed
using Kaplan-Meier curves showed that patients treated with
fosfomycin combinational therapy had higher survival rates at 45
days after diagnosis (Fig. 1,
log-rank test t=3.96, P=0.047). However, in a multivariate
analysis, no correlation between fosfomycin combination and outcome
was observed (Table VII,
P=0.141).
 | Table VII.Multivariate models of risk factors
for mortality in patients with carbapenem-resistant Klebsiella
pneumoniae sepsis. |
Table VII.
Multivariate models of risk factors
for mortality in patients with carbapenem-resistant Klebsiella
pneumoniae sepsis.
|
| Unstandardized
Coefficients | Standardized
Coefficients | 95% CI |
|---|
|
|
|
|
|
|---|
| Variable | B | Std. Error | Beta | Sig | Up | Down |
|---|
| (Constant) |
1.371 | 0.592 | 5.369 | 0.020 |
|
|
| Gender |
1.279 | 0.614 | 4.349 | 0.037 | 3.594 | 1.080 |
| History of
COPD | −1.644 | 0.608 | 7.311 | 0.007 | 0.193 | 0.059 |
| Peripheral
catheter | −1.660 | 0.543 | 9.345 | 0.002 | 0.190 | 0.066 |
| Fosfomycin
combination |
1.202 | 0.817 | 2.167 | 0.141 | 3.326 | 0.671 |
Discussion
CRKP has become a major hospital pathogen worldwide,
and infections due to this organism have been associated with high
mortality (9,11–13). The
increasing prevalence and the high mortality associated with the
organism underscore the importance of effective antimicrobial
therapy for these serious infections. However, optimal treatment
for infections caused by CRKP has not yet been defined (14).
The present retrospective study involved 104 unique
patients with bacteremia due to CRKP with overall mortality rate of
25%, which is substantially lower than the rates reported for CRKP
in previous studies (11,15,16).
Alternatively, the difference may be resulted from unrecognized
confounding variables. Nonetheless, the mortality still remains
considerably higher than for bacteremia due to CRKP (17).
We identified gender, peripheral catheter and
history of COPD as independent clinical risk factors for mortality.
The present study demonstrated that survival in patients with CRKP
was significantly improved when combinational therapy rather than
monotherapy was administered. Fosfomycin combinational therapy is
successful in the present study compared with other monotherapy and
combination regimens. Alternatively, as monotherapy, fosfomycin
could drive resistance develops rapidly (18). In the present study, no patients were
included that received fosfomycin monotherapy. Fosfomycin is a
broad spectrum antibiotic that inhibits peptidoglycan synthesis
(19). Fosfomycin inhibits bacterial
cell wall biogenesis by inactivating the enzyme
UDP-N-acetylglucosamine-3-enolpyruvyltransferase which is also
known as MurA (20). This enzyme is
able to catalyze the committed step in peptidoglycan biosynthesis,
namely the ligation of phosphoenol pyruvate to the 3′-hydroxyl
group of UDP-N-acetyl glucosamine (20), which may provide a route for
meropenem to enter the bacteria. It can be administered in
combination with some other antimicrobial agents, since there is
low level of or no cross resistance (21). This agent is well tolerated and has a
few side effects. It has an increased role as a therapeutic option
against multidrug-resistant Enterobacteriaceae (19,20,22,23).
Cumulative susceptibility of fosfomycin against ESBL positive
Enterobacteriaceae is reported as 87.7% according to CLSI criteria
(24). However, lower susceptibility
rates (<50%) was showed in some studies, and in a study this
agent was shown to be active against only 50% of Klebsiella
(22,25).
Qureshi et al (3) reported that either colistin-polymyxin B
or tigecycline in combination with a carbapenem appeared to be
effective. These combination regimens were more successful than
monotherapy with either colistin-polymyxin B or tigecycline, even
when in vitro testing confirmed susceptibility to the
respective antimicrobials. While the mechanisms underlying the
effectiveness of these combinations are not known, synergistic
activity between carbapenems and colistin has been observed in
vitro among their CRKP isolates, which is consistent with the
clinical observation, at least for this particular combination.
