Introduction
Erectile dysfunction (ED) is a prevalent health
problem worldwide and its incidence is rising (1,2). The
clinical features of ED are strongly associated with age; although
a number of techniques have been used to treat ED (3–5), the
incidence rates of ED in men aged <59 years, 60–69 years and
>69 years are 12, 22 and 30%, respectively (2,6). ED not
only has a significant impact on psychosocial and physical health,
but also affects the life quality of patients and their partners.
Furthermore, ED is an emerging risk marker for cardiovascular
disease, including ischemic heart disease, heart failure and
peripheral vascular disease (7).
Therefore, investigating the molecular mechanisms of age-associated
ED may facilitate the prevention of disease progression and the
development of novel therapies for treatment of this disease.
ED is characterized by the persistent inability to
attain or maintain a penile erection sufficient to permit
satisfactory sexual intercourse (6).
Numerous factors have been found to be closely associated with
erectile function. For instance, neuronal nitric oxide synthase
(nNOS) has been shown to regulate the recovery of erectile function
following partial cavernous nerve injury and exerts a suppressive
role by inducing apoptotic change in penile tissue (8). Hurt et al (9) also reported that cyclic AMP-dependent
phosphorylation of nNOS mediated the initiation as well as the
maintenance of penile erection. In addition, the expression of
Rho-associated kinase (Rho-kinase) was found to be increased in the
penises of aged rats (10) and it
has been demonstrated that inhibiting Rho-kinase protein improves
erectile function in aging rats (11). Previous studies have confirmed that
inhibition of Rho-kinase in a rat model of cavernous nerve injury
decreases penile cell apoptosis and prevents the development of ED
(12,13). Although it has been observed that the
expression of nNOS protein is decreased in the penis of aged
Sprague-Dawley (SD) rats (14), the
association between decreased nNOS and increased Rho-kinase levels
with age-associated ED in SD rats has not been fully
investigated.
To address this issue, in the current study, the
expression of Rho-kinase and nNOS was assessed and the erectile
functional index intracavernosal pressure/mean arterial pressure
(ICP/MAP) was determined in SD rats of different ages (5–24
months). A correlation analysis was performed to evaluate the
association between age and Rho-kinase or nNOS, and between ICP/MAP
and Rho-kinase, nNOS or nNOS/Rho-kinase. The present study aimed to
determine the changes in Rho-kinase and nNOS expression in
aged-associated ED in SD rats, as well as elucidate their
regulatory mechanisms. The findings of the present study may
provide a theoretical basis for drug therapy of age-associated
ED.
Materials and methods
Animals
A total of 100 intact male SD rats (age, 5–24
months; weight, 325–375 g) were obtained from the Experimental
Center of Jinan Clinical Institute, Medical College of Taishan
(Taishan, China). Rats were divided into 20 groups (n=5 in each)
according to their age (5–24 months; rats that were the same age in
months were put in the same group). All rats were housed at a
constant temperature (23±1°C) and humidity (50±5%) with free access
to food and tap water under a 12-h light/dark cycle prior to the
experiments. The experimental protocol was in accordance with the
‘Guiding Principles in the Care and Use of Animals’ endorsed by the
State Department of China. The present study was approved by the
Ethics Committee and Animal Management Committee of the Fourth
People's Hospital of Jinan (Jinan, China).
Erectile response measurements
Rats were anesthetized by intraperitoneal injection
of 10% chloral hydrate (0.3 ml/100 g body weight, Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany). The experimental animals were
fixed on a console. Following neck disinfection, the skin was
incised near the trachea and thyroid cartilage to expose the neck
muscles. The carotid artery was separated along the trachea, below
the thyroid cartilage. The carotid artery was then pulled out using
a 4-gauge wire and cannulated by insertion of a 25-gauge needle.
