Introduction
Drug-eluting stents (DESs) have been widely used for
interventional therapy in patients with coronary artery disease,
since they remarkably reduce in-stent restenosis compared with bare
metal stents (1,2). However, the risk of late and very late
stent thrombosis remains and is associated with delayed vascular
healing and impaired endothelialization due to the lack of
biodegradation of the polymer coatings and potentially
polymer-related inflammation and hypersensitivity following stent
implantation (3–5). To improve the biosafety, several
biocompatible or biodegradable polymers within novel DESs have been
developed. The polymer-free DES has since been proven to be an
appropriate method to suppress neointimal proliferation and
minimize polymer-associated adverse effects. Furthermore, excellent
mechanical flexibility and biocompatibility, in addition to stable
drug release performance, have been demonstrated in DESs with a
nanostructured coating in vitro (6–8). The
nano polymer-free sirolimus-eluting stent (SES) is a novel
polymer-free DES with a nanoporous surface that can act as an
adjuvant for drugs and provide better re-endothelialization and
inhibition of neointimal proliferation (9). Optical coherence tomography (OCT) is a
high resolution imaging modality and has been considered as the
ideal tool for the evaluation of neointimal tissue and vascular
responses following stent implantation in vivo (10–13).
However, it is unclear whether a lack of polymer induces a
different vascular response in drug-eluting stents and vascular
response profiles following nano polymer-free SES implantation
assessed by OCT have not been investigated.
Therefore, the aim of our study was to determine the
effects of nano polymer-free SES on neointimal formation using by
OCT.
Materials and methods
Study design
A total of 8 male juvenile Chinese pigs with a body
weight between 20 and 30 kg were used in the present study. These
were were obtained from the Experimental Pig Production Institute
(Fangshan District, Beijing, China) and were maintained at 18–25°C,
40–60% humidity, with >10 h of light every day; food was
provided twice a day at 2–3% of the pigs' total weight, and free
access to water was provided. Nano polymer-free SES (Lepu Medical
Technology Co., Ltd., Beijing, China) were randomly implanted in
the right coronary artery, left anterior descending or left
circumflex coronary artery with two stents in each porcine model
(one stent per vessel). In order to assess different stages of
neointimal formation, all animals were divided into two groups:
Follow-up after stent implantation of 3 (n=4) or 6 months (n=4). At
the follow-up time point, the animals were sacrificed following
completion of coronary angiography and OCT evaluation to obtain
specimens for histological analysis of stented arterial segments.
The study protocol was approved by the Institutional Animal Care
and Use Committee at Beijing Tiantan Hospital (Beijing, China).
Coronary interventional procedure
Three days prior to the coronary procedure, animals
were administered 300 mg aspirin (Bayer, Newbury, UK) and 75 mg
clopidogrel (Sanofi S.A., Paris, France). Thereafter, antiplatelet
therapy of 75 mg clopidogrel and 100 mg aspirin was administered
daily throughout the study in all animals.
Animals were anesthetized with 0.1–0.2 mg/kg
midazolam (Nhwa Pharmaceutical Co., Ltd., Jiangsu, China) and
0.25–0.3 ml/kg xylazine (Sangon Biological Engineering Co. Ltd.,
Shanghai, China) and an arterial sheath (6 French; Cordis
Corporation, Hialeah, FL, USA) was placed in the right femoral
artery by cut-down with a sterile technique. Coronary
catheterization was performed following administration of
intravenous heparin (5,000 units). Baseline angiography was
acquired and all stents were placed at a targeted 1.1:1 to 1.2:1
stent-to-vessel ratio compared with the reference vessel diameter
to induce a moderate vessel injury and promote neointimal
formation. Stents of 14, 15 or 18 mm length and diameters of 2.5,
2.75 and 3.0 mm were implanted according to the coronary artery
size.
