Introduction
The development of modern marine industry and naval
warfare has made human activities on the sea increasingly frequent.
Thus, there has been an increase in the occurrence of various blast
injuries being immersed by seawater, which may increase tissue
damage and thus affect wound healing. The prompt and effective
treatment of seawater-immersed blast-injury wounds (SIBIW) has
become a key topic in the field of trauma.
SIBIW is a class of damage caused under special
circumstances, thus exhibiting various pathophysiological
differences compared with ordinary trauma. Firstly, the explosion
responsible for the wounding would rapidly generate a large
quantity of high-pressure gas, heat and shock waves, resulting in
lacerations, blast injuries and burns (1,2). This
trauma may result in skin and soft tissue loss, as well as causing
damage to the surrounding tissues and body functions (1,2).
Secondly, the characteristics of seawater, including high salt,
high alkali, easily permeating tissue and low temperature, may
aggravate the infection of a wound, in addition to causing systemic
microcirculation disorders, making the wounds difficult to heal or
delay the healing, increasing the risk of mortality and morbidity
(3). Vacuum sealing drainage (VSD)
was first applied clinically by Dr Fleischmann (Ulm University,
Ulm, Germany) in 1993 (4), and
subsequent clinical practice has indicated that VSD may enhance the
treatment of acute and chronic wounds and promote successful skin
grafting (5–7). However, to date there are no reports
investigating the application of VSD to SIBIWs.
The present study aimed to determine the effects of
VSD treatments on SIBIWs under various negative pressure values by
observing the general appearance, histology and vascular
endothelial growth factor (VEGF)-immunohistochemistry of wounds, as
well as the expression of angiogenesis-promoting regulation gene
miRNA-17-5p and the wound microenvironment changes. In addition,
the optimized pressure range of VSD in treating SIBIW was
investigated, thus providing the basis for the clinical treatment
of such trauma.
Materials and methods
Establishment of animal model and
grouping
A total of eight No. I white Chinese miniature pigs
were obtained (3 months of age; weight, 25–30 kg; male and female;
provided by Liulihe Kexing Experimental Animal Breeding Center,
Beijing, China) and received and intramuscular injection of
sumianxin 0.2 ml/kg and 0.5 ml midazolam (both Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) as anesthesia. Then, the spine of
each pig was set as the midline, and a blast-injury wound was
formed on each limb and 10 cm from the midline, respectively, as
previously described (8). After
normal rapid debridement, the wounds were immersed into 10°C
seawater for 1 h, then rewarmed to body temperature. The animals
were randomly divided into four groups: VSD treatment groups were
administered 120, 180 and 240 mmHg VSD, respectively; the control
group wounds received conventional dressing and covered with saline
gauze. VSD was performed for nine days, and the pigs were
sacrificed after 58 days following anesthesia by intravenous 3%
sodium barbitone injection (1 ml/kg; Sigma-Aldrich; Merck
Millipore). Samples with a diameter of 0.2–0.3 cm from the wound
edge and central basal tissues were consecutively collected on days
1, 3, 5, 7, 9, 16, 23 and 58 postoperation for the dynamic
observation of wound healing situations. This study was conducted
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The animal use protocol has been reviewed and approved by
the Institutional Animal Care and Use Committee of the 309th
Hospital of PLA (Beijing, China).
General morphological observation
Wound size, swelling degree, wound edge, granulation
tissue growth in the basilar wound part, epithelial coverage and
wound healing degree were observed daily until day 58 following the
operation.
Histological observation
The full-thickness skin tissues at the wound edge
were sampled on days 1, 3, 5, 7 and 9 postoperation to assess wound
formation using hematoxylin and eosin (HE) staining. The conditions
of edema, inflammatory cell infiltration, angiogenesis, granulation
tissue growth, epidermal proliferation and migration of the wound
edge tissues were observed using light microscopy.
Immunohistochemical analysis
Paraffin sections (2 µm) of samples were dewaxed and
then washed with distilled water. After soaking in
phosphate-buffered saline (PBS) for 5 min, they were incubated with
3% H2O2 at room temperature for 15 min. After
washing with PBS, the primary rabbit anti-VEGF polyclonal antibody
(1:4,000; bs-1313R; Beijing Zhong Shan Jinqiao Biotechnology
Development Co., Ltd., Beijing, China) was added and incubated at
37°C for 1–2 h, followed by washing with PBS. Then, the
biotinylated goat anti-rabbit IgG secondary antibody (1:200;
bs-2331R; Beijing Zhong Shan Jinqiao Biotechnology Development Co.,
Ltd.) added and incubated at 37°C for 30 min. After washing with
PBS, 3,3′-diaminobenzidine staining was performed, followed by
flushing with water, restaining, dehydration and mounting. The VEGF
expression was observed using a fluorescence microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for determination of
miRNA-17-5p expression
Total RNA was extracted from the granulation tissues
collected on days 1, 5, 9, 16 and 23 postoperation using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
digested with RNase-free DNase (Promega Corporation, Madison, WI,
USA). The reverse transcription reaction with 300 ng total RNA was
performed using the PrimeScript First-strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's instructions. The primer sequences used in RT-qPCR
were as following: miRNA-17-5p forward, 5′-GCTTCAACCCCTTCAAATGC-3′
and reverse, 5′-GACGCAGAAGCGGTGTTATTG-3′; and β-actin forward
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG-3′. The PCR system was as follows: SYBR
Green 1 dye (Thermo Fisher Scientific Inc.), 5 µl; Premix Ex
Taq (2X; Sangon Biological Engineering Co., Ltd., Shanghai,
China), 7.5 µl; forward primer (10 µmol/l), 0.25 µl; reverse primer
(10 µmol/l), 0.25 µl; cDNA (5 ng/µl), 3 µl; and dH2O, 4
µl. The PCR thermal cycling conditions were as follows: 95°C for 20
sec; 40 cycles at 95°C for 15 sec; 93°C for 30 sec; 55°C for 40
sec; and final extension at 72°C for 5 min. The amplification and
melting curve was recorded, and the expression of miRNA-17-5p was
analyzed.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The miRNA-17-5p detection data underwent statistical analysis using
SPSS software, version 20.0 (IBM SPSS, Armonk, NY, USA). The
comparison of miRNA-17-5p was performed using one-way analysis of
variance. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Visual general observation
The SIBIW surface was dirty with residual necrotic
tissue in all groups, with a poorly-defined necrotic area, the
substratum was pale, with less bleeding and a pale wound edge.