Subsequently, the carotid artery was fixed and connected to a
pressure transducer (Gould Electronics, Cleveland, OH, USA) for
continuous monitoring of the MAP. In addition, 5–10 mm from the
distal section of the rat penis, the corpus cavernosum was
cannulated by insertion of a 25-gauge needle and then connected to
a pressure transducer for continuous monitoring of ICP. Heparinised
saline (100 U/ml, Sigma-Aldrich; Merck Millipore) was added into a
connection pipe between the needle and pressure transducer to
prevent coagulation. The abdominal cavity was then opened, exposing
the major pelvic ganglion (MPG), on which a platinum bipolar
electrode was positioned. During stimulation, the position of the
electrode was adjusted until a maximal voltage-induced response was
achieved. All experimental rats were given a 5-V stimulus and the
MAP, ICP and ICP/MAP were recorded (15).
Rho-kinase and nNOS protein
detection
The expression levels of Rho-kinase and nNOS
proteins in the corpus cavernosum of rats of different ages were
detected by western blot analysis as described previously (14), with certain modifications. Following
blocking with 5% skimmed milk, the membrane was incubated with
primary antibodies, including rabbit anti-rat Rho-kinase (1:1,000,
orb312940, Shanghai Qifa Bio-technology Ltd., Shanghai, China),
rabbit anti-rat nNOS (1:1,000, SRP08644b; Tianjin Saier
Bio-technology Ltd., Tianjin, China) and GAPDH polyclonal antibody
(1:5,000, ABP57259; Wuhan Amyjet Scientific Inc., Wuhan, China)
overnight at 4°C. Subsequently, the membranes were incubated with
goat anti-rabbit horseradish peroxidase-linked secondary antibody
(1:5,000, A21020; Wuhan Amyjet Scientific Inc.) at 37°C for 2 h,
followed by enhanced chemiluminescence with diaminobenzidine
tetrahydrochloride substrate (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) in the dark. GAPDH served as a loading
control. The band intensity was then quantified using a
computerized image analysis system (16). The expression levels of Rho-kinase
and nNOS protein were presented as the ratio of Rho-kinase and nNOS
protein normalized to GAPDH.
Correlation analysis
Pearson's correlation analyses between ICP/MAP and
Rho-kinase, ICP/MAP and nNOS as well as between ICP/MAP and
nNOS/Rho-kinase were performed. In addition, the correlation
between the age of experimental rats and Rho-kinase as well as nNOS
was assessed.
Statistical analysis
The data obtained from multiple experiments were
presented as the mean ± standard deviation. Pearson's correlation
analysis was performed to assess the correlation between ICP/MAP,
nNOS, Rho-kinase and nNOS/Rho-kinase. All statistical analyses were
performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). A
two-sided t test was performed to analyze significant differences
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Penile erectile functional
measurements of SD rats of different ages
