Introduction
The liver is involved in the major biochemical and
signaling pathways associated with homeostasis (1) and plays a crucial role in interacting
with and fighting against xenobiotics. Among these, bisphenol A
(BPA) is a fairly ubiquitous compound with certain risks to human
health, which have aroused public concern.
In the liver, BPA is metabolized and eliminated
essentially as a monoglucuronide (the major metabolite) and/or as a
sulfate conjugate. However in Fischer-344 rat hepatocytes, BPA is
converted primarily to a diconjugate (glucuronide/sulfate)
(2). The hepatic capacity for BPA
glucuronidation is predicted to be greater in humans than in rats
and mice (2).
Structurally, BPA imitates estrogen and causes
dysfunction of the reproductive organs (3,4), having
a potential role in severe pathologies such as breast cancer
(5). Additionally, exposure to BPA
has been associated with various diseases, including cardiovascular
disease (6), type II diabetes,
altered insulin homeostasis, abnormal liver function (6) and cancer (7). In particular, the detrimental
interaction of BPA with genes associated with adipose tissue and
obesity has been linked with metabolic syndrome and associated
pathological conditions (8,9). In this context, low-grade chronic
inflammation has been identified as a key pathogenic connection
between metabolic syndrome, obesity and insulin resistance
(10,11). Interleukin (IL)-6 and tumor necrosis
factor (TNF)-α are considered pro-inflammatory markers, useful for
the identification of low-grade inflammation associated with
visceral adiposity (11).
Additionally, concerns regarding the presence of BPA in food and
the environment have been constantly rising since the
dose-dependent incidence of hepatic tumors following perinatal
exposure to BPA was reported in isogenic mouse models (12). Notably, it has previously been shown
that BPA has the ability to dysregulate cytokines (13), induce cellular apoptosis in
hepatocytes (14) and produce
oxidative stress in liver tissue (15,16).
Natural compounds extracted from plants have often
been found to have efficient protective and preventive effects
against pathological conditions. A flavonoid with important
antioxidant properties isolated from the milk thistle Silybum
marianum seeds is silymarin. Apart from its anti-inflammatory
effect (17), silymarin has been
shown to protect against hepatotoxicity induced by numerous
different agents: Excess iron (18),
manganese (19), ethanol (20), carbon tetrachloride (21,22),
acetaminophen (paracetamol) (23)
and anticancer agents as cisplatin (24), epirubicin (25) and doxorubicin (26). The hepatoprotective and antioxidant
activity of silymarin has been attributed to its capacity to
eliminate free radicals produced during the hepatic metabolism of
toxic substances through the inhibition of the cyclooxygenase cycle
and leukotrienes (27). Moreover,
this flavonoid has a phenolic structure, which allows electron
donation to free radicals and reactive oxygen species (ROS) in
order to stabilize them and prevents lipid peroxidation by
interaction with intracellular glutathione (27,28).
Additionally, silymarin has previously been reported to have
anti-proliferative, anti-fibrotic, anti-apoptotic, antiviral and
immunomodulatory properties (25,29) and
has been shown to inhibit TNF-α expression (30).
The present study aimed to quantify the
pro-inflammatory effects of BPA on mouse liver tissue and to
propose a potential counteracting agent against the hepatotoxic and
inflammatory stress exerted by BPA, namely silymarin. To the best
of our knowledge, this is the first study investigating i) the
connection between BPA-induced toxicity and low-grade chronic
inflammatory markers in the liver and ii) the potential beneficial
effect of silymarin in protecting against BPA-induced liver
damage.
Materials and methods
Chemicals
Bisphenol A 99%, silymarin 98% and carboxymethyl
cellulose were purchased from Sigma-Aldrich Chemie GmbH (Munich,
Germany). TNF-α (sc-52746) and IL-6 (sc-1265-R) antibodies were
supplied by Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). A
Novocastra kit for immunohistochemistry (Novolink Max Polymer
Detection System; RE7280-K) was purchased from Leica Microsystems
GmbH (Wetzlar, Germany).
Animals
Male CD-1 mice (n=40; age, 8–10 weeks; body weight,
25±3 g) from a breeding colony at the Animal Facility at ‘Vasile
Goldiș’ Western University of Arad (Arad, Romania) were fed a
standard rodent diet and maintained under a 12 h light/dark cycle,
with a constant temperature (20±1°C) and humidity (50±5%). All
experimental procedures were performed in compliance with the
appropriate laws and institutional guidelines, and were approved by
the Institutional Ethics Committee of Vasile Goldiș Western
University of Arad (Arad, Romania).
Experimental design
For analyzing the effect of silymarin on BPA-induced
hepatotoxicity, mice were randomly divided into four groups (n=10):
Control, BPA, bisphenol A+silymarin (BPA+SY) and silymarin (SY)
groups. Silymarin powder was dissolved in 0.7% carboxymethyl
cellulose and BPA in 5% ethanol, diluted with corn oil. Vehicles
were administered by gavage daily for 10 days to mice in the
control group. A dose of 200 mg/kg BPA (31) was administered daily by gavage to
mice in the BPA and BPA+SY groups, between days 1 and 10. Silymarin
was administered daily for 10 days, at a dose of 200 mg/kg, to mice
in the BPA+SY and SY groups.
All mice were sacrificed on day 11 of the
experiment. The mice were anesthetized with ketamine (100 mg/kg)
and xylazine (10 mg/kg) administered intraperitoneally and
sacrificed by cervical dislocation. Liver samples were collected
and preserved in a buffered formalin solution for histology and
immunohistochemistry or in glutaraldehyde solution for electron
microscopy examination.
Histopathology
Liver specimens were fixed in 4% phosphate-buffered
formalin, embedded in paraffin and cut into 4-µm sections. Sections
for histopathological examination were stained with hematoxylin and
eosin, and analyzed by light microscopy using an Olympus BX43
System Microscope (Olympus, Hamburg, Germany) and photographic
images were captured using a digital camera (Olympus XC30).
