Introduction
Inflammation not only induces discomfort or pain,
but it may also cause physiological dysfunction and psychiatric
disorders, including sleep disorders and depression (1,2). There
are two main types of chronic pathological pain, neuropathic pain
caused by nerve damage and inflammatory pain caused by tissue
damage (3,4). Inflammatory pain is common in clinical
therapy, characterized by spontaneous pain and hyperalgesia
(5,6). From a therapeutic perspective,
inflammatory pain often exhibits tolerance to the existing methods
of treatment (7,8). Therefore, it is essential that the
cellular and molecular mechanisms of inflammatory pain are
elucidated in order to develop novel treatments for pain.
Inflammatory pain refers to the nerve pain caused by
local acute or chronic inflammation (9,10). There
are two main mechanisms of inflammatory pain, one of which is the
release of inflammatory mediators, such as prostaglandins,
bradykinin and P substance (11,12).
These mediators cause swelling and fever in the inflammation sites
through the dilation of blood vessels, in addition to inducing
hyperalgesia by acting on the corresponding receptors. On the other
hand, due to inflammation-induced cell damage and metabolic
abnormalities, local pH in inflammatory sites can be reduced to pH
6.0 to form an acidic environment (13,14).
H+ can be generated by activating the outer peripheral
nociceptors, which have an important role in inflammatory pain.
During inflammatory pain conduction, noxious stimulation signals
are transmitted by the primary sensory neurons in trigeminal
ganglia (TG) and dorsal root ganglion to the dorsal horn of the
lumbar spinal cord by synaptic transmission (15,16).
It is thought that pain is only regulated by neurons
and is not associated with glial cells of the spinal cord (17,18).
However, recent studies demonstrated that subcutaneous injection of
formaldehyde, complete Freund's adjuvant (CFA), phospholipase A2
and zymosan activated the spinal microglia and astrocytes (19–21). In
the present study, rat model of inflammatory pain were constructed
in order to further investigate whether bupivacaine was able to
activate spinal microglia and astrocytes. Furthermore, the role of
local anesthetic in the suppression of inflammatory pain was
evaluated, which may provide experimental evidence to support the
alleviation of the occurrence and development of chronic pain.
Materials and methods
Apparatus and antibodies
von Frey aesthesiometer (2390CE) and rotating rod
apparatus were purchased from IITC Life Science (Woodland Hills,
CA, USA). Anti-IκB-α rabbit polyclonal IgG (C-21; 200 µg/ml;
sc-371) and OX42 mouse monoclonal IgG2a (200 µg/ml; sc-53086)
primary antibodies and goat anti-rabbit IgG-horseradish peroxidase
(HRP) (sc-2030) secondary antibody were purchased from Santa Cruz
Biotechnology Inc., (Dallas, TX, USA). Anti-nuclear factor (NF)-κB
p65 (ab17742), anti-glial fibrillary acidic protein (GFAP; ab7260)
and anti-β-actin (ab8227) rabbit polyclonal primary antibodies and
goat anti-rabbit IgG-HRP and HRP-conjugated goat anti-mouse IgG
H&L secondary antibody (ab6789) were purchased from Abcam
(Cambridge, UK).
Rat model establishment
A total of 32 rats (age, 6–8 weeks; weight, 180–210
g) were obtained from the Department of Physiology at the
Affiliated Hospital of Weifang Medical College (Weifang, China).
Rat models of inflammation-induced pain were established by
subcutaneous intraplantar injection of CFA. Rats were randomly
divided into four groups (n=8): CFA, CFA plus bupivacaine, CFA plus
saline and untreated. Rats were deeply anesthetized by
intraperitoneal injection of sodium pentobarbital (50 mg/kg;
n=8/group). Subsequently, the right hind paw of the rat was
sterilized with iodophor and CFA (1 µl/g) were injected into the
rear portion of the plantar site. Once the rats had regained
consciousness, they were returned to captive animal management.
Rats were maintained at 20–25°C with a normal circadian rhythm, no
light glare or strong noise stimulation, and free access to water
and food.
Mechanical withdrawal threshold (MWT)
determination
The MWT of the rats was assessed between 9:00–11:00
in the morning. Mechanical paw withdrawal threshold was detected
using an electronic von Frey aesthesiometer. In a quiet
environment, the rats were placed in the bottom of a plexiglass
container with metal mesh cages (10×10×15 cm), and were allowed to
adapt to the environment for 20 min. Once the rats exhibited a calm
state, MWT was measured. The central skin of the foot was
vertically stimulated by a Von-Frey filament from bottom to top.
The duration of stimulation was set at 20 sec and stimulus
gradually increased from 0 to 50 g. Stimulation was automatically
terminated in response to rapid paw withdrawal. The experiment was
repeated in triplicate, with 5-min measurement intervals. The mean
of these three repeats was recorded as the mechanical withdrawal
threshold.
Rotarod test
The rotarod test was used to assess the motor
activity of the rats using rotarod apparatus. Data were recorded
prior to treatment (day 0) and on days 1, 3, 5 and 7
post-treatment. Briefly, rats were placed on the round bar of the
rotarod apparatus and the rotating rods were set in the Uniformly
Accelerating mode. Rotational speed increased from 3 to 30 rev/min
over a period of 180 sec. The duration that the rats were able to
remain on the rods was recorded. Experiments were repeated in
triplicate and the mean was subsequently calculated. Two days prior
to the experiment, the rats were trained on the device in order to
ensure they were familiar on the day of assessment.
