Introduction
Parkinson's disease (PD) is a degenerative disorder
affecting the central nervous system (CNS), which results from the
death of dopamine-generating cells in the substantia nigra of the
midbrain. Thus, PD is characterized by depletion of dopaminergic
cell bodies in substantia nigra that are subsequently lost in the
nigrostriatal system. The nigrostriatal system is composed of the
Substantia Nigra and Corpus striatum and is the affected
dopaminergic system in cases of PD. The reported incidence rates of
PD vary largely. The lowest PD incidence was reported to be
4.5/1,000000 in Libya, while the highest incidence was reported in
the USA at 20/1,000000 (1). The
early symptoms of PD include slowness of movement, rigidity,
shaking and walking difficulties. Symptoms presented at later
stages of the disease may include thinking and behavioral problems,
with dementia and depression arising in the advanced stages
(1). Other symptoms, including
sensory, sleep and emotional problems, may also occur. The majority
of PD cases occur after the age of 50 years, and the disorder is
more prevalent among men rather than women. The pathological
hallmark of the disease is accumulation of the protein α-synuclein
into inclusions known as Lewy bodies in neurons (2). The intensity of the Lewy bodies is
directly associated with the clinical symptoms of each individual.
Mechanisms underlying PD may include mitochondrial dysfunction,
oxidative stress, inflammation and defective protein handling. PD
is the second most common neurodegenerative disorder after
Alzheimer's disease (3). Upon
clinical diagnosis of PD, the loss in dopaminergic neurons has
already reached 80%, and thus neuroprotective therapies for PD are
of little significance as the majority of dopaminergic neurons are
lost. By contrast, the use of regenerative agents in PD patients
appears to be more promising (4).
Cell-based therapy has been advocated for PD due to
the high failure rate of other therapeutic strategies (5). Despite the improvement imparted by
developing novel dopamine receptors agonists, these agents are
considered weak in comparison with L-DOPA, thus resulting in their
use as a complementary treatment rather than a substitute to the
classical L-DOPA therapy (6).
Cellular therapy, which was initially investigated using fetal
tissues with limited long-term success, is currently the central
component of regenerative treatment for PD. However, the
identification of stem cells increased the possibility of more
successful cellular therapy for neuroregeneration (7). However, to date, advances in stem cell
research have failed to offer a successful regenerative therapy for
PD patients due to various limiting factors. The most important
problem for stem cell therapy in neurodegenerative diseases such as
PD is the method of administration. The first mode of stem cell
transplantation is through their direct introduction into the
corpus striatum using stereotaxis, with the corpus being the
preferred site for transplantation over the substantia nigra and
subthalamic nucleus (8). However,
this route is considered inapplicable in humans due to
inconvenience, risk of several possible complications and high
costs (9). By contrast, systemic
administration of stem cells has not demonstrated sufficient
encouraging results, which may be due to the difficulty of cells
crossing the blood-brain barrier (BBB) (10).
The search for efficient delivery route for
neurological diseases appears to be crucial. The majority of
candidate drugs for CNS diseases that showed promising results on
in vitro and in vivo studies failed to show similar
efficacy in humans, leading to high attrition rates of novel CNS
active drugs in clinical trials (11). The main reason for such failure is
the presence of the BBB, which prevents the passage of the right
concentration of the drug to the target tissue (12).
One approach for resolving this issue is targeted
intranasal (IN) delivery, which is an applicable method used to
circumvent BBB rather than attempting to cross it (13). The nasal passage is the only direct
connection between the brain and the external environment. This
connection occurs through the extension of axons from the olfactory
bulb to the nose, allowing direct contact with the external
environment. Another potential route is the nose to brain pathway,
which is a controversial pathway suggesting the passage of
medication through the deep structures in the nose that are
innervated by cranial nerves (14).
Based on this pathway, the IN route has been used for the delivery
of a variety of agents for the treatment of different CNS
conditions. For instance, drugs delivered using IN delivery system
include growth factors, neuropeptides, genes and small molecules
(9). Notably, previous animal
studies showed the successful IN delivery of mesenchymal stem cells
(MSCs) to the brain. In addition, animal models of Parkinson's
disease have been successfully treated through IN administration of
L-DOPA (15).
Based on these previous findings, the present study
aimed to investigate the use of the IN route for administration of
MSCs in a mouse model of PD.