During the animal experiment, in 5 groups, one out
of the 5 rats in each group succumbed. As a result, by the end of
the experiment, 5 groups only consisted of 4 rats, while in the
other 15 groups there were 5 rats in each group. Analysis of the
penile erectile functional measurements of all SD rats of different
ages (5–24 months) showed that the baseline ICP increased with age
and was 8.2±4.3 mmHg, and the baseline MAP was 13.8±5.7 mmHg (data
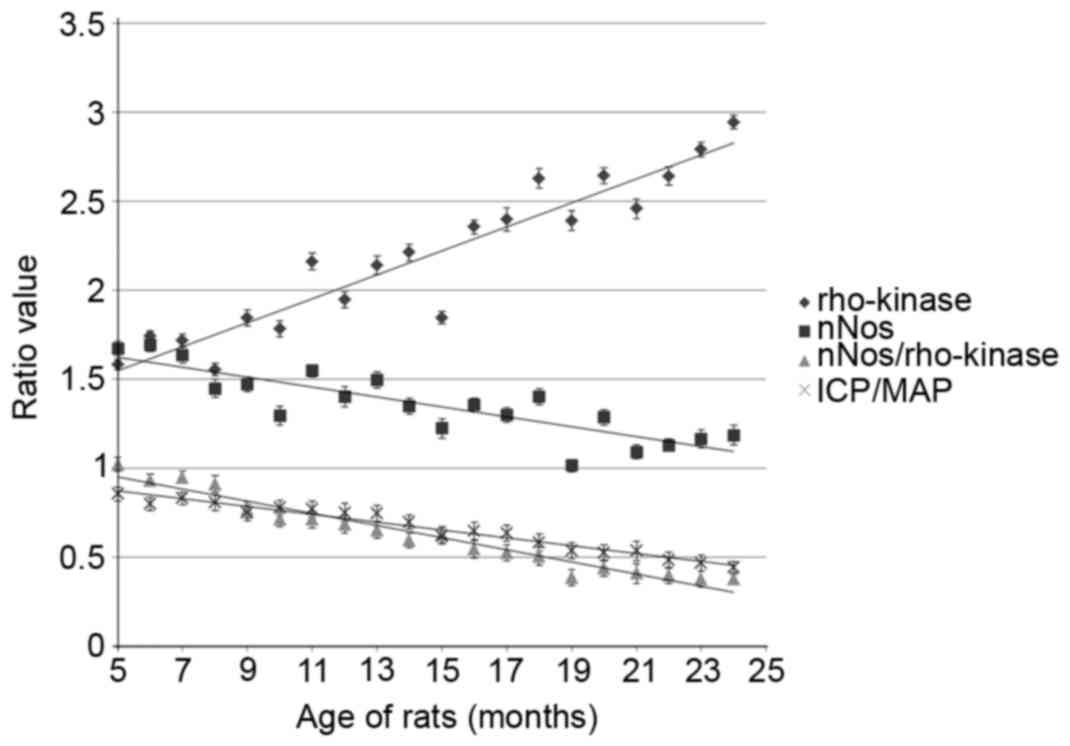
not shown). As shown in Fig. 1, the
penile erectile functional index, ICP/MAP, decreased with age. For
instance, ICP/MAP was 0.85±0.04 at 5 months, 0.62±0.05 at 15 months
and 0.46±0.05 at 24 months, confirming that penile erectile
function decreased with age. In addition, levels of the nNOS
protein markedly decreased with age (1.65±0.04, 1.23±0.06 and
1.19±0.06 at 5, 15 and 24 months, respectively), while levels of
the Rho-kinase protein increased with age (1.59±0.03, 1.85±0.04 and
2.95±0.04 at 5, 12 and 24 months, respectively). Notably, the
nNOS/Rho-kinase ratio decreased with age (1.02±0.04, 0.63±0.05 and
0.37±0.04 at 5, 12 and 24 months, respectively). Statistical
analysis indicated that significant differences existed between
groups of different age rats, and that the ICP/MAP ratio, nNOS
levels and the nNOS/Rho-kinase ratio were decreased, while levels
of Rho-kinase increased with age (P<0.05, Table I).
 | Table I.Analysis of the differences of penile
erectile functional indexes between groups of different age using
t-test. |
Table I.
Analysis of the differences of penile
erectile functional indexes between groups of different age using
t-test.
| Penile erectile
functional indexes | Age, months | T value | P-value |
|---|
| ICP/MAP ratio | 5 vs.
15 | 7.172 | P=0.002 |
|
| 15 vs. 24 | 4.545 | P=0.010a |
| nNOS | 5 vs.
15 | t=11.366 |
P<0.001a |
|
| 15 vs. 24 | t=0.889 | P=0.424 |
| nNOS/Rho-kinase | 5 vs.
15 | t=11.302 |
P<0.001a |
| ratio | 15 vs. 24 | t=7.576 | P=0.002a |
| Rho-kinase | 5 vs.
15 | t=9.875 | P=0.001a |
|
| 15 mo vs. 24 mo | t=35.585 |
P<0.001a |
Correlation analysis
A correlation analysis was performed to investigate
whether levels of Rho-kinase and nNOS were associated with the
erectile function of SD rats, as well as to validate the
association between age and nNOS or Rho-kinase. As presented in
Table II, the correlation analysis
revealed that Rho-kinase was negatively correlated with ICP/MAP
ratio (Pearson's r=−0.917; P<0.01). Moreover, nNOS was
positively correlated with the ICP/MAP ratio (Pearson's r=0.853;
P<0.01). Similarly, the nNOS/Rho-kinase ratio was also
positively correlated with the ICP/MAP ratio (Pearson's r=0.937;
P<0.01). In addition, the correlation analysis showed that nNOS
was significantly negatively correlated with age (Pearson's
r=−0.855; P<0.01), while Rho-kinase was positively correlated
with age (Pearson's r=0.943; P<0.01).
 | Table II.Correlation analysis. |
Table II.