OCT acquisition and analysis
Frequency domain (FD)-OCT imaging was performed
using the C7-XR OCT intravascular imaging system (LightLab Imaging,
Inc., Westford, MA, USA). During FD-OCT image acquisition, a
continuous non-occlusive contrast as a flush was administrated to
replace coronary blood flow and automatic pullbacks were performed
at a rate of 20 mm/sec and 100 frames/sec. Off-line OCT analysis
was performed with LightLab Imaging software (LightLab Imaging,
Inc.).
For quantitative analysis, cross-sectional OCT
images were analyzed at 1-mm longitudinal steps throughout the
pullback from distal stent edge to proximal stent edge. OCT images
were excluded from the analysis if stent struts were not visible on
the screen, bifurcation cross-sections had side branches or if
residual blood was mistaken for neointimal tissue. The lumen and
stent were manually traced and stent struts were positioned
manually in the center of the stent strut which showed a bright
‘blooming’ appearance (14). The
following parameters were measured: Minimal luminal diameter,
luminal area, stent area, neointimal area (stent area - lumen area)
and neointimal burden (mean neointimal area / mean stent area ×
100%). To analyze neointimal thickness, the distance between the
center of each stent strut and the luminal border was measured in
the direction of the center of gravity.
Quantitative analyses of all OCT images were
performed by two independent investigators who were blinded to the
angiographic data and clinical presentations.
Interobserver variability
Two independent investigators analyzed 50
cross-sectional images without artifacts. Inter-observer agreement
was determined by calculating values for differences in
measurements of neointimal area and neointimal thickness.
Histological analysis
All animals were sacrificed following follow-up
coronary angiography and OCT imaging; this was performed using
intravenously administered 30 ml 10% potassium chloride.
Immediately following sacrifice, the hearts were excised and the
stented coronary artery segments were harvested from the heart by
careful dissection, fixed by immersion in 10% formalin, dehydrated
in a graded series of ethanol and embedded in methyl methacrylate
resin (Huayi Acrylic Acid Co., Ltd., Shanghai, China). Following
polymerization, sections measuring ~1.3 mm were sawed from each
stent, beginning at the distal stent edge. The artery-stent
specimens were cut on a rotary microtome at 100 µm from the
proximal through the distal margin of the stent and stained with
hematoxylin and eosin, and elastic Van Gieson stains. There were no
histological sections lost due to processing, and all sections were
of excellent quality. Morphometric analysis was performed by
Image-Pro Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA). Morphologic analysis of strut-associated
inflammation, stent endothelialization and arterial injury were
completed using previously described methods (3,15,16).
Briefly, the inflammatory score for each individual strut was
graded as follows: 0, No inflammatory cells surrounding the strut;
1, light, non-circumferential lymphohistiocytic infiltrate
surrounding the strut; 2, localized, moderate-to-dense cellular
aggregate surrounding the strut noncircumferentially; and 3,
circumferential dense lymphohistiocytic cell infiltration of the
strut. The stent endothelialization score was defined as the extent
of the circumference of the arterial lumen covered by endothelial
cells and was scored between 1 and 3 (1, 25%; 2, 25 to 75%; 3,
>75%). The intimal fibrin content was graded as follows: 1,
Focal residual fibrin involving any portion of the artery and for
moderate fibrin deposition adjacent to the strut involving 25% of
the circumference of the artery; 2, moderate fibrin deposition
involving 25% of the circumference of the artery or heavy
deposition of fibrin adjacent to and between stent struts involving
25% of the circumference of the artery; or 3, heavy deposition of
fibrin involving 25% of the circumference of the artery. The
intimal smooth muscle cell (SMC) content was scored as follows: 1,
Sparse SMC density involving any portion of the artery and for
moderate SMC infiltration less than the full thickness of the
neointima involving <25% of the circumference of the artery; 2,
moderate SMC infiltration less than the full thickness of the
neointima involving >25% of the circumference of the artery, or
dense SMC content the full thickness of the neointima involving
<25% of the circumference of the artery; or 3, dense SMC content
the full thickness of the neointima involving >25% of the
circumference of the artery.
All histomorphological analyses were performed by a
single independent investigator blinded to the study.