Treatment of specimen
In the CFA-induced inflammatory pain model, symptoms
of pain were evident on days 1–3 following establishment.
Postoperative pain lasted ~2 weeks. Spinal cord samples were
harvested at 3 or 5 days post-CFA injection. Briefly, rats were
anesthetized with 10% chloral hydrate (3 ml/kg) by intraperitoneal
injection. Following the onset of anesthesia (diethyl ether), the
rats were fixed on the small animal anatomy desk. The lumbar
enlargement part of the rat's spinal cord was harvested and
weighed. Samples were kept at −80°C.
Western blot analysis
In order to examine the protein expression levels of
OX42, which is a spinal microglial marker, and GFAP, which is an
astrocyte marker, western blotting was performed. Samples were
lysed with RIPA buffer and whole proteins were obtained in cell
lysates. A total of 15 µg/well protein was separated by 10%
SDS-PAGE and subsequently transferred onto polyvinylidene
difluoride membranes. Membranes were blocked with 5% bovine serum
albumin for 30 min at room temperature prior to washing three times
with PBS buffer for 5 min. Following this, membranes were incubated
with anti-IκB-α rabbit polyclonal IgG (C21), anti-OX42 mouse
monoclonal IgG2a, anti-NF-κB p65 and anti-GFAP primary antibodies
(all 1:1,000) overnight at 4°C. Following washing with
Tris-buffered saline with Tween 20 three times (10 min each) at
room temperature, the membranes were incubated with goat
anti-rabbit IgG-HRP and goat anti-mouse IgG H&L (HRP) secondary
antibodies for 40 min at room temperature. Images were captured and
the blots were visualized using a gel imaging system (Bio-Rad
GelDoc XR; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA expression levels of TNF-α, IL-1β and IL-6
proinflammatory cytokines were detected by RT-qPCR. Total RNA was
extracted from the lumbar spinal cord by TRIzol (Takara
Biotechnology Co., Ltd., Dalian, China). Subsequently, cDNA samples
were transcribed using the PrimeScript® RT reagent kit
(Takara Biotechnology Co., Ltd.) according to the kit protocol. The
contents in the kit included PrimeScript RTase, 5X PrimeScript
buffer, RNase inhibitor, dNTP mixture, oligo dT primer, Ex Taq HS
DNA polymerase (5 U/µl) and RNase free dH2O. Following DNase
treatment (D2215; Takara Biotechnology Co., Ltd.), qPCR analysis
was performed to evaluate the expression levels of inflammatory
cytokines on an ABI 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The 20-µl reaction volume
contained 2 µl template, 0.25 µM of each pair of primers and 12.5
µl SYBR Green real-time PCR MasterMix (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Thermal cycling was performed as follows:
95°C for 10 min followed by 35 cycles of 95°C for 15 sec, 58°C for
20 sec, and 72°C for 30 sec. Primers were as follows: TNF-α,
forward ACT GAA CTT CGG GGT GATTG and reverse GCT TGG TGG TTT GCT
ACG AC; IL-1β, forward CAC CTT CTT TTC CTT CAT CTT TG and reverse
GTC GTT GCT TGT CTC TCC TTGTA; IL-6, forward TGA TGG ATG CTT CCA
AACTG and reverse GAG CAT TGG AAG TTG GGGTA; and β-actin, forward
CAT GTA CGT TGC TAT CCA GGC and reverse CTC CTT AAT GTC ACG CAC
GAT. mRNA expression levels of target genes were normalized to
those of β-actin according to the 2−∆∆Cq method
(22)
Statistical analysis
SPSS software (version 18.0, SPSS, Inc., Chicago,
IL, USA) was used for data analysis. Differences in the data were
analyzed with Student's t-test and were presented as the mean ±
standard error of the mean. P<0.01 was considered to indicate a
statistically significant difference.
Results
MWT significantly decreases after CFA
injection
A total of 100 µl CFA was subcutaneously injected
into the right hind foot of the rats to establish rat models of
inflammation-induced pain. Within 10 min, the right rear foot
appeared red and swollen. Spontaneous pain-related behaviors were
observed, including raising and licking of the rear foot on the
right side. MWT was detected in rats daily prior to and following
CFA injection. Variations in the MWT in the untreated left rear
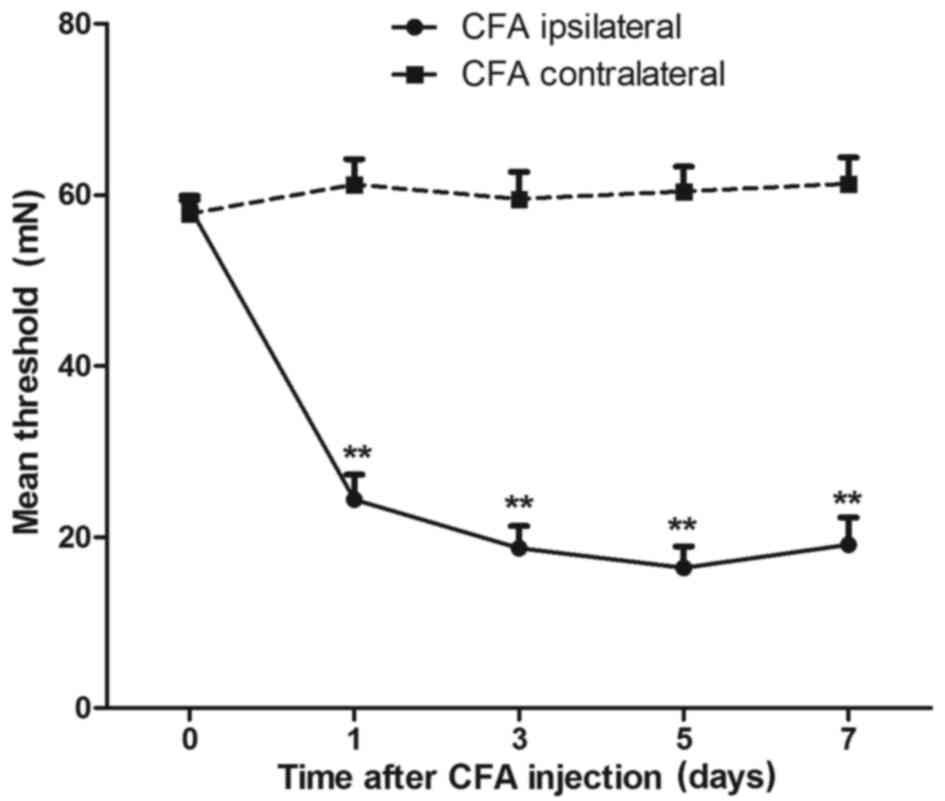
foot were used as controls. As shown in Fig. 1, the results demonstrated that the
MWT in the right rear foot significantly decreased since the 1st
day of CFA injection (P<0.01; n=8), and the MWT in the left rear
foot exhibited no obvious variation. The lowest MWT was detected 3
to 5 days after CFA injection (P<0.01; n=8). Therefore, the
subsequent experiments were conducted 3 to 5 days after CFA
injection.