Correlation analysis.
| Comparison | Pearson's r | P-value |
|---|
| Rho-kinase vs.
ICP/MAP ratio | −0.917 | P<0.01 |
| nNOS vs. ICP/MAP
ratio | 0.853 | P<0.01 |
| nNOS/Rho-kinase ratio
vs. ICP/MAP ratio | 0.937 | P<0.01 |
| nNOS vs. Age | −0.855 | P<0.01 |
| Rho-kinase vs.
Age | 0.943 | P<0.01 |
Discussion
Physiological aging is a significant risk factor for
ED. The present study investigated the mechanisms of age-associated
ED by assessing changes in erectile function as well as the ICP/MAP
ratio, nNOS and Rho-kinase protein levels in SD rats. The results
indicated that the levels of nNOS protein and the ICP/MAP ratio
decreased with age, while levels of Rho-kinase protein increased
with age. Furthermore, the study also demonstrated that the ICP/MAP
ratio was positively correlated with nNOS and the nNOS/Rho-kinase
ratio and negatively correlated with Rho-kinase.
A previous study found that older rats have
decreased relative nNOS protein levels compared with younger rats
(1±0.15 vs. 1.7±0.15) (14). Another
study demonstrated that there is ~13% decrease in the gene
expression of nNOS in the penises of aged rats (17). In addition, increasing evidence has
shown that endothelial and neuronal-derived nitric oxide acts as an
important mediator of corpus cavernosal smooth-muscle relaxation
and penile erection in rats with ED (18). nNOS was also shown to be involved in
mediating initiation as well as maintenance of penile erection via
cyclic adenosine monophosphate-dependent phosphorylation (9). Furthermore, changes in the
dimethylarginine dimethylaminohydrolase/asymmetric
dimethylarginine/NOS pathway were found to accompany lower ICP in
an older group of rats (18 months old), compared with that of a
younger group (3 months old) (19).
The ICP/MAP ratio is the common functional index to determine
erectile function (10). Qiu et
al (20) also demonstrated that
immediate and delayed treatment with stromal vascular fraction
resulted in a significantly increased ICP/MAP ratio and increased
expression of nNOS compared with the vehicle-treated group,
implying that increased expression of nNOS may enhance recovery of
erectile function in a rat model of cavernous nerve injury via
increasing the ICP/MAP ratio. In the present study, decreased
expression of nNOS was found in aged rats and the ICP/MAP ratio
decreased with age. Additionally, the expression of nNOS was
positively correlated with the ICP/MAP ratio (r=0.853). Therefore,
the results of the present study are in line with previous findings
and imply that the decreased erectile function of SD rats is
associated with increasing age and that decreased nNOS may be a
reason for age-associated ED.
Furthermore, accumulating evidence has demonstrated
that age-associated ED is likely to be associated with an increased
Rho-kinase activity and antagonism of Rho-kinase activity can
attenuate the age-associated decline in male erectile function
(11). Rho-kinase was found to
affect the erectile response via enhancing the vasoconstriction
state of the penile smooth muscle in lean and obese-diabetic Zucker
rats (21). Rho-kinase may have an
important role in maintaining penile detumescence and
vasoconstriction of the corporal vasculature (22). Meanwhile, increased RhoA/Rho-kinase
signaling activation is involved in impaired erectile function
during the ageing process (10). A
study by Gao et al (14)
confirmed that the expression of Rho-kinase protein was increased
in old rats (age, 20–22 months) compared to that in young rats
(age, 6–9 months). Furthermore, inhibition of Rho-kinase by a
selective Rho kinase inhibitor, Y-27632, was reported to increase
ICP in a rat model of erection, thus preventing the development of
vasculogenic ED (23). An increase
in the ICP/MAP ratio in response to electrical stimulation of the
MPG has been shown to be blocked by inhibitors of nitric oxide
production or signaling, which is in line with the hypothesis that
nitric oxide induces penile erection via inhibition of
RhoA/Rho-kinase signaling (24). The
present study found a negative correlation between Rho-kinase and
age. Furthermore, Rho-kinase was negatively associated with the
ICP/MAP ratio (r=−0.917). All of these findings suggested that
increased Rho-kinase may contribute to age-associated ED in SD
rats.