Materials and methods
Rotenone mouse model and stem cells
administration
A total of 30 B57BL/6 mice (age, 8 weeks; weight,
16–20 g) were provided by the Medical Experimental Research Center
of Mansoura University (Mansoura, Egypt) and maintained in
conditions of 21–23°C, with a humidity of 40–55% and a 12 h
dark/light cycle. All animal experiments were performed according
to the Guidelines for the Care and Use of Mammals in Neuroscience
(2003), and were approved by the Ethical Committee for research at
Mansoura University. All efforts were made to minimize animal
suffering and to reduce the number of animals used.
A PD model was developed in mice through the
intraperitoneal administration of 3 mg/kg/day body weight rotenone
(Sigma-Aldrich, St. Louis, MO, USA), for 60 consecutive days. The
mice were divided into three groups (10 mice in each) as follows:
Control group, which received daily intraperitoneal injection of
0.5% carboxymethyl cellulose (El-Nasr Chemicals Co., Cairo, Egypt);
PD model group (rotenone group), receiving rotenone (3 mg/kg body
weight) dissolved in 0.5% carboxymethyl cellulose
intraperitoneally; and rotenone+MSC group, which received rotenone
administration similarly to the PD model group, followed by IN bone
marrow MSCs derived on day 60. All animals were sacrificed by
perfusion through the aorta with 50 ml of 10 mM phosphate-buffered
saline (PBS), followed by 150 ml of a cold fixative consisting of
4% paraformaldehyde, 0.35% glutaraldehyde and 0.2% picric acid in
100 mM phosphate buffer, under deep anesthesia with pentobarbital
(100 mg/kg, intraperitoneally). Animals were sacrificed on day 70,
thus after 10 days of MSCs treatment for the rotenone+ MSC
group.
Stem cells isolation
MSCs were isolated from the mononuclear cell
fraction of pooled bone marrow from healthy C57BL/6 mice. Mice were
sacrificed by cervical dislocation and their femurs and tibiae were
carefully cleaned from the skin and cut at the ankle bone. The
muscle and connective tissue were scraped, and the bones were
placed in 10% ethyl alcohol for sterilization and left for a few
seconds. Next, the ends of the tibia and femur were cut by sharp
scissors and a 27-gauge needle was inserted, after which the sample
was flushed with Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and collected in a 15-ml
tube. The cell suspension was then filtered through a 70-µm filter
mesh. Bone marrow cells were cultured in DMEM supplemented with 10%
fetal bovine serum and 1% antibiotic-antimycotic solution (Thermo
Fisher Scientific, Inc.) in a 25-cm2 tissue culture
flask and incubated at 37°C and 5% CO2. Subsequent to
adhesion to the plastic wall of the dish and culture in DMEM, the
stem cells were tested for plasticity through in vitro
differentiation, including investigation of adipogenesis,
chondrogenesis and osteogenesis (16). Isolated MSCs were then incubated
overnight with micrometer-sized iron oxide (MPIO) particles (Bangs
Laboratories, Inc., Fishers, IN, USA). For IN application, a 20 µl
cell suspension (5×105 cells) was carefully placed on
one nostril of each animal, allowing the mice to insufflate the MSC
suspension, as previously described (17).
Behavioral evaluation
Behavioral assessment
To evaluate the therapeutic effects of IN delivery
of MSCs, neurobehavioral investigation of all animals was performed
by monitoring their general activity, presence of tremors, akinesia
and cataplexy, as well as using the open field and parallel rod
tests.
Tremors
Tremors were monitored immediately after the
administration of rotenone. Three trained examiners monitored the
animals in a blind study. Tremors were quantified on a modified
intensity-score basis in a scale of 0–5, as described previously
(18).
Akinesia
Akinesia was measured by recording the latency (in
seconds) of the animals to move all four limbs, and the test was
terminated when the latency exceeded 180 sec (19).
Catalepsy
The term catalepsy indicates the inability of
rodents to correct an externally imposed posture (19). It was measured by placing the animals
on a flat horizontal surface with the two hind limbs on a square
wooden block (3-cm high), and the latency to move the hind limbs
from the block to the ground was estimated in seconds.
Open field analysis
Mice were adapted to the open field enclosure prior
to the test, and monitored. All tests were performed for 1 h, using
an ANY-maze video tracking system (Stoelting Co., Wood Dale, IL,
USA). This software, with the complementary Open Field Cage, was
used to analyze the behavioral changes in the mice objectively,
including the following parameters: Average speed, total time
immobile, total number of immobility episodes, rotations of body
and efficient paths.