It is considered that treatment with fosfomycin is
free from the nephrotoxicity that characterizes treatment with
aminoglycosides. A previous animal study demonstrated that
fosfomycin has a protective effect against nephrotoxicity as a
result of treatment with aminoglycosides by inhibiting
aminoglycoside-induced histamine release from mast-cell destruction
(26).
The present study is limited by its observational
nature and relatively small number of cases, since we limited the
analysis to cases with bacteremia only. In conclusion, mortality
associated with bacteremia due to CRKP continues to be high. The
use of combinational therapy, with fosfomycin combinational therapy
in particular, seems to have a survival benefit in this critically
ill population.
Acknowledgements
This study was partially supported by grants from
the National Natural Science Foundation of China (grant nos.
81403029, 81000311 and 81270831).
References
|
1
|
Tzouvelekis LS, Markogiannakis A,
Psichogiou M, Tassios PT and Daikos GL: Carbapenemases in
Klebsiella pneumoniae and other Enterobacteriaceae: An evolving
crisis of global dimensions. Clin Microbiol Rev. 25:682–707. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Duin D, Kaye KS, Neuner EA and Bonomo
RA: Carbapenem-resistant Enterobacteriaceae: A review of treatment
and outcomes. Diagn Microbiol Infect Dis. 75:115–120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qureshi ZA, Paterson DL, Potoski BA,
Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM and
Doi Y: Treatment outcome of bacteremia due to KPC-producing
Klebsiella pneumoniae: Superiority of combination antimicrobial
regimens. Antimicrob Agents Chemother. 56:2108–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borer A, Saidel-Odes L, Riesenberg K,
Eskira S, Peled N, Nativ R, Schlaeffer F and Sherf M: Attributable
mortality rate for carbapenem-resistant Klebsiella pneumoniae
bacteremia. Infect Control Hosp Epidemiol. 30:972–976. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeli Y, Akova M, Cornaglia G, Daikos
GL, Garau J, Harbarth S, Rossolini GM, Souli M and Giamarellou H:
Controlling the spread of carbapenemase-producing Gram-negatives:
Therapeutic approach and infection control. Clin Microbiol Infect.
16:102–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee GC and Burgess DS: Treatment of
Klebsiella pneumoniae carbapenemase (KPC) infections: A review of
published case series and case reports. Ann Clin Microbiol
Antimicrob. 11:322012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ni W, Wei C, Zhou C, Zhao J, Liang B, Cui
J, Wang R and Liu Y: Tigecycline-amikacin combination effectively
suppresses the selection of resistance in clinical isolates of
KPC-producing Klebsiella pneumoniae. Front Microbiol. 7:13042016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewis RE: Using carbapenems for
carbapenem-resistant Klebsiella pneumoniae - are we flogging a dead
(work) horse antibiotic? Virulence. 3:1–2. 2016.
|
|
9
|
Daikos GL and Markogiannakis A:
Carbapenemase-producing Klebsiella pneumoniae: (When) might we
still consider treating with carbapenems? Clin Microbiol Infect.