At day 1 after treatment, the effects of the 120,
180 and 250 mmHg VSD treatment groups were improved compared with
the control group; with reduced swelling, dry wound surface and
rosy basal tissues. By contrast, the wound edge of the control
group had swelled, with increased necrotic tissues and pale
substratum.
At day 3 after treatment, the wound surface of the
120 mmHg treatment group was clean. By contrast, the other
treatment groups exhibited secondary necrosis, with gray necrotic
tissues in the wounds and evident exudate; the wound infection was
severe, and the control group had increased necrotic tissue.
At day 5 after treatment, the wound substratum of
the 120 mmHg treatment group was rosy, the subcutaneous tissues
under the scab were red. The wounds of the 180 and 240 mmHg
treatment groups were drier than previously, with reduced exudate
and swelling, while the red swelling and exudation of the control
group did not show any improvement.
At day 9 after treatment, all wound surfaces in the
120, 180 and 250 mmHg VSD treatment groups were clean and dry, the
substratum was pink, the granulation tissues were proliferated and
the epidermal edge of wound began to migrate forward. Among which,
the granulation tissues of the 120 and 180 mmHg groups were plump
and smooth, and the epidermis of these groups migrated the
fastest.
Morphological observation
At day 1 after treatment the wound cells exhibited
edema, partial cytoplasm transparency and mild staining. Focal
inflammatory cell infiltration was visible, consisting
predominantly of neutrophils and eosinophils. Compared with the
control group, the VSD treatment group exhibited fewer inflammatory
cells.
At day 3 after treatment the 120 mmHg VSD treatment
group exhibited no significant changes of inflammatory cells, while
the edema of the other groups remained obvious, and the
inflammatory cells were increased, exhibiting a diffused
distribution.
At day 5 after treatment the VSD treatment group
showed the growth of small quantity granulation tissue, among which
the 120 mmHg group was relatively obvious. The control group
continued to exhibit a numerous inflammatory cells, with no
significant granulation tissue growth.
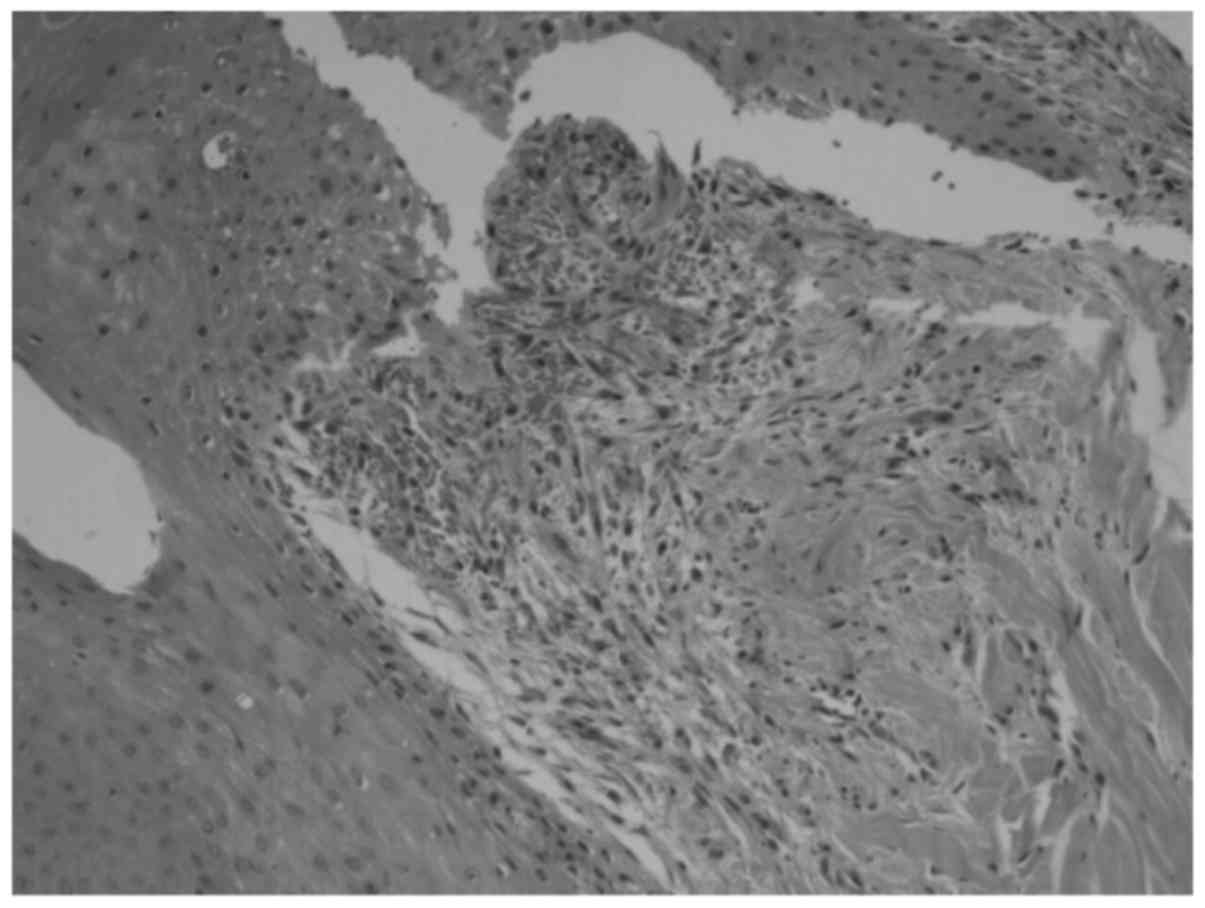
At day 9 after treatment the cells had no
significant swelling, with fewer inflammatory cells. All the
treatment groups exhibited growth of granulation tissues, and the
120 mmHg group exhibited the most growth (Fig. 1).
VEGF expression
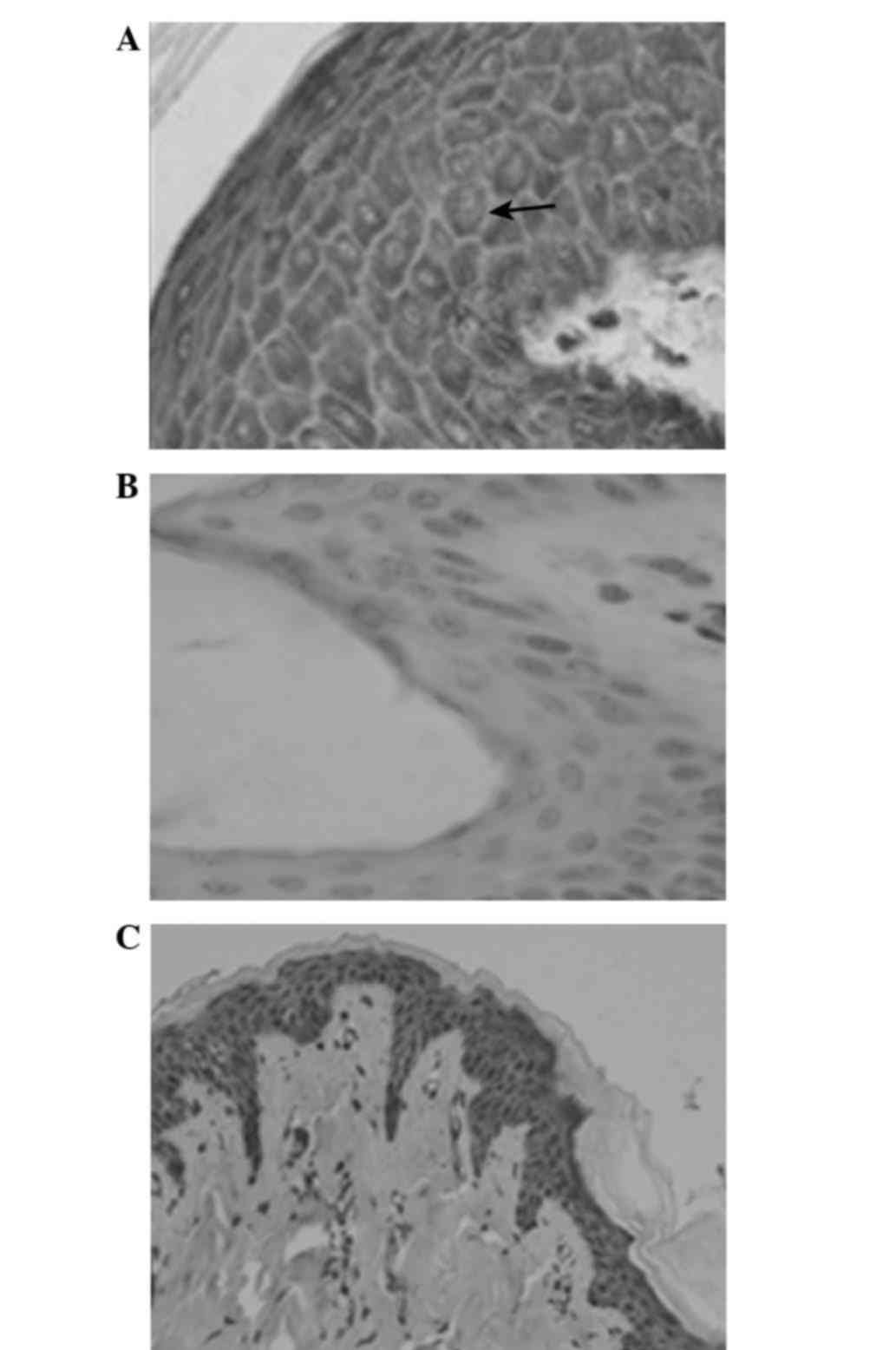
At 2 h following trauma, the positive VEGF
expression increased in the epidermal cells, which was indicated by
yellow/brown granules in the cytoplasm (Fig. 2A). There was only trace positive VEGF
expression in normal skin tissue (Fig.