Parallel rod test
Mice were adapted to the parallel rod enclosure
prior to the assessment, and monitored. All tests were performed
for 15 min, using an ANY-maze video tracking system. This software,
with the complementary Open Field Cage, was used to analyze
locomotor changes in mice in the form of number of slips.
Tissue analysis
After the mice were sacrificed on day 70, all
animals were evaluated for stem cells tracking and tyrosine
hydroxylase (TH) antibody binding. TH is the key enzyme in the
dopamine synthesis process, staining against TH is the preferred
antibody-based method to detect dopaminergic neurons. For stem cell
tracking, brain sections of IN MSC-treated mice were stained with
Prussian blue (Sigma-Aldrich) in order to detect the MPIO-labeled
MSCs (18). After perfusion, the
brain was quickly removed and post-fixed for 2 days with
paraformaldehyde in 100 mM PBS, and then transferred to 15% sucrose
solution in 100 mM PBS containing 0.1% sodium azide at 4°C. The
brain specimens were processed into paraffin blocks, and then cut
by a microtome at 4–5 micron on glass slides. Next, the specimens
were deparaffinized, and then endogenous peroxidase was blocked
using 30% hydrogen peroxide in methanol for 10 min, followed by
serum blocking solution (10% non-immune serum) for 10 min. Antigen
retrieval was performed with EDTA solution for 20 min at 90°C in a
water bath. Subsequently, the slides were incubated with primary
mouse monoclonal anti-TH antibody (dilution, 1:1,000; cat no.
AMAB91112; Sigma-Aldrich) overnight at 4°C. Following several
washes with PBS, the samples were incubated with the required
biotinylated secondary antibody (1:10,000; cat no. A4416;
Sigma-Aldrich) for 10 min, followed by the avidin-biotin-peroxidase
complex for 10 min at room temperature. All the sections were
washed several times with PBS between each incubation, and labeling
was then revealed by addition of diaminobenzidine, which was used
as a chromogen. Slides were counterstained with Meyer's hematoxylin
(Sigma-Aldrich), dehydrated and covered with the cover slip.
Immunohistochemical analysis was also performed to
investigate TH antibody binding in substantia nigra pars compacta
(SNpc) and corpus striatum. Subsequently, TH immunostained brain
sections were evaluated as follows: i) Striatal TH-fiber density
measurement was performed using Image J software (http://imagej.nih.gov/ij/) as previously described
(19); and ii) number of
dopaminergic neurons in the SNpc were counted as previously
described (20), and were reported
as percentages of the control neurons (with the control set to
100%).
Image analysis
All analyses were performed by an investigator
blinded to the experimental design. Various areas were subjected to
image analysis. In order to bilaterally evaluate the TH-positive
fiber innervation in the striatum, mean optical density
measurements were performed using the Image J software (version
1.33–1.34; National Institutes of Health, Bethesda, MD, USA). In
addition, images of coronal sections were captured at seven
rostral-caudal levels, in order to cover the entire striatal
complex. The striatum was included from the lateral ventricle to
the external capsule and a horizontal line connecting the ventral
end of the ventricle via the anterior commissure to the external
capsule. The data are expressed as percentage of the controls and
represent the average of the seven levels (20). Furthermore, assessment of
dopaminergic neurons in the substantia nigra pars compacta (SNpc)
was determined by counting the number of TH-positive cells in the
SNpc of both hemispheres, in every fourth section throughout the
entire nucleus. The anatomical levels considered in the
anteroposterior (AP) extension were within −5.20 and −5.80 mm with
respect to bregma. Results are expressed as the percentage of
TH-positive cells in the lesioned SNpc with respect to the control
(21).
Statistical analysis
All data are presented as the mean ± standard
deviation. Two groups of data were analyzed by Student's t-test.
Three groups of data were analyzed by analysis of variance with a
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioral evaluation
Behavioral assessment revealed progressive
deterioration in the locomotor functions of the rotenone group (PD
model group) compared with the control group. However, IN
administration of MSCs (rotenone+MSC group) was found to improve
the locomotor performance of the animals when compared with the
rotenone-only treated animals. In general, increased salivation and
hyperpnea were observed following injections, but with no
accompanied convulsions or mortality in any group.