Inflammation-induced pain does not
affect body weight
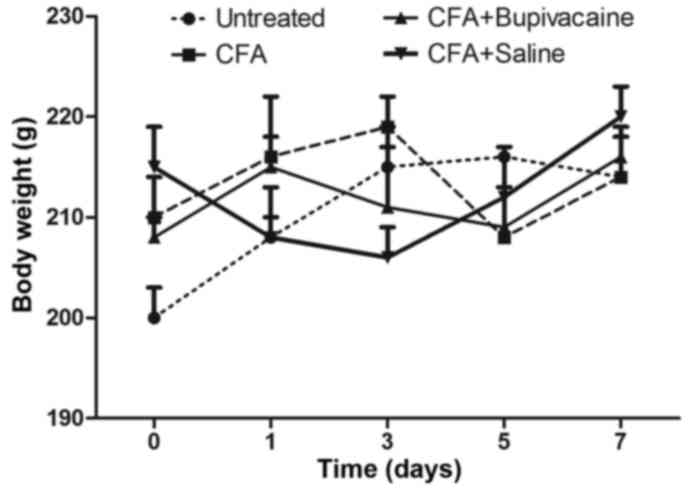
The respective body weights of the rats in the
various groups were determined prior to CFA injection and every two
days following injection. As shown in Fig. 2, the body weight of the rats in the
CFA, CFA plus bupivacaine and CFA plus saline solution groups
exhibited no statistical variations, compared with the untreated
group. The stable increase of the body weight of rats observed
among the different groups suggested that 100 µl CFA was an
appropriate dosage to construct the inflammatory pain model.
Bupivacaine increases the MWT of rats
treated with CFA
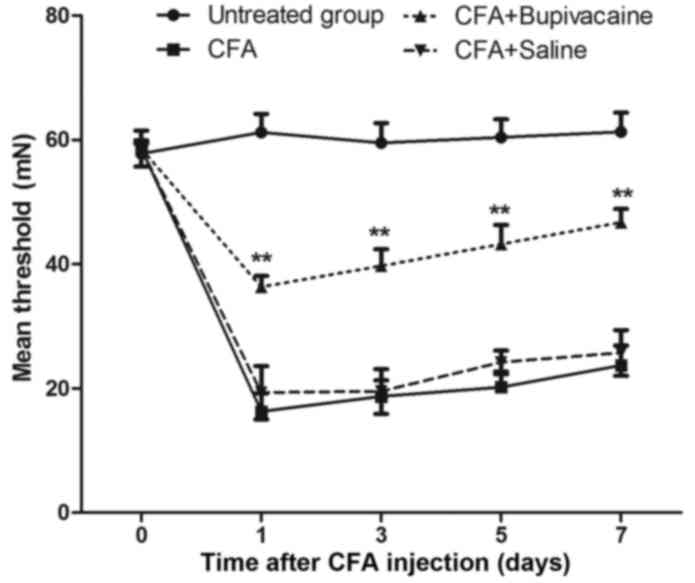
Following the successful construction of the rat
models of inflammation-induced pain. In order to test the effects
of bupivacaine on the inflammation-induced pain response, rats were
randomly divided into four equal groups (n=8): CFA, CFA plus
bupivacaine, CFA plus saline solution and untreated groups. As
shown in Fig. 3, the mean MWT was
significantly increased in the CFA plus bupivacaine group, as
compared with the CFA group (P<0.01). The MWT in the CFA plus
bupivacaine group remained lower than the untreated group.