Statistical analysis
Variables were analyzed for normal distribution
using the Kolmogorov-Smirnov test. Continuous values following a
normal distribution were expressed as the mean ± standard
deviation, and those not normally distributed were indicated as the
median with interquartile range. Categorical variables were
expressed as frequencies and percentages. Differences between the
two groups were determined using Student's t-test or Mann-Whitney U
test, as appropriate for continuous variables. The χ2
test or Fisher exact test was used as appropriate to compare
categorical variables. Agreement between the two observers
(interobserver variability) was investigated using kappa analysis.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS version
20.0 software (IBM SPSS, Armonk, NY, USA).
Results
General findings
A total of 16 nano polymer-free SES were
successfully implanted in the coronary arteries of 8 pigs. All pigs
survived without problems until sacrifice. The survival time of
pigs and stent characteristics are presented in Table I.
 | Table I.Pig survival time and stent
characteristics. |
Table I.
Pig survival time and stent
characteristics.
| Pig no. | Survival time | Stent-1 location | Stent-1 size | Stent-2 location | Stent-2 size |
|---|
| 1 | 6 months | LCX | 2.75×18 mm | RCA | 2.75×18 mm |
| 2 | 6 months | LCX | 2.75×14 mm | RCA | 2.5×14 mm |
| 3 | 6 months | LAD | 2.5×18 mm | RCA | 2.5×15 mm |
| 4 | 6 months | LCX | 2.5×15 mm | RCA | 2.5×18 mm |
| 5 | 3 months | LCX | 2.75×15 mm | RCA | 2.75×15 mm |
| 6 | 3 months | LCX | 2.5×15 mm | RCA | 3.0×18 mm |
| 7 | 3 months | LCX | 2.75×18 mm | RCA | 2.75×18 mm |
| 8 | 3 months | LAD | 2.5×18 mm | RCA | 2.5×18 mm |
OCT quantitative analysis
The results of OCT quantitative analysis of the
stents are presented in Table II.
Lumen area (2.73±0.66 vs. 3.00±0.65 mm2, P<0.001) and
stent area (3.86±0.39 vs. 4.05±0.59 mm2, P<0.001) at
6 months were significantly smaller compared with those at 3
months. In addition, at 6 months, neointimal thickness (193.3±109.5
vs. 167.2±119.7 µm, P=0.023) and neointimal burden (29.3±14.3 vs.
24.8±17.4%, P=0.006) significantly increased compared with at 3
months; neointimal area increased from between 3 and 6 months, but
this difference was not significant.
 | Table II.Quantitative optical coherence
tomography analysis. |
Table II.
Quantitative optical coherence
tomography analysis.
| Parameter | 3 months (274
sections) | 6 months (305
sections) | P-value |
|---|
| Lumen area,
mm2 | 3.00±0.65 | 2.73±0.66 | <0.001 |
| Stent area,
mm2 | 4.05±0.59 | 3.86±0.39 | <0.001 |
| Neointimal area,
mm2 | 1.06±0.80 | 1.12±0.55 | 0.35 |
| Neointimal burden,
% | 24.8±17.4 | 29.3±14.3 | 0.006 |
| Neointimal thickness,
µm | 167.2±119.7 | 193.3±109.5 | 0.023 |
Reproducibility of qualitative OCT
analysis
Interobserver variability for the quantitative OCT
assessment showed good concordance: k=0.91 for neointimal area and
k=0.81 for neointimal thickness.
Histological analysis
The histomorphometry and semi-quantitative scoring
for arterial injury, inflammation and stent endothelialization at 3
and 6 months after nano polymer-free SES implantation are
summarized in Table III. Marked
stent endothelialization was achieved at 3 and 6 months. However,
the endothelialization score was greater at 6 months compared with
at 3 months (2.85±0.36 vs. 2.52±0.60, P<0.001). In addition, at
3 months, nano polymer-free SES showed a significantly higher
inflammatory score [0 (0, 1) vs. 0 (0, 0), P<0.001] and fibrin
score [0 (0, 1) vs. 0 (0, 0), P<0.001] compared with at 6
months. There were no significant differences in injury score and
intimal smooth muscle cell content between 3 and 6 months.