The maximum tremor intensity was only up to a score
of 4, with a maximum tremor duration of 20 min and a peak in
intensity at 10 min. Tremors were rarely observed in the
MSC-treated group, and the intensity did not exceed a score of
1.
Rotenone administration resulted in akinesia in the
rotenone treated mice at 34+7 sec, while the MSC-treated group
displayed an improved performance (12+5 sec). In addition,
catalepsy was evident in the animals treated with rotenone, with a
latency period was 41+5 sec. By contrast, the MSC-treated group
displayed a better performance (13+4 sec).
The results of the open field test indicated a
significant deterioration between the control and the rotenone
treated groups with regard to various parameters, including the
average speed, total time immobile, total immobility episodes,
rotations of body and efficient paths (Table I). However, mice in the IN
MSC-treated group showed results that were comparable to the
control mice, revealing behavioral improvement. These parameters
were determined by the original software ANY-maze, compatible with
the open field test.
 | Table I.Open field test parameters. |
Table I.
Open field test parameters.
| Parameter | Group 1 | Group 2 | Group 3 |
|---|
| Average speed | 0.11±0.03 |
0.07±0.01a | 0.10±0.02 |
| Total time
immobile | 4.21±2.14 |
13.03±5.24a | 6.02±4.03 |
| Total immobile
episode | 2.01±1.21 |
7.21±3.22a | 3.91±2.12 |
| Clockwise
rotations | 1.03±0.63 |
5.89±1.90a | 2.10±0.74 |
| Efficient path | 0.04±0.02 |
0.01±0.00a |
0.02±0.02a |
Furthermore, a parallel rod test was performed, and
the results indicated a significant increase in the number of slips
in the rotenone group (6 slips) compared with the control group,
which had an average of 1 slip. IN MSC treatment improved the
performance of the rotenone-treated mice to an average of 2
slips.
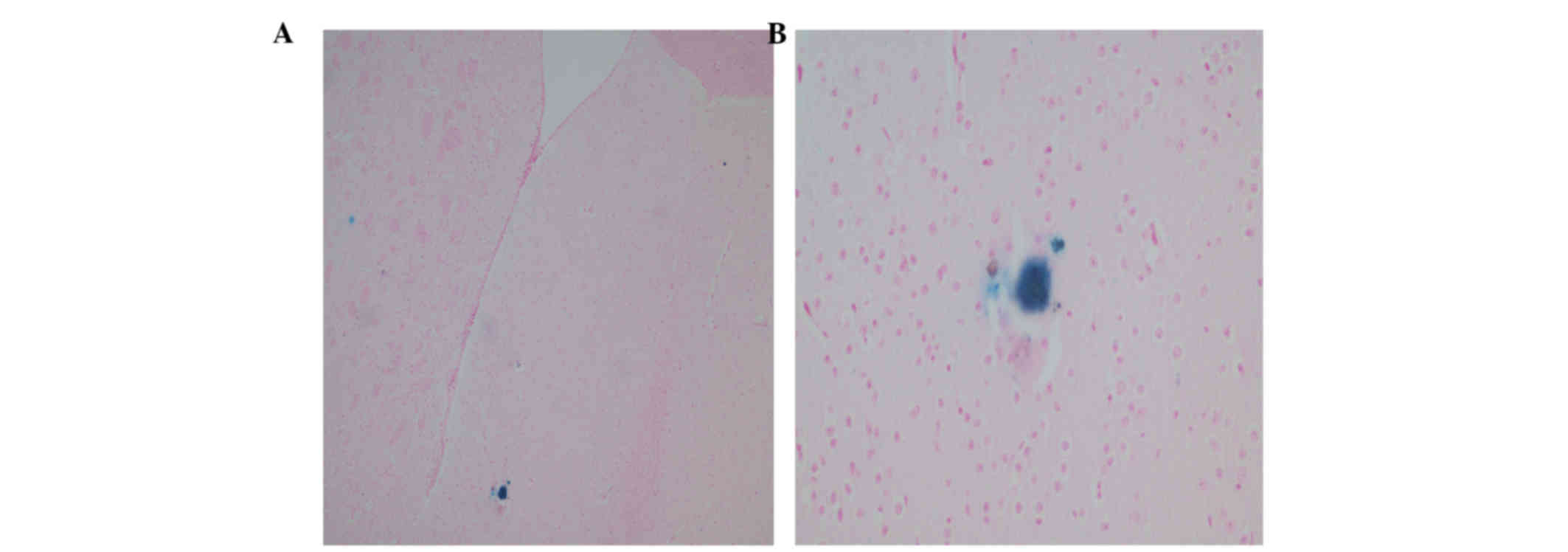
Stem cell tracking
Histopathological evaluation of treated animal brain
sections revealed successful IN delivery of stem cells to the brain
tissues of the rotenone+MSC mice. This successful delivery was
evidenced by the positive staining with Prussian blue in different
areas of the brain tissues (Fig.