Inflammation-induced pain does not
affect motor activity
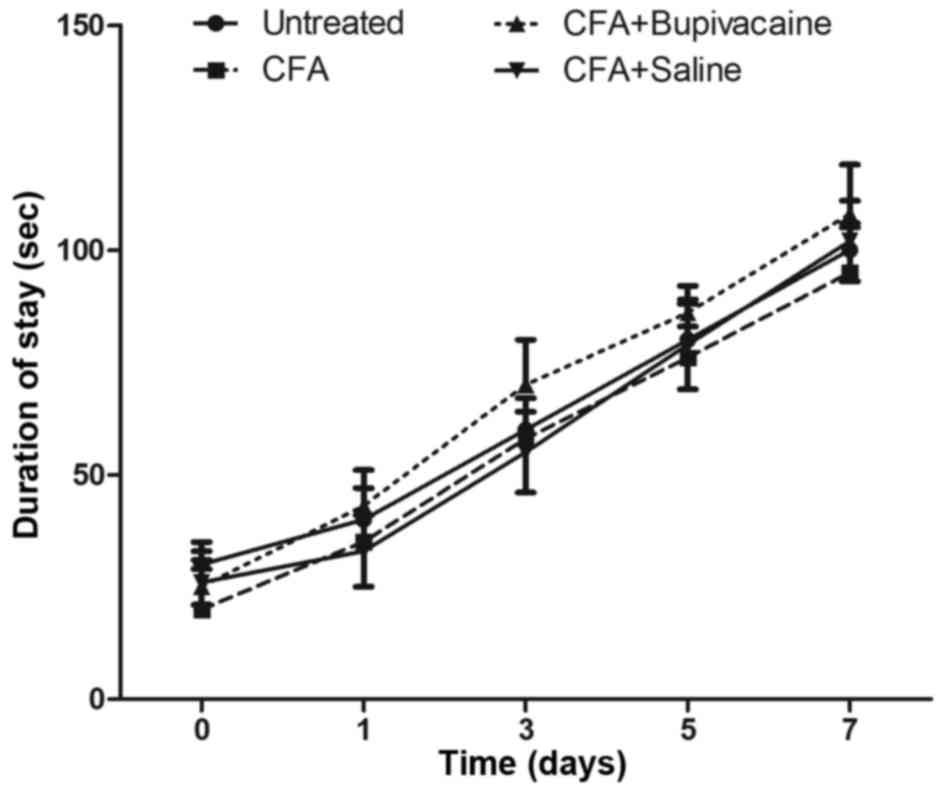
In order to test the effects of bupivacaine on the
functional exercise capacity of the rats, the rotarod test was used
to assess motor activity among the four groups. As shown in
Fig. 4, as the number of experiments
increased, the duration that the rats were able to remain on the
bar gradually increased. No statistical differences were detected
between the CFA plus bupivacaine group and the other groups
(P>0.05). These findings demonstrated that the motor activity of
rats in the CFA and CFA plus bupivacaine groups was not obviously
affected by the inflammatory response.
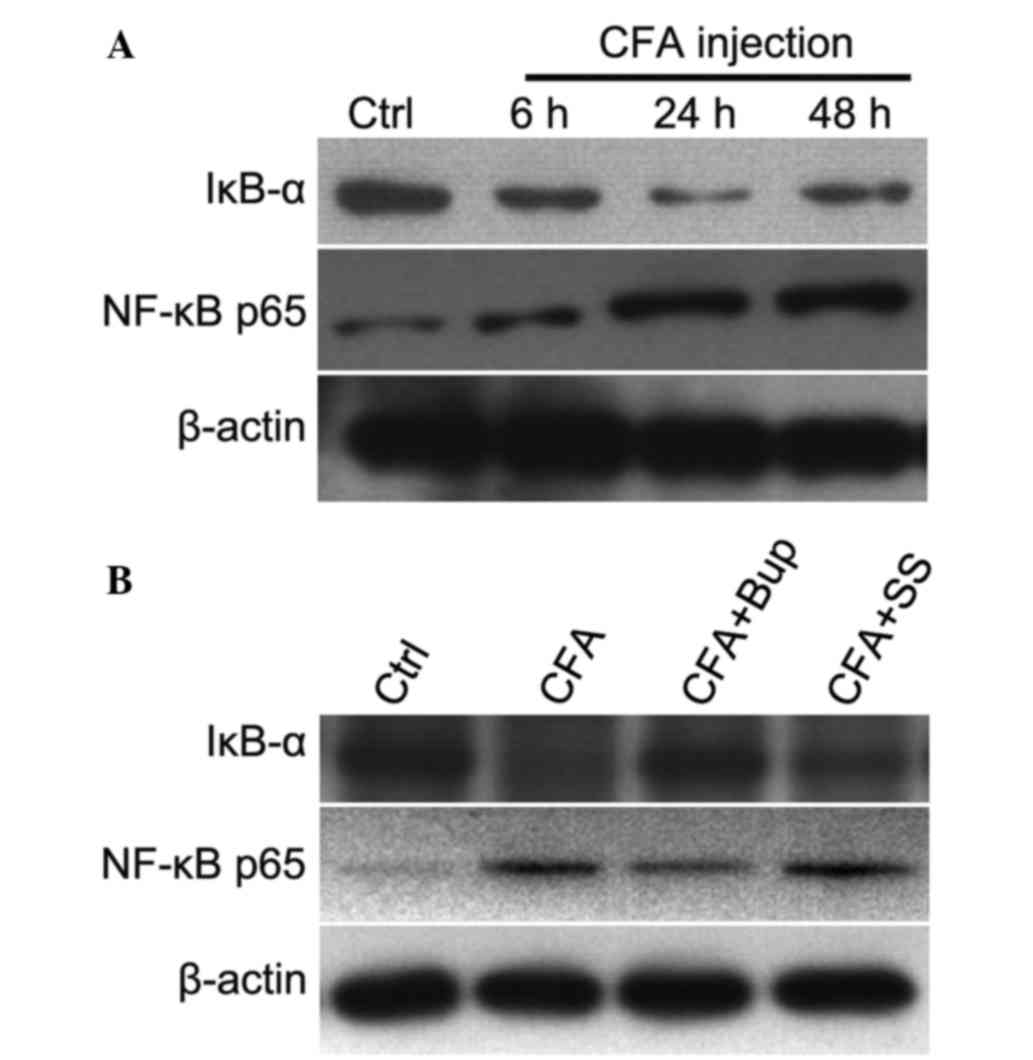
Bupivacaine inhibits NF-κB activation
in the dorsal horn of the lumbar spinal cord of model rats
NF-κB has an important role in various physiological
and pathological processes associated with pain; therefore, NF-kB
signaling pathway intervention may have an effective
antinociceptive effect (23,24). Activation of spinal NF-κB/p65 has
been demonstrated to contribute to peripheral inflammation, and
inflamed tissue may increase the excitability of spinal dorsal horn
neurons (25,26). In the present study, the expression
levels of IκB and nuclear NF-κB were detected in the dorsal horn of
the lumbar spinal cord after CFA injection. As shown in Fig. 5A, IκB expression levels decreased as
the time after CFA injection increased, as compared with the
untreated group. Conversely, the expression levels of the p65
subunit of NF-κB in the nucleus increased in a time-dependent
manner after CFA injection. Notably, bupivacaine treatment
increased the expression levels of IκB and decreased nuclear NF-κB
expression levels, as compared with the inflammatory group.