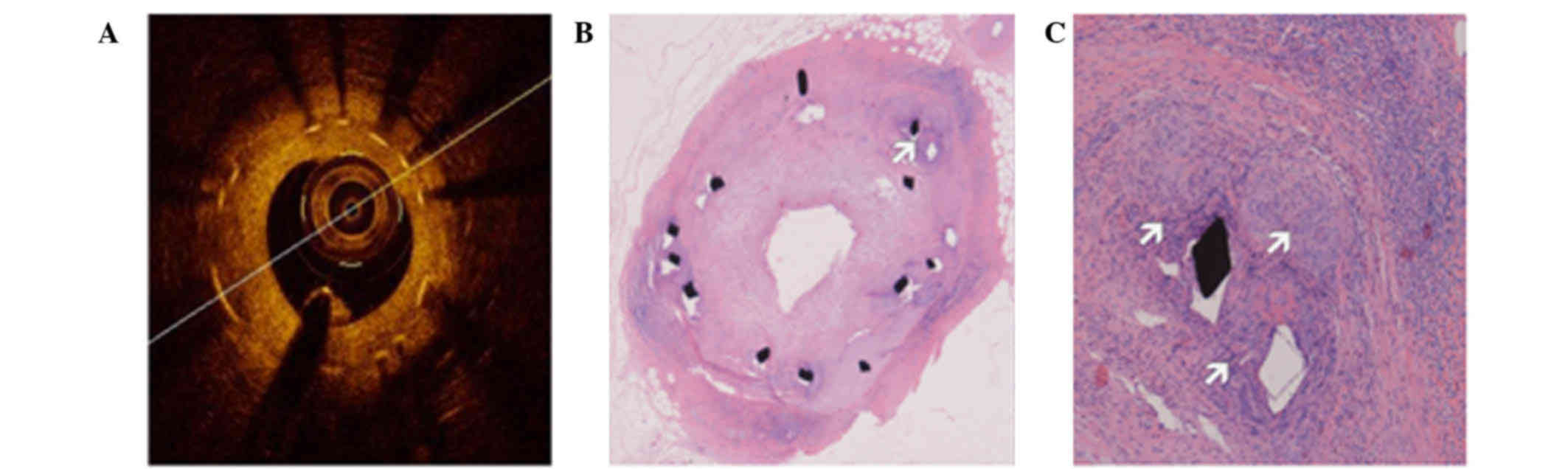
Representative images of OCT and histomorphologic sections at 3
months after nano polymer-free SES implantation are presented in
Fig. 1.
 | Table III.Histological analysis. |
Table III.
Histological analysis.
| Parameter | 3 months (189
sections) | 6 months (151
sections) | P-value |
|---|
| Inflammation
scorea | 0 (0, 1) | 0 (0, 0) | <0.001 |
| Injury score | 1.26±1.07 | 1.25±1.01 | 0.91 |
| Endotheliazation
score | 2.52±0.60 | 2.85±0.36 | <0.001 |
| Intimal SMC
content | 2.84±0.37 | 2.76±0.58 | 0.14 |
| Fibrin
scorea | 0 (0, 1) | 0 (0, 0) | <0.001 |
Discussion
The present study reports the vascular response to
nano polymer-free SES in a pig coronary model. The primary findings
of the present study were that following nano polymer-free SES
implantation (1), endothelialization
was achieved at 3 months but neointimal proliferation was more
significant at 6 months; (2) In
addition, strut-associated inflammation was more frequently
observed at 3 months compared with at 6 months.