1).
Immunohistochemical analysis
Rotenone treatment resulted in a significant
decrease in dopaminergic neuron number in the SNpc (37±8% of
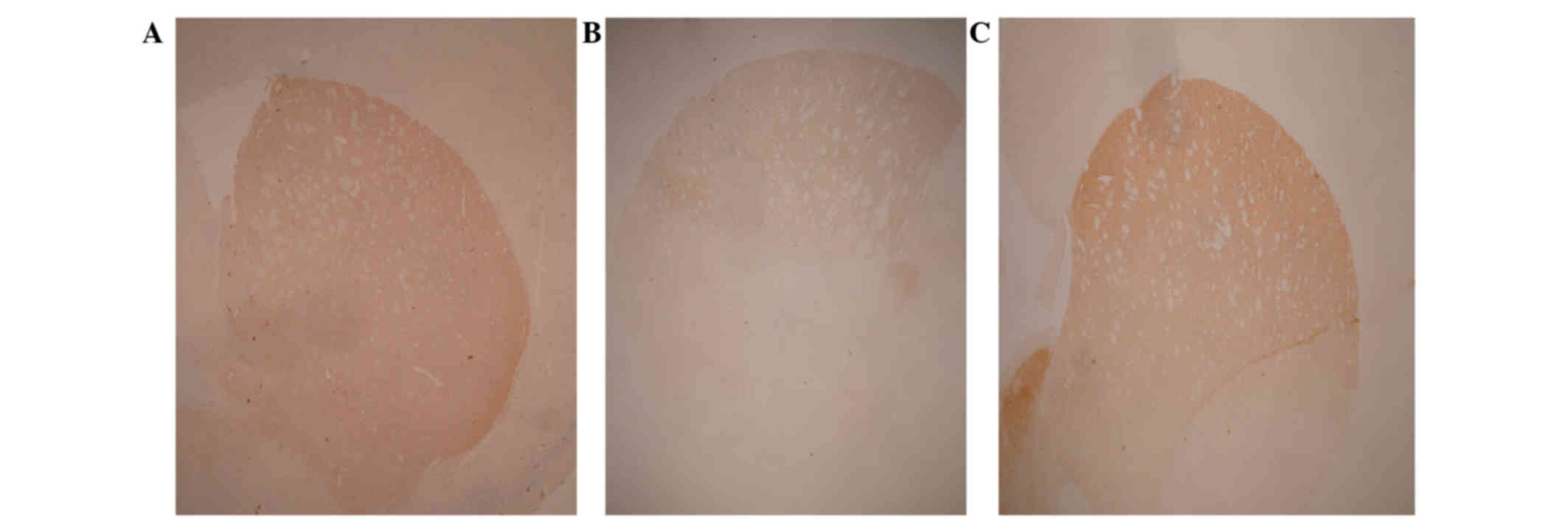
neurons compared with the control; Table II). Similarly, rotenone treatment
reduced the corpus striatum fiber density to 53±7% of the control
value (Table I; Fig. 2A and B). However, in the animals
receiving IN MSCs, the degeneration caused by rotenone treatment
was significantly counteracted by stem cells administration, with a
SNpc neuron number at 80±6% and corpus striatum fiber density at
91±5% of the values reported in the control group (Table I; Fig. 2A
and C).
 | Table II.SNpc neuronal counting and stratial
OD. |
Table II.
SNpc neuronal counting and stratial
OD.
| Parameter | Rotenone group | Rotenone+MSC
group |
|---|
| SNpc neurons,
% | 37±8 | 80±6a |
| Stratial OD, % | 53±7 | 91±5a |
Discussion
Cellular therapy for PD has been previously
suggested as a promising treatment option (22) However, the limitations of cellular
transplantation methods render this approach unsuitable for
clinical applications (23).