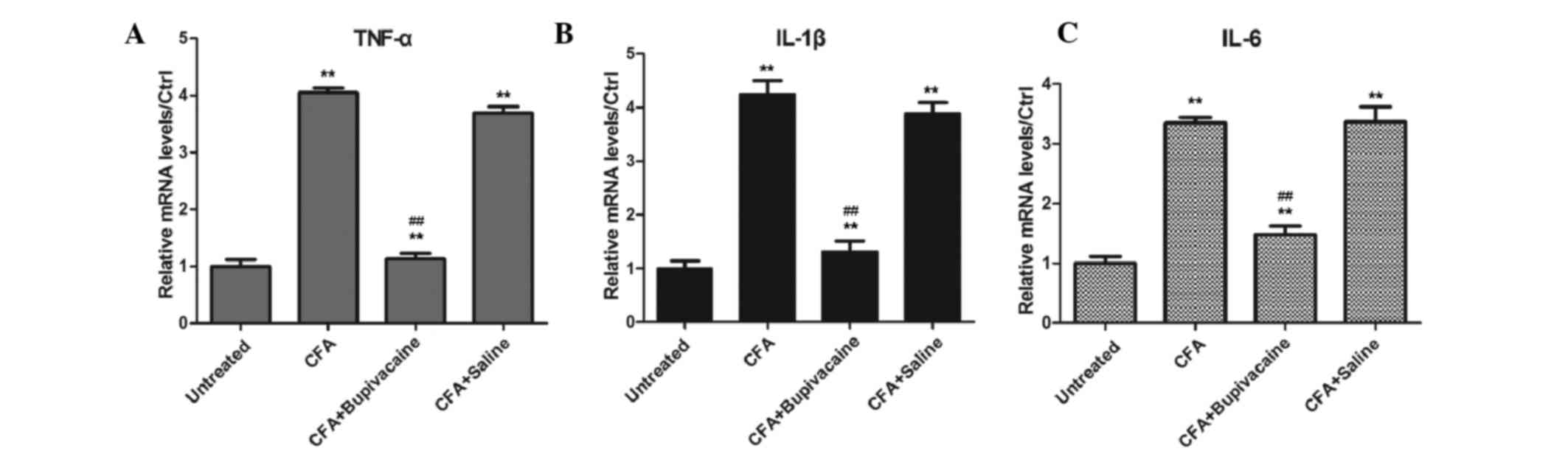
Bupivacaine inhibits the secretion of
inflammatory cytokines
The mRNA expression levels of inflammatory cytokines
were detected by RT-qPCR. As shown in Fig. 6, rats in the CFA group exhibited
significantly increased expression levels of TNF-α, IL-1β and IL-6,
as compared with the untreated group (P<0.05). Bupivacaine
treatment decreased the levels of inflammatory cytokines in CFA
plus bupivacaine group than CFA group (P<0.01).