DESs have markedly reduced restenosis compared with
bare metal stents, owing to the controlled release of
anti-proliferative drugs from polymers (17). However, there is evidence that the
durable polymers may lead to delayed vascular healing and
reendothelialization, and localized hypersensitivity reactions and
inflammation, resulting in a high frequency of in-stent restenosis
and/or late stent thrombosis (4,18–20). The
most effective solution appears to be the application of drugs to
the stent surface without polymers to eliminate the adverse effects
associated with the polymer (21).
Previously, the polymer-free DES as an emerging technology has been
proved to be a feasible and valuable method to inhibit neointimal
proliferation without the potential of late polymer-related adverse
effects (7,8). The nano polymer-free SES is a
polymer-free DES utilizing nanometer-size pore technology, with
nanoporous cavities that can be used as drug carriers to store and
control the release of anti-proliferative drugs; kinetic release
data indicate that ~85% of the drug is released within 1 month
after nano polymer-free SES implantation (22). Several intravascular ultrasound and
histological observations have demonstrated that polymer free SES
shows a sustained neointimal suppression and reduced inflammation
compared with polymer coated SES (9,23).
However, a higher quality of intravascular imaging is required to
fully evaluate vascular response following the implantation of nano
polymer-free SES.
A previous study performed by the authors of the
present study revealed that, as a novel intravascular imaging
modality, OCT can provide insights into the characteristics of
atherosclerotic plaques and neointimal coverage (24). In addition, the recently developed FD
interferometry analysis OCT system allows for faster image
acquisition (≤20 mm/sec) and greater scan depths that enable its
application in large caliber vessels (25). As a result, FD-OCT is the preferred
intravascular imaging modality for the in vivo atheromatous
plaque characterization, and post-stenting assessment in patients
with coronary disease (26).
Therefore, the present study evaluated nano polymer-free SES for
vascular response, endothelialization and inhibition of neointimal
hyperplasia by FD-OCT and pathology in an animal model. The results
of the current study demonstrated that nano polymer-free SES
effectively inhibits neointimal formation for the first 3 months
and that late neointimal formation occurs at 6 months, in part
owing to inflammation and cellular proliferation. Previous
pharmacokinetic studies have revealed that nano polymer-free SES
can effectively deliver drugs to the local coronary artery and
release the drug rapidly; such a release profile was favorable for
rapid endothelialization of nano polymer-free SES (27). Although a previous study demonstrated
that polymer free SES showed less inflammation and improved
arterial healing in rabbits (22),
histology analysis at 3 months in the present study demonstrated a
markedly higher level of inflammation. Notably, the fibrin score in
the current study increased progressively in 3 months after
implantation of nano polymer-free SES. Therefore, the present study
demonstrates that chronic inflammation is associated with
neointimal formation and may result in further neointimal
proliferation over time following nano polymer-free SES
placement.
There are a number of limitations of the present
study. Firstly, the experimental animal did not develop
atherosclerosis and nano polymer-free SESs were implanted in normal
coronary arteries. Thus, the results may not interpret arterial
response in real-world coronary artery lesions. Secondly, vascular
responses of nano polymer-free SES were investigated but were not
compared with common polymer-coated SES. Thirdly, the sample size
was small and bias existed; therefore, the results can not be
simply transferred to clinical practice. Finally, the follow-up
duration was short and longer follow-up is required.
In conclusion, nano polymer-free SES achieves
endothelialization at 3 months but neointimal proliferation is more
significant at 6 months and may be attributed to strut-associated
inflammation.
Acknowledgements
The authors wish to acknowledge the expert technical
assistance of the staff at Beijing Pinggu Hospital (Beijing, China)
with the animal experimental phases of this study. The present
study was funded by Beijing Municipal Commission of Health and
Family Planning (grant no. 2011-3-026).
References
|
1
|
Moses JW, Leon MB, Popma JJ, Fitzgerald
PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams
DO, Teirstein PS, et al: SIRIUS Investigators: Sirolimus-eluting
stents versus standard stents in patients with stenosis in a native
coronary artery. N Engl J Med. 349:1315–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stone GW, Ellis SG, Cox DA, Hermiller J,
O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J,
et al: TAXUS-IV Investigators: A polymer-based, paclitaxel-eluting
stent in patients with coronary artery disease. N Engl J Med.