2B).
At day 1 after treatment, VEGF expression was
detected in the treatment and control groups. Numerous yellow
granules were visible within the epidermal cellular cytoplasm of
wound peripheral tissues. The expression of VEGF in the 120 mmHg
group was higher in comparison with the other treatment groups and
the control group.
Between days 5 and 9 after treatment, the VEGF
expression in the cytoplasm continued to increase, peaking on day
9. The 120 mmHg group continued to exhibit the most significant
expression, with brown granules uniformly distributed within the
cytoplasm. At day 58 following treatment, the wound healed and the
expression of VEGF in the cytoplasm was significantly decreased,
less expressed in the VSD group, and that in the 20 mmHg group was
close to the normal skin tissues; while there still was moderate
expression in the control group (Fig.
2C).
miRNA-17-5p detection
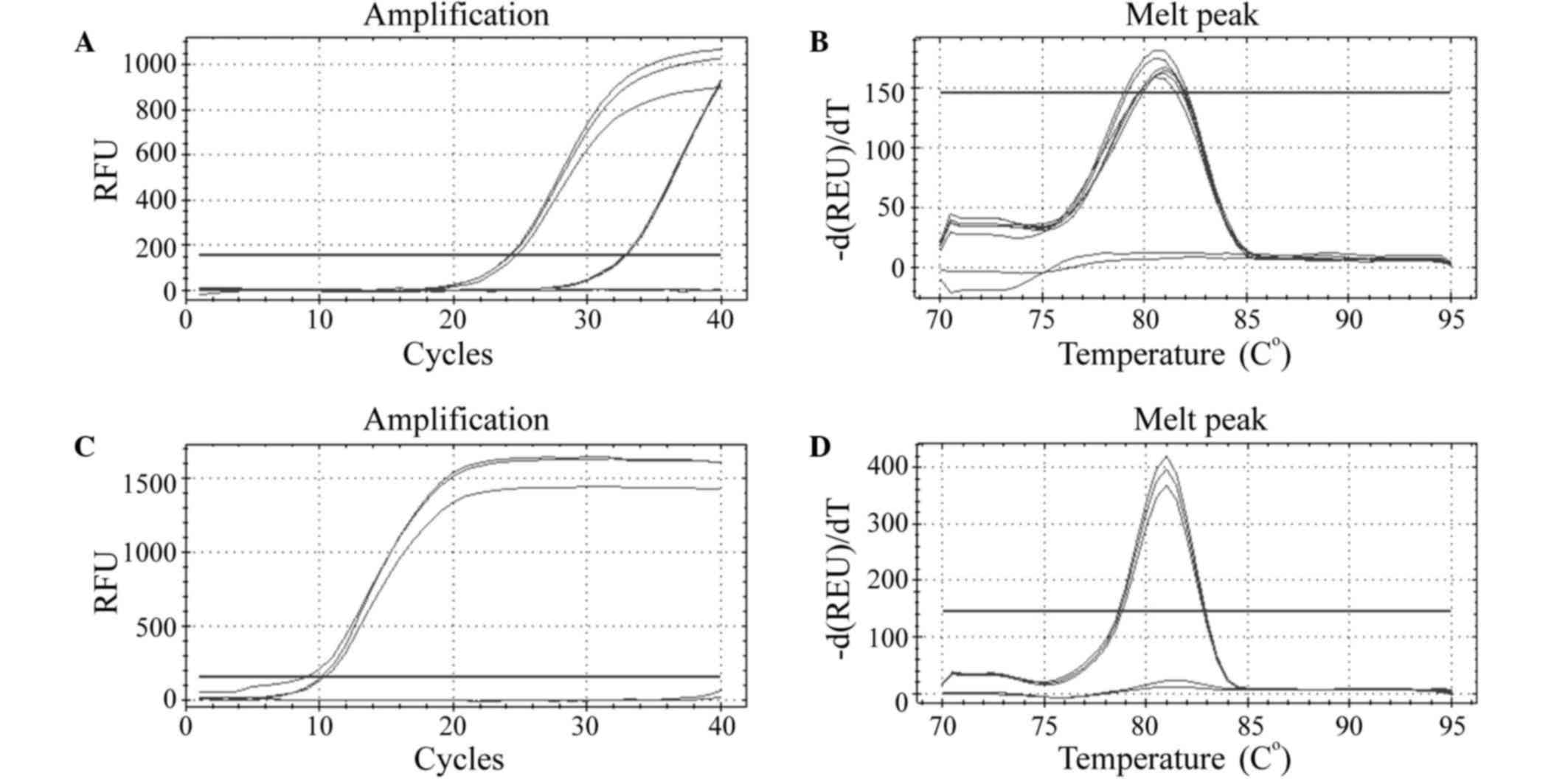
The quality detection of RNA showed that the RNA
expression was stable and within the normal limits, and the gel
electrophoresis indicated that the RNA was integral (Fig. 3). RT-qPCR identification revealed
that the amplification of ssc-17-5p primer was good, and the qPCR
primers could effectively and specifically amplify the
corresponding RNA fragments (Fig.