Immunohistochemistry
Paraffin-embedded liver tissue sections of 5-µm
thickness were deparaffinized and rehydrated in decreasing
concentrations of ethyl alcohol. Rabbit polyclonal anti-TNF-α and
anti-IL-6 antibodies (1:100 dilution) were used as primary
antibodies. Immunoreactions were visualized employing a Novocastra
immunohistochemistry kit with peroxidase and diaminobenzidine
chromogen according to the manufacturer's instructions. Negative
control slides were processed by the substitution of primary
antibodies with irrelevant immunoglobulins of matched isotype,
under the same conditions as those used for the primary antibodies.
Stained sections were analyzed by light microscopy (Olympus
BX43).
Gene expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Liver samples were collected from the mice and total
RNA was isolated using an RNeasy Mini kit (Qiagen GmbH, Hilden,
Germany) according to the manufacturer's protocol. Extracted RNA
was tested for integrity using a 2100 BioAnalyzer instrument
(Agilent Technologies GmbH, Waldbronn, Germany) and purity using a
NanoDrop spectrophotometer (Thermo Scientific, Inc., Waltham, CA,
USA), then reverse transcribed to corresponding cDNA using an
iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). qPCR was performed on a Rotor-Gene thermocycler using a
Rotor-Gene SYBR Green RT-PCR Kit (Qiagen GMBH, Hilden, Germany) and
the following thermal cycling conditions: 3 min at 95°C, followed
by 40 cycles of 30 sec at 95°C, 30 sec at 62°C 45 sec at 72°C, 60
sec at 95°C, 10 sec at 55°C and cooling to 20°C. The sequences of
the primers used for TNF-α and IL-6 mRNA detection are presented in
Table I. mRNA levels of target genes
were normalized to the levels of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), which was used as reference gene and was
assessed under the same experimental conditions. Additionally,
transcript levels of the target inflammation markers were compared
with the mRNA levels of the same markers from the control
group.
 | Table I.Primer sequences used to identify
TNF-α and IL-6 inflammatory markers by RT-qPCR. |
Table I.
Primer sequences used to identify
TNF-α and IL-6 inflammatory markers by RT-qPCR.
| Target | Primer sequence (5′
to 3′) |
|---|
| TNF-α | F:
CAGGTTCTCTTCAAGGGACAAG |
|
| R:
GCAGAGAGGAGGTTGACTTTC |
| IL-6 | F:
GCAGAGAGGAGGTTGACTTTC |
|
| R:
GACAGGTCTGTTGGGAGTGGTATC |
| GAPDH | F:
AGGTCGGTGTGAACGGATTTG |
|
| R:
TGTAGACCATGTAGTTGAGGTCA |
Electron microscopy
Samples were prefixed with 2.7% glutaraldehyde
solution in 0.1 M phosphate buffer at 4°C for 1.5 h, washed in 0.15
M phosphate buffer and postfixed in 2% osmic acid solution in 0.15
M phosphate buffer. Dehydration was performed in acetone and the
sections were embedded in the epoxy embedding resin Epon 812.
Sections of thickness 60 nm were cut with a Leica EM UC7
ultramicrotome (Leica Microsystems GmbH) and analyzed with a Tecnai
12 Biotwin transmission electron microscope.
Statistical analysis
Gene expression data were statistically evaluated
using GraphPad Prism 3.03 software (GraphPad Software, Inc., La
Jolla, CA, USA), and one-way analysis of variance, followed by a
Bonferroni test. P<0.05 was considered to indicate a
statistically significant difference. The experiments were
performed with n=10 biological replicates and each data set is
presented as the average of three replicates (mean ± standard
deviation).
Results
Protective effects of silymarin
against hepatotoxicity induced by BPA
Histopathological examination and comparison with
the control (Fig. 1A), revealed that
BPA administration induced necrotic changes of hepatocytes, which
were particularly pronounced in the centrilobular area (Fig. 1B). Inflammatory cell infiltration and
vascular congestion were also present. In the BPA+SY group, 10 days
of 200 mg/kg silymarin administration markedly reduced the
hepatocellular changes, as compared with those in the BPA group,
and the morphology was not dissimilar to that of the SY group
(Fig. 1C). The liver morphology of
the SY group (Fig. 1D) was
comparable with that of the control group (Fig. 1A).