Experimentally, the most successful approach for cell
transplantation is the intralesional route, performed by
stereotaxis-aided injection of cells into the corpus striatum
(24). However, despite the
efficiency of this route, it requires complex surgical procedures
that makes it clinically difficult to advocate for PD cases.
Furthermore, longitudinal follow-up of transplanted cells revealed
the need of booster doses, due to the loss of the majority of
transplanted cells with time (25).
The requirement for repetitive doses results in difficult repeat
surgical procedures in human cases. Systemic administration of
cells has not been proven to be effective in PD, and certain
positive results have been attributed to systemic effects due to
growth factor upregulation rather than local regenerative effects
imparted by the transplanted cells (26).
A new and attractive alternative route is the
intranasal administration of drugs for CNS diseases (27). The IN route efficiently introduces
drugs intracranially through different suggested pathways.
Initially, direct delivery of IN therapeutics was attributed to the
olfactory nerve, but more recently, the trigeminal nerve was
suggested as another contributing route. In addition, the quick
time required for therapeutics to reach the brain through the IN
route suggests extracellular delivery rather than axonal transport.
Recently, diffusion within perineural and lymphatic channels or
perivascular space has been suggested as a more reliable hypothesis
(28).
The ease and efficacy of IN administration, besides
the rapid delivery of therapeutic agents, resulted in several
trials investigating the use of this route in various CNS
disorders, particularly in neurodegenerative diseases. The IN
administration of therapeutic agents was attempted with numerous
vehicles and tracking methodologies (29,30). In
the majority of studies, IN administration (alone or assisted with
drug delivery agents) successfully reached the intracranial region
and led to significant improvement in the disease (31). In addition, investigation of the role
of IN stem cell delivery in PD has been attempted in several animal
models, with promising results. Although long-term follow-up of
transplanted stem cells revealed disappearance of cells after a
certain period of time, this is predicted in neurodegenerative
diseases, which mandates repetitive doses, thus further supporting
the use of the easy IN route.
In spite of the positive results obtained from
previous studies addressing the use of IN stem cells in PD,
translation into clinical practice has yet to be achieved. This is
due to the lack of an ideal animal model of PD that can
recapitulate the pathology that occurs in human cases (32). Therefore, it is important to study
new therapeutic agents for PD on different animal models.
Previously, the IN route was assessed in 6-OHDA rat model (21) and in transgenic mice (22). The main issue with animal models of
PD is the absence of an ideal model that can recapitulate all PD
pathological findings (23).
Therefore, it appears that investigating new therapeutic approaches
on different animal models may be more effective (24).
Although the 6-OHDA and transgenic models are
important tools to study PD in animals, they present major
limitations that may reduce the credibility of their use for
therapeutic agent testing (33). The
6-OHDA model involves the local injection of the agent into the
nigrostriatal system, leading to immediate and severe degeneration
in this area. With the exception of damage caused in the
dopaminergic system, this model is not associated with other
characteristics of PD. It lacks the cascade of events or pathogenic
pathways that lead to PD in human cases, including lack of the
neuroinflammatory nature of PD which is important when studying
cell transplantation. Furthermore, it does not represent the
progressive nature of the disease, and since PD is a unilateral
disease, the model does not accurately represent the locomotor
disturbances occurring in PD. By contrast, transgenic models of PD
can serve as successful models for the rare familial type of the
disease; however, they lack the sequence of events that lead to the
development of typical PD. In idiopathic PD, the role of
environmental exposure is major, and thus this is a limiting point
of transgenic models. An alternative model that carries numerous of
the PD characteristics is the rotenone-induced model. Rotenone can
induce a PD model in animals that has a chronic and progressive
nature. The disease process is accompanied by various features of
PD pathogenesis, particularly the neuroinflammatory effects and BBB
influence, which are critical points in evaluating cell
therapy.
Based on the aforementioned observations and
limitations, the present study used the IN route for delivering
MSCs in a rotenone-induced PD model in mice. To evaluate the
therapeutic efficiency of this route, animals were evaluated
behaviorally using a variety of neurobehavioral tests (such as the
open field and parallel rod tests) and histopathological evaluation
of brain sections through immunostaining against TH, which is the
main marker of dopaminergic cells. Behavioral assessment assists in
the study of the symptom-relieving effects of therapy, as
behavioral tests can be translated into clinical performance in
human cases. In addition, histopathological evaluation helps to
study the improvement of disease pathology following treatment. In
the present study, the transplanted stem cells were tracked to
ensure their successful delivery intracranially and that they
reached the site of the lesion. This step is of paramount
importance to verify that any therapeutic effects of MSCs are
caused by their direct regenerative effects and not due to a
systemic body reaction.