17:1135–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zarkotou O, Pournaras S, Tselioti P,
Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki
K and Tsakris A: Predictors of mortality in patients with
bloodstream infections caused by KPC-producing Klebsiella
pneumoniae and impact of appropriate antimicrobial treatment. Clin
Microbiol Infect. 17:1798–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pournaras S, Vrioni G, Neou E, Dendrinos
J, Dimitroulia E, Poulou A and Tsakris A: Activity of tigecycline
alone and in combination with colistin and meropenem against
Klebsiella pneumoniae carbapenemase (KPC)-producing
Enterobacteriaceae strains by time-kill assay. Int J Antimicrob
Agents. 37:244–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bratu S, Landman D, Haag R, Recco R, Eramo
A, Alam M and Quale J: Rapid spread of carbapenem-resistant
Klebsiella pneumoniae in New York City: A new threat to our
antibiotic armamentarium. Arch Intern Med. 165:1430–1435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gasink LB, Edelstein PH, Lautenbach E,
Synnestvedt M and Fishman NO: Risk factors and clinical impact of
Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect
Control Hosp Epidemiol. 30:1180–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchaim D, Chopra T, Perez F, Hayakawa K,
Lephart PR, Bheemreddy S, Blunden C, Hujer AM, Rudin S, Shango M,
et al: Outcomes and genetic relatedness of carbapenem-resistant
enterobacteriaceae at Detroit medical center. Infect Control Hosp
Epidemiol. 32:861–871. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirsch EB and Tam VH: Detection and
treatment options for Klebsiella pneumoniae carbapenemases (KPCs):
An emerging cause of multidrug-resistant infection. J Antimicrob
Chemother. 65:1119–1125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonzalez-Padilla M, Torre-Cisneros J,
Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A,
Natera C, Rodriguez M, Salcedo I, Rodriguez-López F, et al:
Gentamicin therapy for sepsis due to carbapenem-resistant and
colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother.
70:905–913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tumbarello M, Viale P, Viscoli C,
Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S,
Ginocchio F, Cristini F, et al: Predictors of mortality in
bloodstream infections caused by Klebsiella pneumoniae
carbapenemase-producing K. pneumoniae: Importance of combinational
therapy. Clin Infect Dis. 55:943–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qureshi ZA, Paterson DL, Peleg AY,
Adams-Haduch JM, Shutt KA, Pakstis DL, Sordillo E, Polsky B,
Sandkovsky G, Bhussar MK and Doi Y: Clinical characteristics of
bacteraemia caused by extended-spectrum β-lactamase-producing
Enterobacteriaceae in the era of CTX-M-type and KPC-type
β-lactamases. Clin Microbiol Infect. 18:887–893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Zheng B, Li Y, Zhu S, Xue F and Liu
J: Antimicrobial susceptibility and molecular mechanisms of
fosfomycin resistance in clinical Escherichia coli isolates in
mainland China. PLoS One. 10:e01352692015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raz R: Fosfomycin: An old - new
antibiotic. Clin Microbiol Infect. 18:4–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown ED, Vivas EI, Walsh CT and Kolter R:
MurA (MurZ), the enzyme that catalyzes the first committed step in
peptidoglycan biosynthesis, is essential in Escherichia coli. J
Bacteriol. 177:4194–4197. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yılmaz N, Ağuş N, Bayram A, Şamlıoğlu P,
Şirin MC, Derici YK and Hancı SY: Antimicrobial susceptibilities of
Escherichia coli isolates as agents of community-acquired urinary
tract infection (2008–2014). Turk J Urol. 42:32–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falagas ME, Kastoris AC, Kapaskelis AM and
Karageorgopoulos DE: Fosfomycin for the treatment of
multidrug-resistant, including extended-spectrum beta-lactamase
producing, Enterobacteriaceae infections: A systematic review.
Lancet Infect Dis. 10:43–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roussos N, Karageorgopoulos DE, Samonis G
and Falagas ME: Clinical significance of the pharmacokinetic and
pharmacodynamic characteristics of fosfomycin for the treatment of
patients with systemic infections. Int J Antimicrob Agents.
34:506–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho YH, Jung SI, Chung HS, Yu HS, Hwang
EC, Kim SO, Kang TW, Kwon DD and Park K: Antimicrobial
susceptibilities of extended-spectrum beta-lactamase-producing
Escherichia coli and Klebsiella pneumoniae in health
care-associated urinary tract infection: focus on susceptibility to
fosfomycin. Int Urol Nephrol. 47:1059–1066. 2014. View Article : Google Scholar
|
|
26
|
Liu CY, Lai CC, Lee MR, Lee YC, Huang YT,
Liao CH and Hsueh PR: Clinical characteristics of infections caused
by Tsukamurella spp. and antimicrobial susceptibilities of the
isolates. Int J Antimicrob Agents. 38:534–537. 2011. View Article : Google Scholar : PubMed/NCBI
|