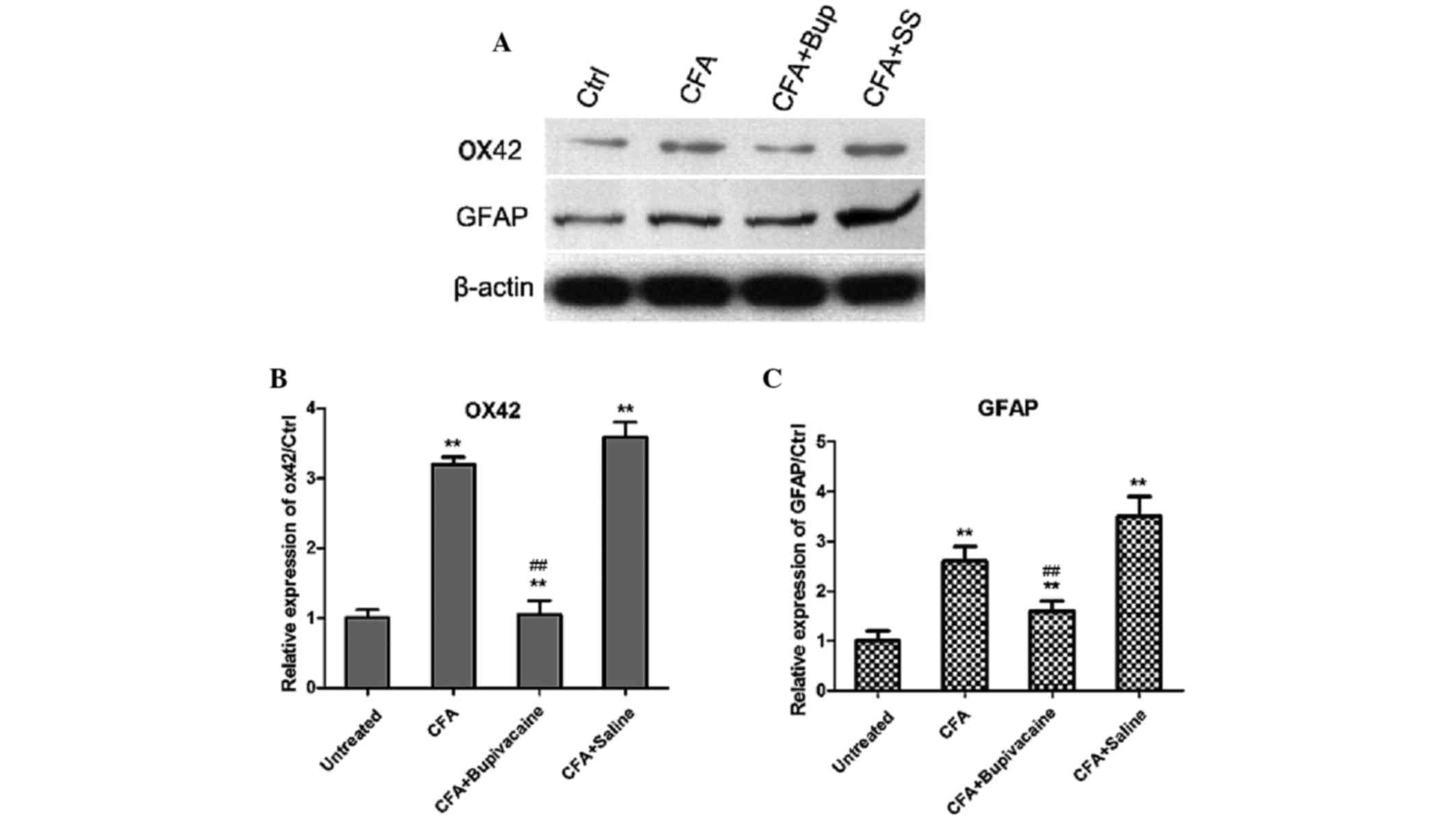
Bupivacaine decreases the expression
levels of OX42 and GFAP
The expression levels of OX42, which is a spinal
microglial marker, and GFAP, which is an astrocyte marker, were
detected by western blotting analysis. As shown in Fig. 7, the expression levels of OX42 and
GFAP were significantly increased in the CFA group, as compared
with the untreated group (P<0.05). However, bupivacaine
treatment significantly decreased the expression levels of OX42 and
GFAP, as compared with the rats in the CFA group (P<0.01). These
findings suggested that bupivacaine administration decreased the
activation of microglia and astrocytes in the rat models of
inflammatory pain.
Discussion
Inflammatory pain is a chronic pain disease caused
by tissue damage, including rheumatoid arthritis, omarthritis and
postoperative pain (9,27,28),
which has a particularly high incidence in China. It has extremely
important clinical implications; therefore, it is crucial that the
pathogenesis and development of inflammatory pain are investigated.
In the present study, rat models of CFA-induced inflammatory pain
were constructed to simulate the human disease. Rats models allow
us to explore the pathogenesis of the disease, clarify the
mechanism of disease progression and effectively investigate the
physiological and pathological processes. The results demonstrated
that the MWT of the right rear foot significantly decreased by ~20
mN on the 1st day of CFA injection, as compared with the untreated
left foot (**P<0.01, n=8). MWT data were stable from the 3rd day
for >2 weeks. The MWT in the untreated left rear foot exhibited
no significant variation. These findings indicated that rat models
of inflammatory pain were successfully constructed.
NF-κB is an important nuclear transcription factor
that regulates the expression of inflammation-related genes
(29). The inactive form of NF-κB,
which is bound by members of the IkB family, is typically located
in the cytoplasm (30). Various
stimuli activate NF-κB, which leads to the phosphorylation of IκB,
followed by ubiquitination and subsequent degradation (31,32).
This leads to the exposure of the nuclear localization signals on
NF-κB subunits; therefore, they translocate to the nucleus. The
present study investigated whether bupivacaine affects NF-κB
activation in the dorsal horn of the lumbar spinal cord. As
hypothesized, in the inflammatory group with CFA injection, western
blotting analysis demonstrated that IκB protein expression levels
were decreased and the p65 subunit of NF-κB translocated to the
nucleus, leading to the activation of inflammatory genes. mRNA
expression levels of inflammatory cytokines, including TNF-α, IL-1β
and IL-6, were also analyzed, and the expression levels of these
cytokines were also increased. Notably, in the CFA plus bupivacaine
group, the activation of NF-κB was suppressed and the expression
levels of inflammatory cytokines were inhibited, as compared with
the CFA group.
Bupivacaine hydrochloride injection, which is a long
acting amide local anesthetic, is a commonly used clinical
anesthesia (33). Due to its rapid
onset and increased duration of action, its application increases
peripheral nerve block, epidural block and subarachnoid block.