Protective effects of silymarin
against TNF-α and IL-6 hepatic overexpression induced by BPA
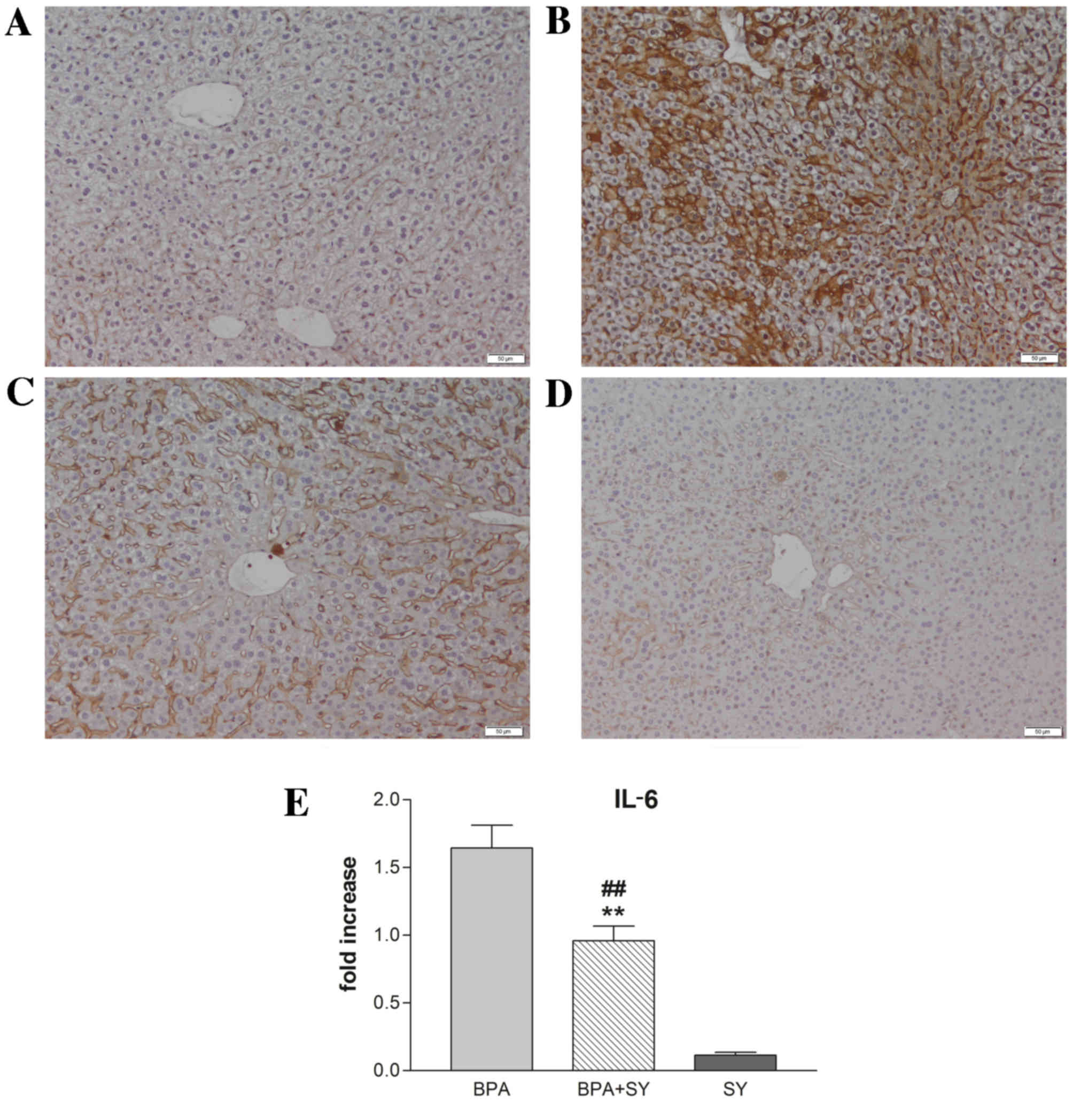
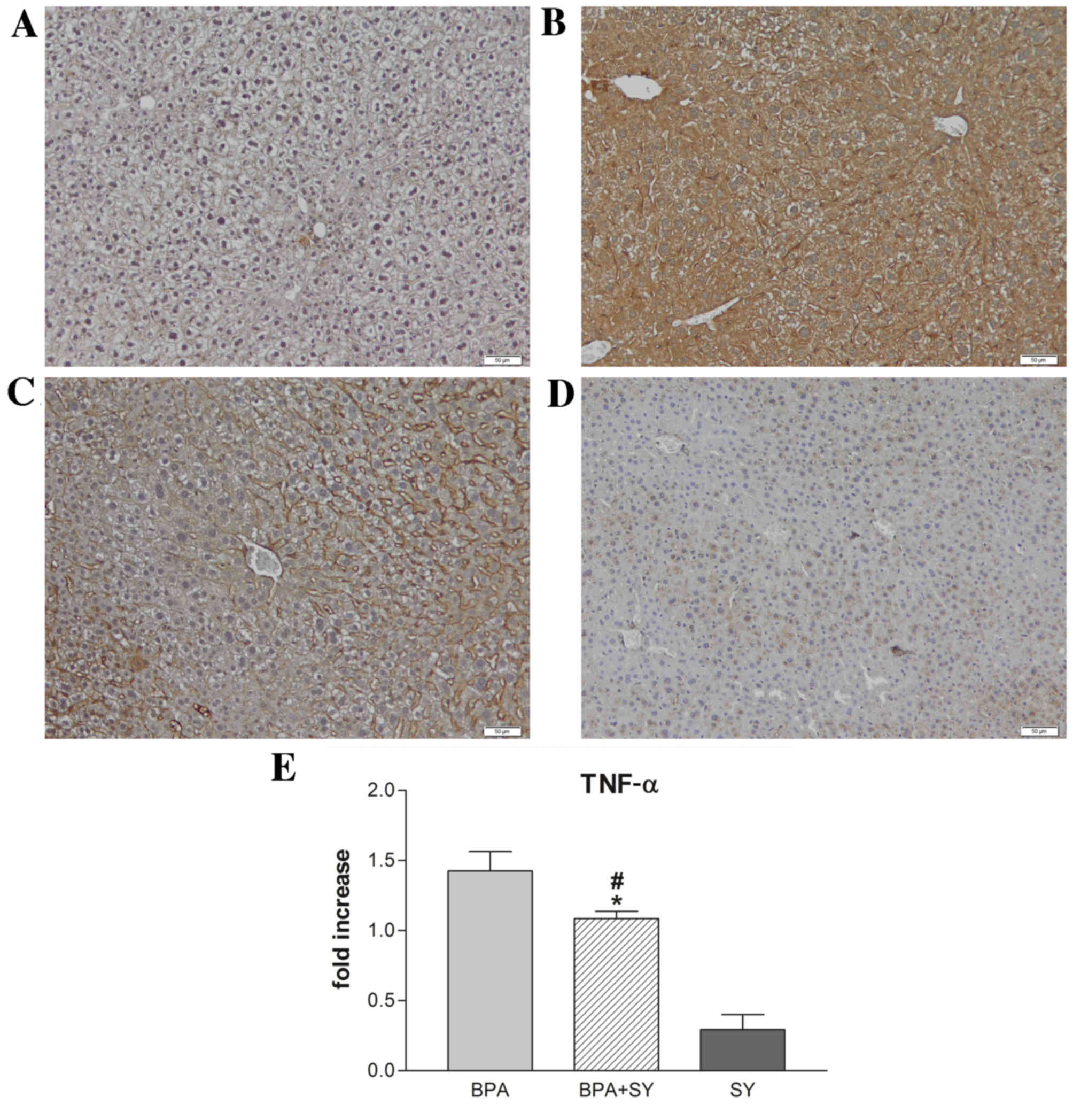
Immunohistochemical and gene expression analyses
were performed to evaluate the impact of silymarin treatment on the
regulation of IL-6 and TNF-α under BPA pro-inflammatory activity.