Of note, intracavernosal gene therapy with penile
nNOS has been shown to correct age-associated ED (25). High expression of RhoA was also shown
to result in a flaccid state of the corpus cavernosum by
RhoA-mediated Ca2+ sensitization, which was activated by
a Rho-kinase inhibitor suitable for the treatment of ED (26). In addition, the ICP/MAP ratio is the
common functional index to determine erectile function (10). The present study found that the
nNOS/Rho-kinase ratio decreased with age and was positively
correlated with the ICP/MAP ratio (r=0.937, P<0.01), suggesting
that a decreased nNOS/Rho-kinase ratio may be a cause of
age-associated ED and the nNOS/Rho-kinase ratio may serve as an
alternative to the ICP/MAP ratio as a promising indicator of
erectile function.
The present study had certain limitations. First,
there were only 5 rats in each of the 20 groups according to their
age (5–24 months). Theoretically, the rats aged 5–10 months were
considered young compared to rats aged 15–24 months. Although there
were only 5 rats in each age group, the sample size was justified
for exploring the effect of age on the association of ED with nNOS
and Rho-kinase expression; however, it is required to verify the
results of the present study by further studies using a larger
number of rats in each group.
In conclusion, the results of the present study
indicated that decreased nNOS and increased Rho-kinase expression
may be important reasons for age-associated ED. Moreover, the
nNOS/Rho-kinase ratio may be an alternative to the ICP/MAP ratio to
serve as a possible indicator of erectile function. In addition,
increasing nNOS and decreasing Rho-kinase expression may represent
potential therapeutic strategies for the treatment of ED.
References
|
1
|
Cai L, Jiang M, Zeng M, Xing W, Wen Y and
Zhang B: Age-specific clinical features of erectile dysfunction.
Health. 6:2014. View Article : Google Scholar
|
|
2
|
Bacon CG, Mittleman MA, Kawachi I,
Giovannucci E, Glasser DB and Rimm EB: Sexual function in men older
than 50 years of age: Results from the health professionals
follow-up study. Ann Intern Med. 139:161–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steers WD: Pharmacologic treatment of
erectile dysfunction. Rev Urol. 4:(Suppl 3). S17–S25.
2002.PubMed/NCBI
|
|
4
|
de Andrade E, de Mesquita AA, de Claro JA,
de Andrade PM, Ortiz V, Paranhos M and Srougi M: Study of the
efficacy of Korean Red Ginseng in the treatment of erectile
dysfunction. Asian J Androl. 9:241–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shabsigh R, Rajfer J, Aversa A, Traish AM,
Yassin A, Kalinchenko SY and Buvat J: The evolving role of
testosterone in the treatment of erectile dysfunction. Int J Clin
Pract. 60:1087–1092. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montorsi F, Adaikan G, Becher E, Giuliano
F, Khoury S, Lue TF, Sharlip I, Althof SE, Andersson KE, Brock G,
et al: Summary of the recommendations on sexual dysfunctions in
men. J Sex Med. 7:3572–3588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inman BA, St Sauver JL, Jacobson DJ,
McGree ME, Nehra A, Lieber MM, Roger VL and Jacobsen SJ: A
population-based, longitudinal study of erectile dysfunction and
future coronary artery disease. Mayo Clin Proc. 84:108–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sezen SF, Lagoda G and Burnett AL:
Neuronal nitric oxide signaling regulates erection recovery after
cavernous nerve injury. J Urol. 187:757–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hurt KJ, Sezen SF, Lagoda GF, Musicki B,
Rameau GA, Snyder SH and Burnett AL: Cyclic AMP-dependent
phosphorylation of neuronal nitric oxide synthase mediates penile
erection. Proc Natl Acad Sci. 109:16624–16629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin L, Liu T, Lagoda GA, Champion HC,
Bivalacqua TJ and Burnett AL: Elevated RhoA/Rho-kinase activity in
the aged rat penis: Mechanism for age-associated erectile
dysfunction. FASEB J. 20:536–538. 2006.PubMed/NCBI
|
|
11
|