4).
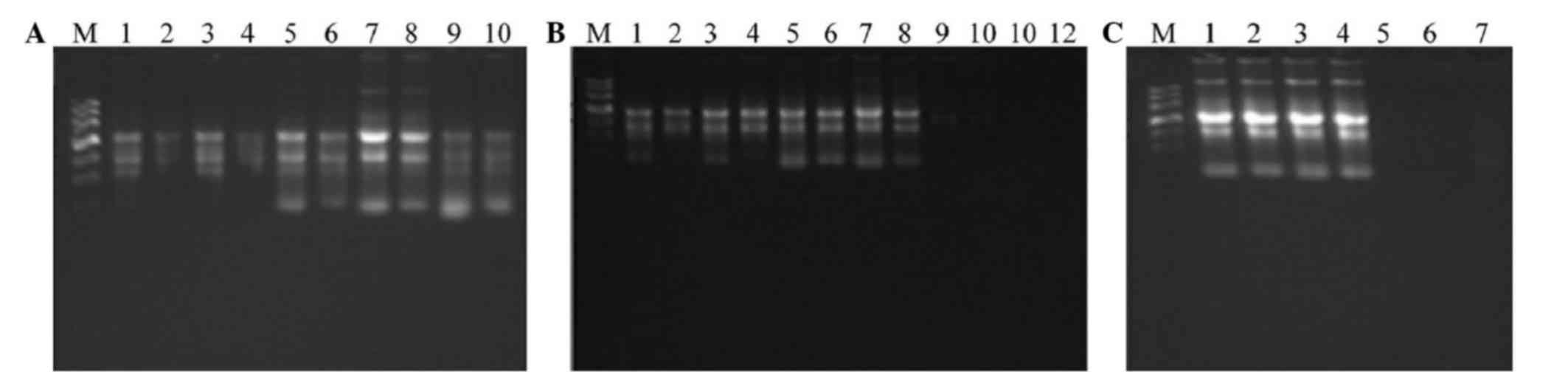 | Figure 3.Electrophoresis of RNA. The numbers
represent the different samples. (A) 1, A5d-2-1, 2, A5d-2-2; 3,
A5d-3-1; 4, A5d-3-2; 5, A5d-4-1; 6, A5d-4-2; 7, A9d-2-1; 8,
A9d-2-2; 9, A9d-3-1; and 10, A9d-3–2. (B) 1, A5d-1-1, 2, A5d-1-2;
3, A9d-1-1; 4, A9d-1-2; 5, A16d-1-1; 6, A16d-1-2; 7, A23d-1-1; 8,
A23d-1-2; 9, A0d-0-1; 10, A0d-0-2; 11, A1d-1-1; and 12, A1d-1–2.
(C) 1, A23d-3-1, 2, A23d-3-2; 3, A23d-4-1; 4, A23d-4-2; 5, A1d-2-2;
6, A1d-3-1; and 7, A1d-4–1. |
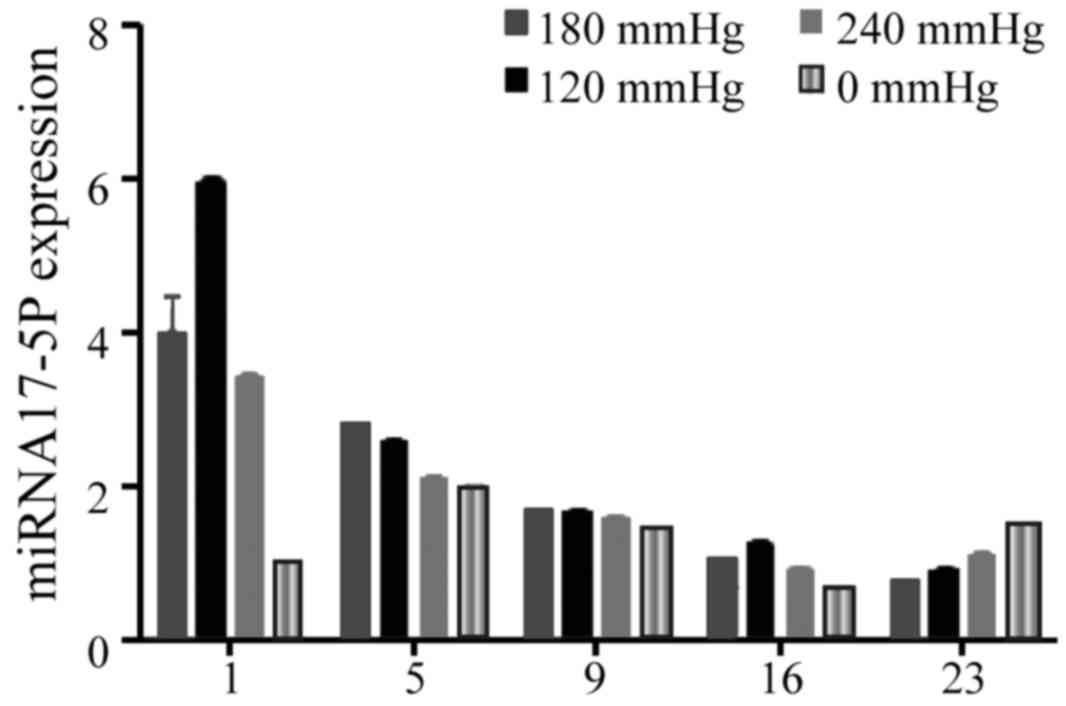
miRNA-17-5p expression was detected in the SIBIW
granulation tissues, and the expression in the VSD treatment group
was higher compared with the control group. The expression in the
VSD treatment group peaked at day 1 after treatment, and the
expression detected in the 180 mmHg group was higher compared with
the other treatment groups, Over time, the miRNA-17-5p expression
gradually decreased, while the expression levels in the 120 mmHg
group remained continuously higher than the other treatment groups,
and the expression of the control group exhibited the dynamic
trend. There was significant difference at each time point between
the 120, 180 and 250 mmHg VSD treatment groups and the control
group (P<0.05) (Fig. 5).