As shown in Figs. 2 and 3, compared with the control (Figs. 2A and 3A), the administration of BPA caused a
significant increase in the number of parenchymal cells
immunopositive for IL-6 and TNF-α (Figs.
2B and 3B). No apparent IL-6 or
TNF-α expression was detected in non-parenchymal cells. In the
BPA+SY group, silymarin treatment decreased the number of cells
labeled with both antibodies, compared with the BPA group (Figs. 2C and 3C). No immunopositivity for the group
treated with SY alone was detected (Figs. 2D and 3D).
These findings were confirmed by the results of IL-6
and TNF-α RT-qPCR analysis (Figs. 2E
and 3E). Evaluation by qPCR
demonstrated that exposure to BPA induced high transcript levels of
IL-6 in mouse liver tissue (a 1.6-fold increase compared with the
control), thus confirming that BPA exerts a hepatotoxic and
pro-inflammatory effect (Fig. 2E).
Treatment with the natural compound silymarin resulted in very low
levels of IL-6 mRNA, comparable with the control. Notably, a
statistically significant lower level of IL-6 transcript
(P<0.01) was found in livers isolated from mice exposed to a 1:1
ratio of BPA and SY for 10 days, when compared with IL-6 expression
in the BPA group. However, a significant difference in IL-6
expression (P<0.01) was observed between mice exposed
simultaneously to SY and BPA and those exposed only to SY.
Furthermore, the pro-inflammatory effect of BPA
triggered similar changes in IL-6 and TNF-α transcript levels in
mouse hepatic tissue after 10 days of treatment. SY administration
for the same interval resulted in a low level of TNF-α expression,
significantly lower than the levels obtained for dual BPA and SY
exposure (P<0.05). Overall, the TNF-α mRNA levels detected by
qPCR in mice treated with both BPA and SY were statistically
significantly lower than those in mice treated only with BPA
(P<0.05; Fig. 3E).
Protective effects of silymarin
against ultrastructural hepatic injuries induced by BPA
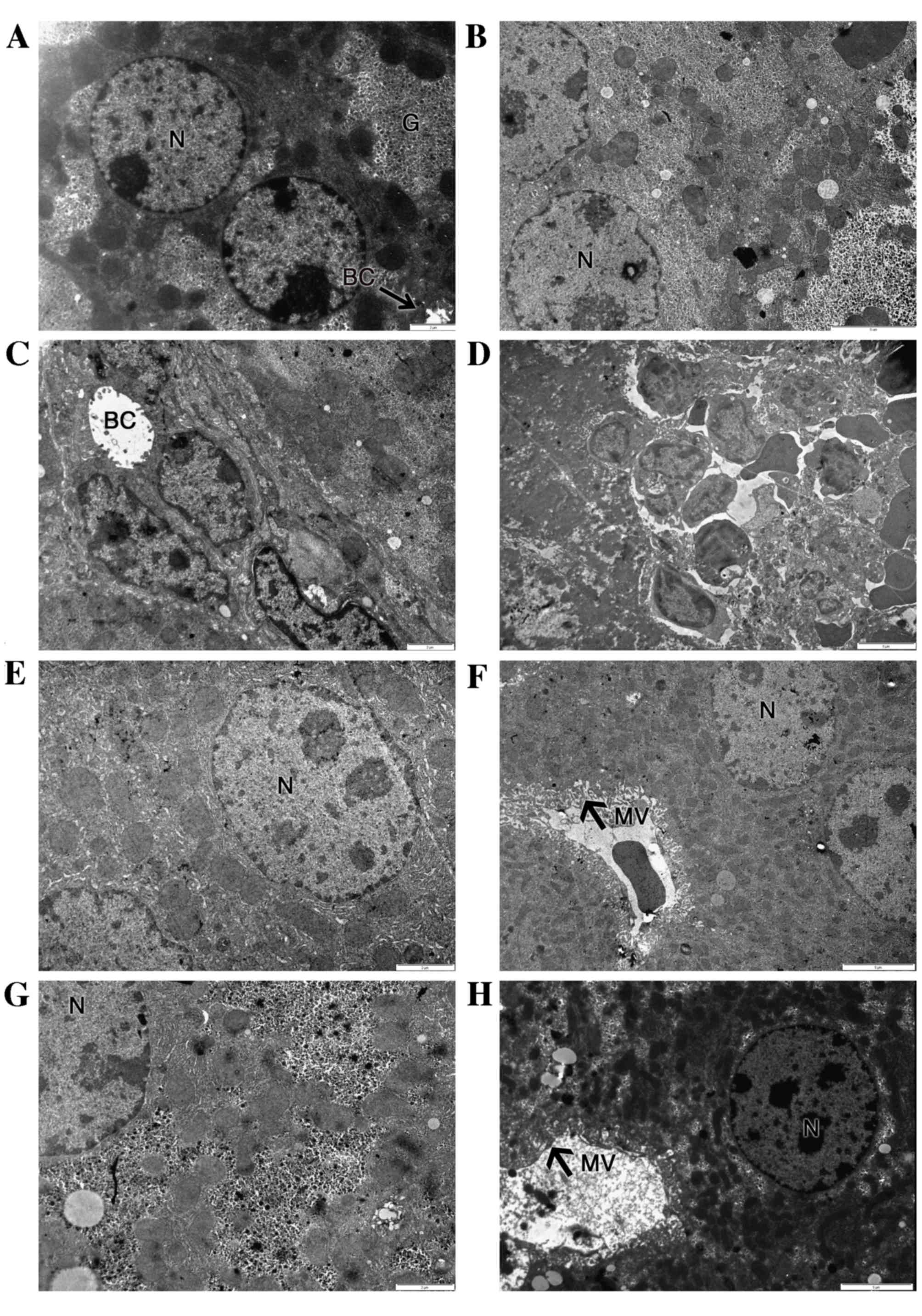
Fig. 4 shows that the
ultrastructure of hepatocytes was normal in the control group
(Fig. 4A) and SY group (Fig. 4G), showing regular organelles and
cytoplasmic glycogen deposition. In the BPA-treated group, the
majority of the hepatocytes exhibited irregular nuclear shapes,
together with damaged rough endoplasmic reticulum (rER), irregular
mitochondria and reduced glycogen deposits (Fig. 4B). Some hepatocytes displayed dilated
bile canaliculi, voided of microvilli (Fig. 4C). Liver-infiltrating lymphocytes
were present in many micrographs (Fig.