350:221–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joner M, Finn AV, Farb A, Mont EK,
Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK and Virmani R:
Pathology of drug-eluting stents in humans: Delayed healing and
late thrombotic risk. J Am Coll Cardiol. 48:193–202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Camenzind E, Steg PG and Wijns W: Stent
thrombosis late after implantation of first-generation drug-eluting
stents: A cause for concern. Circulation. 115:1440–1455; discussion
1455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakazawa G, Finn AV, Vorpahl M, Ladich ER,
Kolodgie FD and Virmani R: Coronary responses and differential
mechanisms of late stent thrombosis attributed to first-generation
sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol.
57:390–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hausleiter J, Kastrati A, Wessely R, Dibra
A, Mehilli J, Schratzenstaller T, Graf I, Renke-Gluszko M, Behnisch
B, Dirschinger J, et al: Investigators of the Individualizable
Durg-Eluting Stent System to Abrogate Restenosis Project:
Prevention of restenosis by a novel drug-eluting stent system with
a dose-adjustable, polymer-free, on-site stent coating. Eur Heart
J. 26:1475–1481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehilli J, Kastrati A, Wessely R, Dibra A,
Hausleiter J, Jaschke B, Dirschinger J and Schömig A: Intracoronary
Stenting and Angiographic Restenosis-Test Equivalence Between 2
Drug-Eluting Stents (ISAR-TEST) Trial Investigators: Randomized
trial of a nonpolymer-based rapamycin-eluting stent versus a
polymer-based paclitaxel-eluting stent for the reduction of late
lumen loss. Circulation. 113:273–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruef J, Störger H, Schwarz F and Haase J:
Comparison of a polymer-free rapamycin-eluting stent (YUKON) with a
polymer-based paclitaxel-eluting stent (TAXUS) in real-world
coronary artery lesions. Catheter Cardiovasc Interv. 71:333–339.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen M, Zheng B, Wu Z, Peng HY, Wang XG,
Zhang B and Huo Y: Efficacy and safety of a novel nano-porous
polymer-free sirolimus-eluting stent in pigs. Chin Med J (Engl).
126:4731–4735. 2013.PubMed/NCBI
|
|
10
|
Gonzalo N, Serruys PW, Okamura T, van
Beusekom HM, Garcia-Garcia HM, van Soest G, van der Giessen W and
Regar E: Optical coherence tomography patterns of stent restenosis.
Am Heart J. 158:284–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prati F, Regar E, Mintz GS, Arbustini E,
Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, et
al: Expert's OCT Review Document: Expert review document on
methodology, terminology and clinical applications of optical
coherence tomography: Physical principles, methodology of image
acquisition and clinical application for assessment of coronary
arteries and atherosclerosis. Eur Heart J. 31:401–415. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murata A, Wallace-Bradley D, Tellez A,
Alviar C, Aboodi M, Sheehy A, Coleman L, Perkins L, Nakazawa G,
Mintz G, et al: Accuracy of optical coherence tomography in the
evaluation of neointimal coverage after stent implantation. JACC
Cardiovasc Imaging. 3:76–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Q, Suzuki N, Kozuma K, Miyagawa M,
Nomura T, Kawashima H, Shiratori Y, Ishikawa S, Kyono H and Isshiki
T: Quantitative optical coherence tomography analysis for late
in-stent restenotic lesions. Int Heart J. 56:13–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guagliumi G, Musumeci G, Sirbu V, Bezerra
HG, Suzuki N, Fiocca L, Matiashvili A, Lortkipanidze N, Trivisonno
A, Valsecchi O, et al: ODESSA Trial Investigators: Optical
coherence tomography assessment of in vivo vascular response after
implantation of overlapping bare-metal and drug-eluting stents.