Discussion
Wound healing involves a series of complex
pathophysiological processes, which includes the inflammation,
proliferation and migration of repaired cells, in addition to the
formation and tissue remodeling of granulation tissues (9). Factors that are able to disrupt these
processes may impact wound healing. SIBIWs are obviously different
to general injuries due to their particular characteristics
(10).
Seawater immersion may increase tissue damage; SIBIW
is a type of complex and comprehensive damage (11). The tissues at the wounds have various
injury factors, and are typically accompanied by extensive damage
ranges, ill-defined necrotic tissues, heavily contaminated wounds,
as well as different ranges of skin and soft tissue defects
(12). Seawater has a complex
composition, and may contain a variety of bacteria, and pH and
osmolality are higher than the normal value in humans. Furthermore,
in the majority of regions, seawater temperature is <20°C, which
is lower than the human body temperature (13). These factors result in seawater
exhibiting high permeability, high alkalinity, diverse flora and
low temperature. The environment of high permeability and
alkalinity could aggravate the damage in wounded tissues and cells,
and enhance edema; various bacteria could induce complex and
diverse infections. Inflammation would be difficult to control and
long-term low temperature could potentially result in severe
cardiovascular disorders, metabolic acidosis and blood circulation
disorders, thus accelerating the wound tissue necrosis and
increasing local wound damage (14).
Since the introduction of VSD into clinics, and its
application to various types of wound treatment, it has achieved
potentially promising results (15–17). A
previous study showed that VSD could perform the adequate drainage,
so that wounds are thoroughly cleaned and fully prevented the
formation of subcutaneous space (18). Thus, the removal of necrotic tissue
is promoted and the growth of bacterial cultures is reduced; reduce
the secretion of inflammatory mediators and toxic substances and
reduce the wound edema and swelling (19). In addition, VSD has been suggested to
increase wound blood flow and expand blood vessel diameter, thus
improving blood circulation and nutrient supply to wounded tissue
(20,21). VSD may also reduce the quantity of
acidic substances in a wound, promote nutrient absorption and
oxygen usage, the growth of granulation tissues, wound fibroblasts
and capillary endothelial cells, thus conducive to regeneration of
dermis (22–24). Huljev (20) and Atay et al (25) found that VSD could also effectively
reduce the production of wound matrix metalloproteinase, and
inhibit its activity, thereby promoting collagen synthesis, which
would be favor for wound healing.
During the wound healing process, the degree of
inflammation and vascular proliferation substantially impact wound
healing. Inflammation is the body's own defensive process; however,
excessive inflammation could exacerbate tissue damages, leading to
mitigated wound healing (26).
Furthermore, angiogenesis may provide more nutrients to the wound,
thus accelerating wound healing.
Through the naked-eye general sample observation,
the present study found that on day 1 following treatment, the
wound swelling of the VSD treatment group was mild, with limited
oozing and no significant necrotic tissues, while the wound
swelling of the control group was significant, with gray necrotic
tissues. On day 3 after treatment, the 180 and 240 mmHg VSD
treatment groups and the control group exhibited secondary
necrosis, with increased wound swelling and significant infection.
On day 5 after treatment, the VSD treatment group exhibited reduced
wound exudate and necrotic tissue, while the control group had
increased necrotic tissue and exudate. On day 9, all wound swelling
regressed, the infections disappeared and the granulation tissues
grew. The wound of the 120 mmHg group remained clean and dry, with
no obvious discharge or secondary necrosis. The granulation tissues
grew well, and epidermal forward-migration was faster.
Histological analysis of tissues collected on day 1
revealed edema and focal inflammatory cells, which were
predominantly neutrophils and eosinophils. On day 3, with the
exception of the 120 mmHg group, there was extensive inflammatory
cell infiltration in the other groups. On day 5, only the 120 mmHg
group exhibited the growth of granulation tissue, the number of
inflammatory cells in the 180 and 240 mmHg groups were decreased,
while the control group continued to exhibit a large number of
inflammatory cells. On day 9, all wounds exhibited the growth of
granulation tissue. These results indicate that VSD may be able to
effectively reduce inflammation and secondary necrosis in SIBIWs,
promote the growth of granulation tissue, and accelerate the wound
healing, among which the 120 mmHg group exhibited the most marked
therapeutic effects.