4D).
Treatment with 200 mg/kg SY for 10 days
significantly reduced the ultrastructural injuries induced by BPA
co-administration (Fig. 4E and F).
Organelle and cytoplasm structures were widely protected against
the effects of BPA. Abnormal irregular nuclear shapes and dilated
bile canaliculi were not visible in the SY+BPA group. Glycogen
deposits were increased, while the cytoplasmic distribution of
mitochondria and rER was restored. However, proliferation of smooth
reticulum vesicles was present (Fig.
4E).
Discussion
BPA is an endocrine-disrupting chemical prevalent in
the environment, and previous studies have focused on its effects
on reproduction (32,33). Due to limited information concerning
the toxic effect on the liver, the present study focused on
evaluating the toxic effect of BPA and investigating the potential
alleviation of BPA-induced injuries using a natural agent. The
present study represents the first report of the ability of
silymarin to reduce hepatic lesions and to counteract inflammation
caused by BPA administration in animal models. The target of the
study was to explore whether a dose of 200 mg/kg silymarin is
sufficient to induce a protective effect against structural and
ultrastructural injuries induced by BPA and to lower the levels of
pro-inflammatory cytokines observed in murine liver tissue
following exposure to BPA for 10 days.
Acute hepatotoxicity induces inflammatory effects
and hepatocyte apoptosis or necrosis. The necrotic
pericentrilobular changes, vascular congestion and inflammation
observed in histological liver sections are naturally occurring
reactions of the system to cell damage (34–36), as
also shown by the histopathological and immunohistochemical results
of the present study.
The hepatic inflammatory response is mediated by
proinflammatory cytokines, especially TNF-α, the release of which
is one of the first events in many types of liver injury, and
further modulates the effects of other cytokines, such as IL-6
(37). IL-6 is the predominant
regu-lator of the hepatic acute-phase response and modulates liver
fibrosis through degrading extracellular matrix proteins by
protease inhibition or cytokine binding (38,39). The
present study showed that IL-6 and TNF-α are stimulatory, as
suggested by higher levels in hepatic tissue and upregulation of
their mRNA in mice with BPA-induced liver acute inflammation.
Previous data have shown a significant correlation between BPA and
inflammatory markers such as IL-6 and TNF-α (11). This previous study, involving a
homogenous adult male population in southern Italy, revealed that
higher BPA plasma levels are associated with higher levels of
circulating IL-6 and TNF-α pro-inflammatory cytokines.
Following the administration of 200 mg/kg daily
doses of silymarin for 10 days, the present study demonstrated an
overall diminished level of IL-6 and TNF-α pro-inflammatory
cytokines in mouse liver tissue and attenuation of the inflammatory
process. By studying the transcript levels and protein expression
of IL-6 and TNF-α pro-inflammatory markers, the present study
confirmed at quantitative and qualitative levels a significant
reduction in the levels of IL-6 (P<0.01) and TNF-α (P<0.05)
in the group exposed to BPA and SY in equal proportions, as
compared with the group that received only BPA. This data is in
accordance with previous results found in the literature,
confirming the anti-inflammatory and hepatoprotective effect of
silymarin against toxic substances (40,41).
Similarly, a study assessing the toxic effects of sodium nitrate
food additive in rat liver (40) has
identified significant increases in hepatic TNF-α and IL-1β levels
in rats exposed to sodium nitrate as compared with their levels in
a control group. The co-administration of 80 mg/kg sodium nitrite
and 25 mg/kg silymarin for 12 weeks resulted in significant
reductions of TNF-α and IL-1β hepatic concentrations, similar to
observations in the present study, suggesting that silymarin has a
dose-dependent beneficial effect against the impairment of liver
function.
The anti-inflammatory effect of silymarin on the
liver is supported by additional research. A study using murine
knock-out cells (41) revealed that
nuclear factor-κB, a key transcription factor considered to play a
crucial role in the cellular response to signal transduction by
TNF-α and IL-6 (42), was inhibited
through reparative stress signaling. It was concluded that
silymarin activates stress and repair responses, which correlates
with the ability to suppress inflammation.