Bupivacaine is capable of combining with the membrane receptor of
the nerve and blocking the sodium ion channels (34). Moreover, bupivacaine is able to raise
the threshold of neural action potentials, slow the spreading of
nerve impulses and reduce the speed of the action potential,
thereby blocking the transmission of nerve impulses (35,36).
Previous studies have demonstrated that the analgesic effects of
isoflurane and ketamine are associated with the glial cells in the
spinal cord (37,38); however, few studies have investigated
the effects of bupivacaine local anesthetic.
In the present study, the degree of glial cell
activation was detected in the L4-5 area of the spinal cord in rat
models of CFA-induced inflammatory pain. The results demonstrated
that the expression levels of OX42 and GFAP significantly
increased, suggesting that the activation of spinal microglia and
astrocytes may be associated with inflammatory pain. Following
treatment with bupivacaine, the expression levels of OX42 and GFAP
were significantly decreased, as compared with the model group,
which demonstrated that bupivacaine was able to reduce the
activation of spinal microglia and astrocytes in the rat models of
inflammatory pain (39). The results
of the present study are consistent with a study by Suter et
al (40), which reported that
activation of spinal cord microglia contributed to the development
of neuropathic pain and microglial activation was associated with
mechanical allodynia. Therefore, the intensity of pain stimulation
and detection time of spinal microglia and astrocytes may be
associated with the activation of microglia and astrocytes. The
present findings demonstrated that treatment with bupivacaine
significantly decreased the activation of microglia and astrocytes
by increasing the expression of IκB and decreasing the expression
of NFκB in rat models of inflammatory pain. These results provide
clarification of the pathogenesis and mechanism of
inflammation-induced pain and may indicate provide novel
therapeutic strategies for the clinical treatment of pain.
References
|
1
|
Hu Y, Yu SY, Zuo LJ, Cao CJ, Wang F, Chen
ZJ, Du Y, Lian TH, Wang YJ, Chan P, et al: Parkinson disease with
REM sleep behavior disorder: Features, α-synuclein, and
inflammation. Neurology. 84:888–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Irwin MR, Olmstead RE, Ganz PA and Haque
R: Sleep disturbance, inflammation and depression risk in cancer
survivors. Brain Behav Immun. 30:(Suppl). S58–S67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andersen HH, Duroux M and Gazerani P:
MicroRNAs as modulators and biomarkers of inflammatory and
neuropathic pain conditions. Neurobiol Dis. 71:159–168. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan J, Ramadan K, Korczeniewska O, Anwer
MM, Benoliel R and Eliav E: Interleukin-10 levels in rat models of
nerve damage and neuropathic pain. Neurosci Lett. 592:99–106. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranoux D, Attal N, Morain F and Bouhassira
D: Botulinum toxin type A induces direct analgesic effects in
chronic neuropathic pain. Ann Neurol. 64:274–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jaggi AS and Singh N: Exploring the
potential of telmisartan in chronic constriction injury-induced
neuropathic pain in rats. Eur J Pharmacol. 667:215–221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zollner C, Mousa SA, Fischer O, et al:
Chronic morphine use does not induce peripheral tolerance in a rat
model of inflammatory pain. J Clin Invest. 118:1065–1073.
2008.PubMed/NCBI
|
|
8
|
Conrozier T, Mathieu P, Bonjean M, Marc
JF, Renevier JL and Balblanc JC: A complex of three natural
anti-inflammatory agents provides relief of osteoarthritis pain.
Altern Ther Health Med. 20:(Suppl 1). 32–37. 2014.PubMed/NCBI
|
|
9
|
Moalem G and Tracey DJ: Immune and
inflammatory mechanisms in neuropathic pain. Brain Res Rev.
51:240–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lorenzini L, Giuliani A, Giardino L and
Calza L: Laser acupuncture for acute inflammatory, visceral and
neuropathic pain relief: An experimental study in the laboratory
rat. Res Vet Sci. 88:159–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McHugh JM and McHugh WB: Pain:
Neuroanatomy, chemical mediators, and clinical implications. AACN
Clin Issues. 11:168–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaible HG, Ebersberger A and Von Banchet
GS: Mechanisms of pain in arthritis. Ann NY Acad Sci. 966:343–354.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gabriel AF, Marcus MA, Honig WM, Helgers N
and Joosten EA: Environmental housing affects the duration of
mechanical allodynia and the spinal astroglial activation in a rat
model of chronic inflammatory pain. Brain Res. 1276:83–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu XN, Zhang T, Qian NS, Guo XD, Yang HJ,
Huang KB, Luo GQ, Xiang W, Deng WT, Dai GH, et al: Antinociceptive
effects of endomorphin-2: Suppression of substance P release in the
inflammatory pain model rat. Neurochem Int. 82:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao X, Zhao XT, Xu LC, Yue LP, Liu FY,
Cai J, Liao FF, Kong JG, Xing GG, Yi M and Wan Y: Shp-1
dephosphorylates TRPV1 in dorsal root ganglion neurons and
alleviates CFA-induced inflammatory pain in rats. Pain.