Good blood circulation is key to the wound healing,
and the formation of granulation tissues may aid in providing
sufficient blood towards the wound. Granulation tissues could
provide the regenerated tissues with oxygen and nutrients, while
repairing tissue defects, promoting the absorption of necrotic
tissues and helping to control inflammation (24). Granulation tissues are composed of
nascent thin-walled capillaries and proliferated fibroblasts, in
which the regeneration of capillaries and the formation of a
vascular network are crucial. VEGF has been shown to be a unique
growth factor, specific to the process of angiopoiesis.
The present results showed that VEGF was positive
expressed in the cytoplasm of VSD-treated tissues, while normal
skin tissues showed a relatively moderate amount of expression.
Comparisons among the various VSD treatment groups and the control
group revealed that the expression of VEGF in the VSD treatment
group at day 1 was higher than the control group, particularly in
the 120 mmHg group, which reached the highest level on day 9 after
treatment. Subsequent to day 9, the wound healing was decreased.
Histological observation revealed that on day 5, the 120 mmHg group
began to exhibit the growth of granulation tissues, while on the
day 9, all the wounds exhibited the growth of granulation tissues.
Therefore, it was hypothesized that VSD could increase the
expression of VEGF, promote the growth of granulation tissues and
accelerate wound healing; particularly at a negative pressure of
120 mmHg.
In previous years, molecular biology studies have
discovered that miRNAs may regulate the expression of multiple
genes inside the organism, thus affecting such physiological
activities as growth and development of the body (27,28).
With regard to wound healing, miRNAs may be able to regulate the
angiogenesis, migration and regeneration functions of epithelial
cells, thus affecting the wound healing process (29). Suárez et al (30) found in a study of ischemia and
reperfusion that miR-17-5p could regulate the functions of
endothelial cells, thus promoting the angiogenesis. Otsuka et
al (31) studied the luteal
functions of infertile mice, and further demonstrated that
miR-17-5p could affect the endothelial cell functions via the
inhibition of metalloproteinase 1 (TIMP-1), thus promoting
angiogenesis. Furthermore, previous studies have indicated that
miR-17-5p plays a role in the promotion of angiogenesis (32,33).
However, previous studies regarding the angiogenic promotion
effects of miR-17-5p have not investigated its expression in
wounds.
The present result showed increased miR-17-5p
expression in the granulation tissues of SIBIWs; with the
expression in the 120 mmHg VSD group significantly higher compared
with the control group. In the 180 mmHg VSD treatment group, the
expression on day 1 was the highest, and decreased gradually over
time. By contrast, the expression in the control group appeared as
a change in the lower level. The naked-eye general and histological
observations suggest that the granulation tissues of the 240 mmHg
VSD treatment group grew significantly, and that wound healing was
more rapid. Therefore, it was hypothesized that miR-17-5p could
regulate endothelial cell functions in the early stage of wound
healing, thus promoting angiogenesis, increasing the growth of
granulation tissues and accelerating wound healing. In addition, at
day 1 after treatment, the miR-17-5p expression in the 180 mmHg
group was significantly increased, while in the subsequent
treatments, the expression in the 120 mmHg group was continuously
higher compared with the other groups. These results indicated that
the expression of miR-17-5p under 120 mmHg treatment produced the
most marked therapeutic effects.
In conclusion, we speculated that VSD could
effectively reduce the inflammation, infection and secondary
necrosis of SIBIWs, in addition to promoting the growth of
granulation tissues, accelerating epithelial migration and
increasing miR-17-5p expression. The therapeutic effects of 120
mmHg were more marked compared with 180 and 240 mmHg; however, it
remains unclear whether this represents the optimal negative
pressure for SIBIW treatment, and further studies are required.
References
|
1
|
Kirkman E and Watts S: Haemodynamic
changes in trauma. Br J Anaesth. 113:266–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ning J, Mo L, Zhao H, Lu K, Wang L, Lai X,
Yang B, Zhao H, Sanders RD and Ma D: Transient regional hypothermia
applied to a traumatic limb attenuates distant lung injury
following biast limb trauma. Crit Care Med. 42:e68–e78. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu P, Liu JC, Lai XN, Peng XL, Wu GP,
Zhang LC and Wang LL: Pathological study of rabbits' femoral
arteries subjected to gunshot wounds combining with seawater
immersion. Chin J Traumatol. 8:186–190. 2005.PubMed/NCBI
|
|
4
|
Fleischmann W, Strecker W, Bombelli M and
Kinzl L: Vacuum sealing as treatment of soft tissue damage in open
fractures. Unfallchirurg. 96:488–492. 1993.PubMed/NCBI
|
|
5
|
Witkowski W, Jawien A, Witkiewicz W and
Zon B: Initial multi-centre observations upon the effect of a new
topical negative pressure device upon patient and clinician
experience and the treatment of wounds. Int Wound J. 6:167–174.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scherer LA, Shiver S, Chang M, Meredith JW
and Owings JT: The vacuum assisted closure device: A method of
securing skin grafts and improving graft survival. Arch surg.
137:930–934; discussion 933–934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Liu DR, Liang Z, Liu F, Lin H and
Guo Z: Reapair of refractory wounds through grafting of artificial
dermis and autologous epidermis aided by vacuum-assistde closure.