The cytotoxic effects of BPA are strongly linked
with the production of ROS in liver, kidney and brain tissue, as
previously documented (15,43). The effects of BPA on the liver have
been particularly tested, since the liver is the major site exposed
to toxicants and oxidative stress, although it possesses an
endogenous antioxidant defense system. A study in rats has
confirmed that liver damage following exposure to BPA is mainly due
to BPA affecting the oxidant/antioxidant balance in the liver
(44). Oxidative stress effects are
followed by ultrastructural injuries. In the present study, BPA
administration caused hepatocyte damage, which resulted in nuclear
changes and other organelle impairments. The changes in hepatocyte
ultrastructure were probably due to injuries of the membrane
structure caused by lipid peroxidation. A parallel study (45) concluded that BPA-induced oxidative
stress is similar to the stress produced by hydrogen peroxide. The
mechanism proposed for the oxidative-stress increase is based on
the lipophilic nature of BPA (46);
BPA is likely to interact with the hydrophobic cell plasma
membrane, initiating hydroxyl radical formation and the induction
of lipid peroxidation (45). Xia
et al (47) demonstrated that
BPA induces mitochondria-mediated apoptosis in hepatic cells.
The aforementioned hepatocyte injuries were
alleviated by silymarin administration, suggesting that silymarin
acts as a free-radical scavenger, while other studies have
demonstrated its liver protection effects against paracetamol
(23,48), carbon tetrachloride (21,22),
anticancer agents (25,26) and lipid peroxidation (27), by enhancement of anti-oxidant
status.
Furthermore, the ultrastructural analysis conducted
in the present study recorded a significant reduction of glycogen
deposits in the BPA group, while a previous study suggested that
BPA treatment impairs hepatic glucose oxidation and glycogen
content through defective insulin signal transduction (49). Consistent with observations in the
present study, significant depletion of glycogen stores has also
been reported in the liver tissue of adult male albino rats
following 2 weeks of exposure to cisplatin (50). Notably, this previous study and the
present one found that silymarin was able to counteract toxicant
activity and restore glycogen to normal levels, thus favoring
hepatic glycogenesis. It is generally considered that a harmful
toxicant produces enough cellular stress in hepatocytes that
glycogenolysis is activated in order to generate increased glucose
serum levels (51), which protects
cells from oxidative injury (52).
This was reported by several groups following cisplatin
administration and monitoring of its effects on hepatocytes
(50,53,54). The
underlying mechanism supporting glycogen formation in the presence
of silymarin is not completely known; however, it has been proposed
that silymarin ameliorates the principal functions of the liver,
which are associated with the regulation of carbohydrate
metabolism, by restoring serum glucose levels to normal values
(50,55).
In conclusion, numerous studies have promoted the
exploration of the potential of natural extracts against the
negative effects produced by toxicants or pharmacologically harmful
compounds in tissues. In this perspective, silymarin has exhibited
efficient results in various types of tissues, but particularly in
the liver where it can control oxidative, glucidic and lipid
metabolism. The current study confirms the hepatoprotective effect
of silymarin against exposure to BPA and highlights for the first
time its ability to counteract low-grade inflammation caused by BPA
administration in animal models. Thus, silymarin holds great
promise as an adjuvant therapy for hepatotoxicity, and could be a
useful tool to further investigate the mechanism by which metabolic
signaling pathways regulate cellular inflammation.
Acknowledgements
This study was supported by a strategic grant (no.
POSDRU/159/1.5/S/133391) Project ‘Doctoral and Post-doctoral
programs of excellence for highly qualified human resources
training for research in the field of Life sciences, Environment
and Earth Science’ cofinanced by the European Social Fund within
the Sectorial Operational Program Human Resources Development
2007–2013. This study was also financed by the Institute for
Research of the University of Bucharest (ICUB), through
‘Scholarships for Excellence in Research for Young Researchers,
2015 Competition’ Project and START Project: BEST-NetWORK
16_PA07-C1.
References
|
1
|
Abdel-Wahab WM: Thymoquinone attenuates
toxicity and oxidative stress induced by bisphenol A in liver of
male rats. Pak J Biol Sci. 17:1152–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pritchett JJ, Kuester RK and Spies IG:
Metabolism of bisphenol A in primary cultured hepatocytes from
mice, rats, and humans. Drug Metab Dispos. 30:1180–1185. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y
and Taketani Y: Positive relationship between androgen and the
endocrine disruptor, bisphenol A, in normal women and women with
ovarian dysfunction. Endocr J. 51:165–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendiola J, Jørgensen N, Andersson AM,
Calafat AM, Ye X, Redmon JB, Drobnis EZ, Wang C, Sparks A, Thurston
SW, et al: Are environmental levels of bisphenol a associated with
reproductive function in fertile men? Environ Health Perspect.
118:1286–1291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pupo M, Pisano A, Lappano R, Santolla MF,
De Francesco EM, Abonante S, Rosano C and Maggiolini M: Bisphenol A
induces gene expression changes and proliferative effects through
GPER in breast cancer cells and cancer-associated fibroblasts.
Environ Health Perspect. 120:1177–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lang IA, Galloway TS, Scarlett A, Henley
WE, Depledge M, Wallace RB and Melzer D: Association of urinary
bisphenol A concentration with medical disorders and laboratory
abnormalities in adults. JAMA. 300:1303–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duan B, Hu X, Zhao H, Qin J and Luo J: The
relationship between urinary bisphenol A levels and meningioma in
Chinese adults. Int J Clin Oncol. 18:492–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubin BS: Bisphenol A: An endocrine
disruptor with widespread exposure and multiple effects. J Steroid
Biochem Mol Biol. 127:27–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vom Saal FS, Nagel SC, Coe BL, Angle BM
and Taylor JA: The estrogenic endocrine disrupting chemical
bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 354:74–84.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez-Hernández H, Simental-Mendía LE,
Rodríguez-Ramírez G and Reyes-Romero MA: Obesity and inflammation:
Epidemiology, risk factors, and markers of inflammation. Int J
Endocrinol. 2013:6781592013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Savastano S, Tarantino G, D'Esposito V,
Passaretti F, Cabaro S, Liotti A, Liguoro D, Perruolo G, Ariemma F,
Finelli C, et al: Bisphenol-A plasma levels are related to
inflammatory markers, visceral obesity and insulin-resistance: A
cross-sectional study on adult male population. J Transl Med.