Rajasekaran M, White S, Baquir A and
Wilkes N: Rho-kinase inhibition improves erectile function in aging
male brown-norway rats. J Androl. 26:182–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hannan JL, Albersen M, Kutlu O, Gratzke C,
Stief CG, Burnett AL, Lysiak JJ, Hedlund P and Bivalacqua TJ:
Inhibition of Rho-kinase improves erectile function, increases
nitric oxide signaling and decreases penile apoptosis in a rat
model of cavernous nerve injury. J Urol. 189:1155–1161. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho MC, Park K, Kim SW and Paick JS:
Restoration of erectile function by suppression of corporal
apoptosis, fibrosis and corporal veno-occlusive dysfunction with
Rho-kinase inhibitors in a rat model of cavernous nerve injury. J
Urol. 193:1716–1723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao BH, Zhao ST, Meng FW, Shi BK, Liu YQ
and Xu ZS: Y-27632 improves the erectile dysfunction with ageing in
SD rats through adjusting the imbalance between nNo and the
Rho-kinase pathways. Andrologia. 39:146–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mills TM, Chitaley K, Wingard CJ, Lewis RW
and Webb RC: Effect of Rho-kinase inhibition on vasoconstriction in
the penile circulation. J Appl Physiol (1985). 91:1269–1273.
2001.PubMed/NCBI
|
|
16
|
Bivalacqua TJ, Champion HC, Usta MF,
Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ and
Kadowitz PJ: RhoA/Rho-kinase suppresses endothelial nitric oxide
synthase in the penis: A mechanism for diabetes-associated erectile
dysfunction. Proc Natl Acad Sci USA. 101:9121–9126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajasekaran M, Kasyan A, Jain A, Kim SW
and Monga M: Altered growth factor expression in the aging penis:
The Brown-Norway rat model. J Androl. 23:393–399. 2002.PubMed/NCBI
|
|
18
|
Cartledge JJ, Minhas S, Eardley I and
Morrison JF: Endothelial and neuronal-derived nitric oxide mediated
relaxation of corpus cavernosal smooth muscle in a rat, in vitro,
model of erectile function. Int J Impot Res. 12:213–221. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JH, Chen D, Zhang KQ, Zhang H and Fu
Q: Effect of DDAH/ADMA/NOS regulation pathway on cavernae corporum
cavernosorum rat penis of different age. Andrologia. 48:262–267.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu X, Fandel TM, Ferretti L, Albersen M,
Orabi H, Zhang H, Lin G, Lin CS, Schroeder T and Lue TF: Both
immediate and delayed intracavernous injection of autologous
adipose-derived stromal vascular fraction enhances recovery of
erectile function in a rat model of cavernous nerve injury. Eur
Urol. 62:720–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wingard C, Fulton D and Husain S: Altered
penile vascular reactivity and erection in the zucker
obese-diabetic rat. J Sex Med. 4:348–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chitaley K, Wingard CJ, Webb R Clinton,
Branam H, Stopper VS, Lewis RW and Mills TM: Antagonism of
Rho-kinase stimulates rat penile erection via a nitric
oxide-independent pathway. Nat Med. 7:119–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park K, Kim SW, Rhu KS and Paick JS:
Chronic administration of an oral Rho kinase inhibitor prevents the
development of vasculogenic erectile dysfunction in a rat model. J
Sex Med. 3:996–1003. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chitaley K, Bivalacqua TJ, Champion HC,
Usta MF, Hellstrom WJ, Mills TM and Webb RC: Adeno-associated viral
gene transfer of dominant negative RhoA enhances erectile function
in rats. Biochem Biophys Res Commun. 298:427–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Magee TR, Ferrini M, Garban HJ, Vernet D,
Mitani K, Rajfer J and Gonzalez-Cadavid NF: Gene therapy of
erectile dysfunction in the rat with penile neuronal nitric oxide
synthase. Biol Reprod. 67:20–28. 2002.PubMed/NCBI
|
|
26
|
Wang H, Eto M, Steers WD, Somlyo AP and
Somlyo AV: RhoA-mediated Ca2+ sensitization in erectile function. J
Biol Chem. 277:30614–30621. 2002. View Article : Google Scholar : PubMed/NCBI
|