JACC Cardiovasc Interv. 3:531–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carter AJ, Aggarwal M, Kopia GA, Tio F,
Tsao PS, Kolata R, Yeung AC, Llanos G, Dooley J and Falotico R:
Long-term effects of polymer-based, slow-release, sirolimus-eluting
stents in a porcine coronary model. Cardiovasc Res. 63:617–624.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Virmani R, Kolodgie FD and Farb A:
Drug-eluting stents: Are they really safe? Am Heart Hosp J.
2:85–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stone GW, Moses JW, Ellis SG, Schofer J,
Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane
AJ, et al: Safety and efficacy of sirolimus- and paclitaxel-eluting
coronary stents. N Engl J Med. 356:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pfisterer M, Brunner-La Rocca HP, Buser
PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F,
Osswald S and Kaiser C: BASKET-LATE Investigators: Late clinical
events after clopidogrel discontinuation may limit the benefit of
drug-eluting stents: An observational study of drug-eluting versus
bare-metal stents. J Am Coll Cardiol. 48:2584–2591. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nebeker JR, Virmani R, Bennett CL, Hoffman
JM, Samore MH, Alvarez J, Davidson CJ, McKoy JM, Raisch DW,
Whisenant BK, et al: Hypersensitivity cases associated with
drug-eluting coronary stents: A review of available cases from the
Research on Adverse Drug Events and Reports (RADAR) project. J Am
Coll Cardiol. 47:175–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finn AV, Nakazawa G, Joner M, Kolodgie FD,
Mont EK, Gold HK and Virmani R: Vascular responses to drug eluting
stents: Importance of delayed healing. Arterioscler Thromb Vasc
Biol. 27:1500–1510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiratori Y, Cola C, Brugaletta S,
Alvarez-Contreras L, Martín-Yuste V, del Blanco BG, Ruiz-Salmeron
R, Díaz J, Pinar E, Martí V, García-Picart J and Sabaté M:
Randomized comparison between polymer-free versus polymer-based
paclitaxel-eluting stent: Two-year final clinical results. Circ
Cardiovasc Interv. 7:312–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Chen F, Muramatsu T, Xu B, Li Z,
Ge J, He Q, Yang Z, Li S, Wang L, et al: Nine-month angiographic
and two-year clinical follow-up of polymer-free sirolimus-eluting
stent versus durable-polymer sirolimus-eluting stent for coronary
artery disease: The Nano randomized trial. Chin Med J (Engl).
127:2153–2158. 2014.PubMed/NCBI
|
|
23
|
John MC, Wessely R, Kastrati A, Schömig A,
Joner M, Uchihashi M, Crimins J, Lajoie S, Kolodgie FD, Gold HK, et
al: Differential healing responses in polymer- and nonpolymer-based
sirolimus-eluting stents. JACC Cardiovasc Interv. 1:535–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen BX, Ma FY, Luo W, Ruan JH, Xie WL,
Zhao XZ, Sun SH, Guo XM, Wang F, Tian T and Chu XW: Neointimal
coverage of bare-metal and sirolimus-eluting stents evaluated with
optical coherence tomography. Heart. 94:566–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mandelias K, Tsantis S, Spiliopoulos S,
Katsakiori PF, Karnabatidis D, Nikiforidis GC and Kagadis GC:
Automatic quantitative analysis of in-stent restenosis using FD-OCT
in vivo intra-arterial imaging. Med Phys. 40:0631012013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bezerra HG, Costa MA, Guagliumi G, Rollins
AM and Simon DI: Intracoronary optical coherence tomography: A
comprehensive review clinical and research applications. JACC
Cardiovasc Interv. 2:1035–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia H, Liu H, Kong J, Hou J, Wu J, Zhang
M, Tian J, Liu H, Ma L, Hu S, et al: A novel polymer-free
paclitaxel-eluting stent with a nanoporous surface for rapid
endothelialization and inhibition of intimal hyperplasia:
Comparison with a polymer-based sirolimus-eluting stent and bare
metal stent in a porcine model. J Biomed Mater Res A. 98:629–637.
2011. View Article : Google Scholar : PubMed/NCBI
|