13:1692015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weinhouse C, Anderson OS, Bergin IL,
Vandenbergh DJ, Gyekis JP, Dingman MA, Yang J and Dolinoy DC:
Dose-dependent incidence of hepatic tumors in adult mice following
perinatal exposure to bisphenol A. Environ Health Perspect.
122:485–491. 2014.PubMed/NCBI
|
|
13
|
Wetherill YB, Akingbemi BT, Kanno J,
McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT and
Belcher SM: In vitro molecular mechanisms of bisphenol A action.
Reprod Toxicol. 24:178–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asahi J, Kamo H, Baba R, Doi Y, Yamashita
A, Murakami D, Hanada A and Hirano T: Bisphenol A induces
endoplasmic reticulum stress-associated apoptosis in mouse
non-parenchymal hepatocytes. Life Sci. 87:431–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bindhumol V, Chitra KC and Mathur PP:
Bisphenol A induces reactive oxygen species generation in the liver
of male rats. Toxicology. 188:117–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY,
Lee KJ, Kim SH, Yoon YC, Cho BJ and Park KS: Bisphenol a impairs
mitochondrial function in the liver at doses below the no observed
adverse effect level. J Korean Med Sci. 27:644–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang JS, Jeon YJ, Park SK, Yang KH and Kim
HM: Protection against lipopolysaccharide-induced sepsis and
inhibition of interleukin-1beta and prostaglandin E2 synthesis by
silymarin. Biochem Pharmacol. 67:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Najafzadeh H, Jalali MR, Morovvati H and
Taravati F: Comparison of the prophylactic effect of silymarin and
deferoxamine on iron overload induced hepatotoxicity in rat. J Med
Toxicol. 6:22–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chtourou Y, Garoui E, Boudawara T and
Zeghal N: Therapeutic efficacy of silymarin from milk thistle in
reducing manganese-induced hepatic damage and apoptosis in rats.
Hum Exp Toxicol. 32:70–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panda V, Ashar H and Srinath S:
Antioxidant and hepatoprotective effect of Garcinia indica fruit
rind in ethanol-induced hepatic damage in rodents. Interdiscip
Toxicol. 5:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia R, Cao L, Du J, Xu P, Jeney G and Yin
G: The protective effect of silymarin on the carbon tetrachloride
(CCl4)-induced liver injury in common carp (Cyprinus carpio). In
Vitro Cell Dev Biol Anim. 49:155–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hermenean A, Stan M, Ardelean A, et al:
Antiioxidant and hepatoprotective activity of milk thistle (Silybum
marianum L. Gaertn.) seed oil. Open Life Sci. 10:225–236. 2015.
|
|
23
|
Das S, Roy P, Auddy RG and Mukherjee A:
Silymarin nanoparticle prevents paracetamol-induced hepatotoxicity.
Int J Nanomedicine. 6:1291–1301. 2011.PubMed/NCBI
|
|
24
|
Mansour HH, Hafez HF and Fahmy NM:
Silymarin modulates Cisplatin induced oxidative stress and
hepatotoxicity in rats. J Biochem Mol Biol. 39:656–661.
2006.PubMed/NCBI
|
|
25
|
Sasu A, Herman H, Mariasiu T, Rosu M,
Balta C, Anghel N, Miutescu E, Cotoraci C and Hermenean A:
Protective effects of silymarin on epirubicin-induced mucosal
barrier injury of the gastrointestinal tract. Drug Chem Toxicol.
38:442–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rašković A, Stilinović N, Kolarović J,
Vasović V, Vukmirović S and Mikov M: The protective effects of
silymarin against doxorubicin-induced cardiotoxicity and
hepatotoxicity in rats. Molecules. 16:8601–8613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vargas-Mendoza N, Madrigal-Santillán E,
Morales-González A, Esquivel-Soto J, Esquivel-Chirino C,
García-Luna Y, González-Rubio M, Gayosso-de-Lucio JA and
Morales-González JA: Hepatoprotective effect of silymarin. World J
Hepatol. 6:144–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karimi G, Vahabzadeh M, Lari P, Rashedinia
M and Moshiri M: ‘Silymarin’, a promising pharmacological agent for
treatment of diseases. Iran J Basic Med Sci. 14:308–317.
2011.PubMed/NCBI
|
|
29
|
Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY,
Shyu JC, Hsieh YS, Tsai CC and Liu YC: Effects of silymarin on the
resolution of liver fibrosis induced by carbon tetrachloride in
rats. J Viral Hepat. 15:508–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmad I, Shukla S, Kumar A, Singh BK,
Kumar V, Chauhan AK, Singh D, Pandey HP and Singh C: Biochemical
and molecular mechanisms of N-acetyl cysteine and
silymarin-mediated protection against maneb- and paraquat-induced
hepatotoxicity in rats. Chem Biol Interact. 201:9–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu HJ, Liu C, Duan WX, Xu SC, He MD, Chen
CH, Wang Y, Zhou Z, Yu ZP, Zhang L and Chen Y: Melatonin
ameliorates bisphenol A-induced DNA damage in the germ cells of
adult male rats. Mutat Re. 752:57–67. 2013. View Article : Google Scholar
|
|
32
|
Roelofs MJ, van den Berg M, Bovee TF,
Piersma AH and van Duursen MB: Structural bisphenol analogues
differentially target steroidogenesis in murine MA-10 Leydig cells
as well as the glucocorticoid receptor. Toxicology. 329:10–20.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rezg R, El-Fazaa S, Gharbi N and Mornagui
B: Bisphenol A and human chronic diseases: Current evidences,
possible mechanisms, and future perspectives. Environ Int.