156:597–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Q and Yaksh TL: A brief comparison of
the pathophysiology of inflammatory versus neuropathic pain. Curr
Opin Anaesthesiol. 24:400–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoki Y, Ohtori S, Takahashi K, Ino H,
Takahashi Y, Chiba T and Moriya H: Innervation of the lumbar
intervertebral disc by nerve growth factor-dependent neurons
related to inflammatory pain. Spine (Phila Pa 1976). 29:1077–1081.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Yuan G, Cendan CM, Nassar MA,
Lagerström MC, Kullander K, Gavazzi I and Wood JN:
Nociceptor-expressed ephrin-B2 regulates inflammatory and
neuropathic pain. Mol Pain. 6:772010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silva GD, Lopes PS, Fonoff ET and Pagano
RL: The spinal anti-inflammatory mechanism of motor cortex
stimulation: Cause of success and refractoriness in neuropathic
pain? J Neuroinflammation. 12:102015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burke NN, Geoghegan E, Kerr DM, Moriarty
O, Finn DP and Roche M: Altered neuropathic pain behaviour in a rat
model of depression is associated with changes in inflammatory gene
expression in the amygdala. Genes Brain Behav. 12:705–713.
2013.PubMed/NCBI
|
|
21
|
Ikeda H, Kiritoshi T and Murase K:
Contribution of microglia and astrocytes to the central
sensitization, inflammatory and neuropathic pain in the juvenile
rat. Mol Pain. 8:432012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puszynski K, Bertolusso R and Lipniacki T:
Crosstalk between p53 and nuclear factor-B systems: Pro- and
anti-apoptotic functions of NF-B. IET Syst Biol. 3:356–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Acquisto F, May MJ and Ghosh S:
Inhibition of nuclear factor kappa B (NF-B): An emerging theme in
anti-inflammatory therapies. Mol Interv. 2:22–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vincenzi F, Targa M, Corciulo C, Tabrizi
MA, Merighi S, Gessi S, Saponaro G, Baraldi PG, Borea PA and Varani
K: Antinociceptive effects of the selective CB2 agonist MT178 in
inflammatory and chronic rodent pain models. Pain. 154:864–873.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiechio S, Zammataro M, Morales ME,
Busceti CL, Drago F, Gereau RW, Copani A and Nicoletti F:
Epigenetic modulation of mGlu2 receptors by histone deacetylase
inhibitors in the treatment of inflammatory pain. Mol Pharmacol.
75:1014–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toulme E, Tsuda M, Khakh BS and Inoue K:
On the Role of ATP-Gated P2X Receptors in Acute, Inflammatory and
Neuropathic PainTranslational Pain Research: From Mouse to Man.
Kruger L and Light AR: Boca Raton, FL: 2010
|
|
28
|
Zhang J, Echeverry S, Lim TK, Lee SH, Shi
XQ and Huang H: Can modulating inflammatory response be a good
strategy to treat neuropathic pain? Curr Pharm Des. 21:831–839.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
HuangFu WC, Matsumoto K and Ninomiya-Tsuji
J: Osmotic stress blocks NF-kappaB-dependent inflammatory responses
by inhibiting ubiquitination of IkappaB. FEBS Lett. 581:5549–5554.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang ED, Wang CY, Xiong Y and Guan KL: A
role for NF-kappaB essential modifier/IkappaB kinase-gamma
(NEMO/IKKgamma) ubiquitination in the activation of the IkappaB
kinase complex by tumor necrosis factor-alpha. J Biol Chem.
278:37297–37305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Starokadomskyy P and Burstein E: Detection
of IkB Degradation dynamics and IkB-α ubiquitination. Methods Mol
Biol. 1280:15–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shambharkar PB, Blonska M, Pappu BP, Li H,
You Y, Sakurai H, Darnay BG, Hara H, Penninger J and Lin X:
Phosphorylation and ubiquitination of the IkappaB kinase complex by
two distinct signaling pathways. EMBO J. 26:1794–1805. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alebouyeh MR, Imani F, Rahimzadeh P and
Faiz SH: Evaluation of the efficacy of intrathecal injection of
amitriptyline and doxepin in spinal anesthesia in comparison with
bupivacaine in rats. Anesth Pain Med. 1:15–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwoerer AP, Scheel H and Friederich P: A
comparative analysis of bupivacaine and ropivacaine effects on
human cardiac SCN5A channels. Anesth Analg. 120:1226–1234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohamed SA and Abdel-Ghaffar HS: Effect of
the addition of clonidine to locally administered bupivacaine on
acute and chronic postmastectomy pain. J Clin Anesth. 25:20–27.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deruddre S, Combettes E, Estebe JP,
Duranteau J, Benhamou D, Beloeil H and Mazoit JX: Effects of a
bupivacaine nerve block on the axonal transport of Tumor Necrosis
Factor-alpha (TNF-alpha) in a rat model of carrageenan-induced
inflammation. Brain Behav Immun. 24:652–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka T, Kai S, Matsuyama T, Adachi T,
Fukuda K and Hirota K: General anesthetics inhibit LPS-induced
IL-1beta expression in glial cells. PloS One. 8:e829302013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyazaki H, Nakamura Y, Arai T and Kataoka
K: Increase of glutamate uptake in astrocytes: a possible mechanism
of action of volatile anesthetics. Anesthesiology. 86:1359–1366;
discussion 1358A. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shin JW, Pancaro C, Wang CF and Gerner P:
Low-dose systemic bupivacaine prevents the development of allodynia
after thoracotomy in rats. Anesth Analg. 107:1587–1591. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suter MR, Berta T, Gao YJ, Decosterd I and
Ji RR: Large A-fiber activity is required for microglial
proliferation and p38 MAPK activation in the spinal cord: Different
effects of resiniferatoxin and bupivacaine on spinal microglial
changes after spared nerve injury. Mol Pain. 5:532009. View Article : Google Scholar : PubMed/NCBI
|