Aesthetic Plast Surg. 38:727–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XY, Li JQ, Chen SZ, Li WZ and Li YJ:
Establishment of animal model of blast injury in pig skin and soft
tissue. Zhong Hua Shi Yan Wai Ke Za Zhi. 12:1551–1553. 2006.(in
Chinese).
|
|
9
|
Pazyar N, Yaghoobi R, Rafiee E, Mehrabian
A and Feily A: Skin wound healing and phytomedicine: A review. Skin
Pharmacol Physio. 2:303–310. 2014. View Article : Google Scholar
|
|
10
|
Cao L, Peng MM, Sun JJ, Yu XC and Shi B:
Application of vacuum-assisted closure in seawater-immersed wound
treatment under different negative pressures. Genet Mol Res.
14:6146–6155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi B, Sun J, Cao Y, Yang F, Wu Y, Liang X
and Li L: Application of vacuum sealing drainage to the treatment
of seawater-immersed blast-injury wounds. Int Wound J. May
8–2015.(Epub ahead of print).
|
|
12
|
Popov VL, Kadochnikov DS and Minaeva PV:
The role of the biological damaging factor in the explosive injury.
Sud Med Ekspert. 58:20–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diaz JH: Skin and soft tissue infections
following marine injuries and exposures in travelers. J Travel Med.
21:207–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diaz JH and Lopez FA: Skin, soft tissue
and systemic bacterial infections following aquatic injuries and
exposures. Am J Med Sci. 349:269–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolvos TA: Negative pressure wound therapy
with instillation: The current state of the art. Surg Technol Int.
24:53–62. 2013.
|
|
16
|
Frick A, Wallmichrath J, Frick G, Giunta
RE and Engelhardt TO: Difficult wounds & vacuum assisted
closure (VAC). MMW Fortschr Med. 155:63–64. 2013.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altintas B, Biber R and Brem MH: The
accelerating effect of negative pressure wound therapy with
Prevena™ on the healing of a closed wound with persistent serous
secretion. Int Wound J. 2014.(Epub ahead of print). PubMed/NCBI
|
|
18
|
Zhang WH, Wu Q, Ma J and Wang JH: Effects
of vacuum drainage combined with heparin irrigation for treatment
of scald burns with seawaterimmersion in rabbits. Nan Fang Yi Ke Da
Xue Xue Bao. 35:1481–1486. 2015.(in Chinese). PubMed/NCBI
|
|
19
|
Ingber DE: Tensegrity I. Cell structure
and hierarchical systems biology. J Cell Sci. 116:1157–1173. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huljev D: Negative pressure
therapy-supportive method in chronic wound treatment. Acta Med
Croatica. 67:(Suppl 1). S89–S94. 2013.(In Croatian).
|
|
21
|
Esteban MP Borrero, Begines R Begines,
Rodríguez Llamas S and Díaz Campos T: Managing complications in
severe traumatic injury with VAC therapy with instillation. Rev
Enferm. 36:42–47. 2013.(In Spanish).
|
|
22
|
Malmsjö M, Lindstedt S, Ingemansson R and
Gustafsson L: Use of bacteria- and fungus-binding mesh in negative
pressure wound therapy provides significant granulation tissue
without tissue ingrowth. Eplasty. 14:e32014.PubMed/NCBI
|
|
23
|
Harish V and Maitz PK: Uninterrupted
continuous negative pressure wound therapy is safe and can
facilitate engraftment of dermal regeneration templates. J Plast
Reconstr Aesthet Surg. 67:1011–1013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pitt KA and Stanley BJ: Negative pressure
wound therapy: Experience in 45 dogs. Vet Surg. 43:380–387. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atay T, Burc H, Baykal YB and Kirdemir V:
Results of vacuum assisted wound closure application. Indian J
Surg. 75:302–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roy S and Sen CK: miRNA in wound
inflammation and angiogenesis. Microcirculation. 19:224–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hildebrand J, Rütze M, Walz N, Gallinat S,
Wenck H, Deppert W, Grundhoff A and Knott A: A comprehensive
analysis of microRNA expression during human keratinocyte
differentiation in vitro and in vivo. Invest Dermatol. 131:20–29.
2011. View Article : Google Scholar
|
|
28
|
Zhang L, Stokes N, Polak L and Fuchs E:
Specific microRNAs are preferentially expressed by skin stem cells
to balance self-renewal and early lineage commitment. Cell Stem
Cell. 8:294–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y and Gorski DH: Regulation of
angiogenesis through a microRNA (miR-130a) that down-regulates
antiangiogenic homeobox genes GAX and HOXA5. Blood. 111:1217–1226.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suárez Y, Fernández-Hernando C, Yu J,
Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager
M and Sessa WC: Dicer-dependent endothelial microRNAs are necessary
for postnatal angiogenesis. Proc Natl Acad Sci USA.
105:14082–14087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Otsuka M, Zheng M, Hayashi M, Lee JD,
Yoshino O, Lin S and Han J: Impaired microRNA processing causes
corpus lustrum insufficiency and infertility in mice. J Clin
Invest. 118:1944–1954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramón LA, Braza-Boïls A, Gilabert J,
Chirivella M, España F, Estellés A and Gilabert-Estellés J:
microRNAs related to angiogenesis is deregulated in endometriosis
endometrial cancer. Hum Reprod. 27:3036–3045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonauer A, Carmona G, Iwasaki M, Mione M,
Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et
al: MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|