64:83–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Markiewski MM, DeAngelis RA and Lambris
JD: Liver inflammation and regeneration: Two distinct biological
phenomena or parallel pathophysiologic processes? Mol Immunol.
43:45–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thoolen B, Maronpot RR, Harada T, Nyska A,
Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U,
et al: Proliferative and nonproliferative lesions of the rat and
mouse hepatobiliary system. Toxicol Pathol. 38(7): Suppl. 5S–81S.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Atsafack SS, Kuiate JR, Mouokeu RS,
Mogtomo ML Koanga, Tchinda AT, De Dieu TJ, Nana H Magnifouet, Etame
RM Ebelle, Biyiti L and Ngane RA Ngono: Toxicological studies of
stem bark extract from Schefflera barteri Harms (Araliaceae). BMC
Complement Altern Med. 15:442015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weiss MJ: Cytokines in liver, biliary and
pancreatic diseaseBlumgart's Surgery of the Liver, Pancreas and
Biliary Tract. Jarnagin W: 5th. Elsevier, Inc.; Philadelphia, PA:
pp. 166–180. 2012, View Article : Google Scholar
|
|
38
|
Choi I, Kang HS, Yang Y and Pyun KH: IL-6
induces hepatic inflammation and collagen synthesis in vivo. Clin
Exp Immunol. 95:530–535. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Norris CA, He M, Kang LI, Ding MQ, Radder
JE, Haynes MM, Yang Y, Paranjpe S, Bowen WC, Orr A, et al:
Synthesis of IL-6 by hepatocytes is a normal response to common
hepatic Stimuli. PLoS One. 9:e960532014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sherif IO and Al-Gayyar MMH: Antioxidant,
anti-inflammatory and hepatoprotective effects of silymarin on
hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw.
24:114–121. 2013.PubMed/NCBI
|
|
41
|
Lovelace ES, Wagoner J, MacDonald J,
Bammler T, Bruckner J, Brownell J, Beyer RP, Zink EM, Kim YM, Kyle
JE, et al: Silymarin suppresses cellular inflammation by inducing
reparative stress signaling. J Nat Prod. 78:1990–2000. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pękalski J, Zuk PJ, Kochańczyk M, Junkin
M, Kellogg R, Tay S and Lipniacki T: Spontaneous NF-kB activation
by autocrine TNFa Signaling: A computational analysis. PLoS One.
8:e788872013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kabuto H, Amakawa M and Shishibori T:
Exposure to bisphenol A during embryonic/fetal life and infancy
increases oxidative injury and causes underdevelopment of the brain
and testis inmice. Life Sci. 74:2931–2940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Korkmaz A, Ahbab MA, Kolankaya D and
Barlas N: Influence of vitamin C on bisphenol A, nonylphenol and
octylphenol induced oxidative damages in liver of male rats. Food
Chem Toxicol. 48:2865–2871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Suthar H, Verma RJ, Patel S and Jasrai YT:
Green tea potentially ameliorates bisphenol a-induced oxidative
stress: An in vitro and in silico study. Biochem Res Int.
2014:2597632014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Doerge DR and Fisher JW: Background paper
on metabolism and toxicokinetics of bisphenol A. Proceedings of the
WHO/HSE/FOS/11.1 Expert Meeting on Bisphenol A (BPA'10). World
Health Organization. Geneva. 2010;
|
|
47
|
Xia W, Jiang Y, Li Y, Wan Y, Liu J, Ma Y,
Mao Z, Chang H, Li G, Xu B, et al: Early-life exposure to bisphenol
a induces liver injury in rats Involvement of mitochondria-mediated
apoptosis. PLoS One. 9:e904432014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muriel P, Garciapiña T, Perez-Alvarez V
and Mourelle M: Silymarin protects against paracetamol-induced
lipid peroxidation and liver damage. J Appl Toxicol. 12:439–442.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jayashree S, Indumathi D, Akilavalli N,
Sathish S, Selvaraj J and Balasubramanian K: Effect of Bisphenol-A
on insulin signal transduction and glucose oxidation in liver of
adult male albino rat. Environ Toxicol Pharmacol. 35:300–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abouzeinab NS: Cytoprotective effect and
antioxidant properties of silymarin on cisplatin induced
hepatotoxicity in rats: A biochemical and histochemical study.
International Journal of Cancer Research. 9:9–23. 2013. View Article : Google Scholar
|
|
51
|
Nelson DL and Cox MM: Lehninger Principles
of Biochemistry. 5th. W.H. Freeman and Co.; New York, NY: pp.
540–550. 2008
|
|
52
|
Ebaid H, Dkhil MA, Danfour MA, Tohamy A
and Gabry MS: Piroxicam-induced hepatic and renal histopathological
changes in mice. Libyan J Med. 2:82–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miyamato Y, Shimada K, Sakaguchi Y and
Miyamoto M: Cisplatin (CCDP)-induced acute toxicity in an
experimental model of hepatic fibrosis. J Toxicol Sci. 32:311–319.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rosner MH and Okusa M: Biomarkers in Renal
Disease. 5th. Nova Publishers; New York, NY: pp. 21–45. 2008
|
|
55
|
Salam OM Abdel, Sleem AA, Omara EA and
Hassan NS: Effect of ribavirin alone or combined with silymarin on
carbon tetrachloride induced hepatic damage in rats. Drug Target
Insights. 2:19–27. 2007.PubMed/NCBI
|