In the current study, IN delivery of MSCs
administered to a rotenone animal model were found to result in
improvements in all affected neurobehavioral tests, which indicates
the efficient therapeutic effect of this treatment. The successful
passage of IN stem cells, as observed by stem cell tracking in the
mouse brain tissue, shows that the therapeutic effects observed on
the behavioral level can be attributed to the physical presence of
MSCs inside the target brain tissues. The therapeutic efficiency of
IN delivery of MSCs was then verified by immunostaining with TH
antibodies, showing reduced degenerative effects compared with the
rotenone-only treated group.
The results reported in the present study complement
previous research findings that denote the success of IN delivery
of stem cells in animal PD models. The use of a rotenone PD model
appears to be of great importance, as this model carries certain
important features of PD, such as environmental contribution and
the chronic progressive pattern of the disease (25).
In conclusion, the present study identified the
positive effects of IN delivery of MSCs in a progressive mouse
model of PD. Thus, this treatment may have a possible similar
effect in clinical practice, suggesting potential application in
human cases of PD. According to the present results along with
those of previous studies, IN delivery of stem cells appears to be
a potential safe, easy and cheap route for stem cell treatment in
neurodegenerative disorders. Although this study offered proof of
the potential therapeutic benefit of IN route for delivering MSCs
as a treatment for PD, further investigation is required prior to
clinical application, such as comparison between different drug
delivery vehicles, evaluation of nanosubstance addition, and
investigation of the ideal type of stem cells and timing of
transplantation.
Acknowledgements
The present study was supported by the Medical
Experimental Research Center of Mansoura University (grant no.
2014-01).
References
|
1
|
Chen RC, Chang SF, Su CL, Chen TH, Yen MF,
Wu HM, Chen ZY and Liou HH: Prevalence, incidence, and mortality of
PD: A door-to-door survey in Ilan county, Taiwan. Neurology.
57:1679–1686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aarsland D, Londos E and Ballard C:
Parkinson's disease dementia and dementia with Lewy bodies:
Different aspects of one entity. Int Psychogeriatr. 21:216–219.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samii A, Nutt JG and Ransom BR:
Parkinson's disease. Lancet. 363:1783–1193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sengupta U, Guerrero-Muñoz MJ,
Castillo-Carranza DL, Lasagna-Reeves CA, Gerson JE,
Paulucci-Holthauzen AA, Krishnamurthy S, Farhed M, Jackson GR and
Kayed R: Pathological interface between oligomeric alpha-synuclein
and tau in synucleinopathies. Biol Psychiatry. 78:672–683. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shulman JM, De Jager PL and Feany MB:
Parkinson's disease: Genetics and pathogenesis. Annu Rev Pathol.
6:193–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toulouse A and Sullivan AM: Progress in
Parkinson's disease-where do we stand? Prog Neurobiol. 85:376–392.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Street V and Stacy M: Dopamine
agonistsHandbook of Parkinson's Disease. Pahwa R and Lyons KE: 4th.
Informa Healthcare; New York, NY: pp. 335–348. 2007
|
|
8
|
Goya R Laguna, Tyers P and Barker RA: The
search for a curative cell therapy in Parkinson's disease. J Neurol
Sci. 265:32–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inden M, Kim D, Qi M, Kitamura Y,
Yanagisawa D, Nishimura K, Tsuchiya D, Takata K, Hayashi K,
Taniguchi T, et al: Transplantation of mouse embryonic stem
cell-derived neurons into the striatum, subthalamic nucleus and
substantia nigra, and behavioral recovery in hemiparkinsonian rats.
Neurosci Lett. 387:151–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanson LR and Frey WH II: Intranasal
delivery bypasses the blood-brain barrier to target therapeutic
agents to the central nervous system and treat neurodegenerative
disease. BMC Neurosci. 9:(Suppl 3). S52008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chao YX, He BP and Tay SS: Mesenchymal
stem cell transplantation attenuates blood brain barrier damage and
neuroinflammation and protects dopaminergic neurons against MPTP
toxicity in the substantia nigra in a model of Parkinson's disease.
J Neuroimmunol. 216:39–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruigrok MJ and de Lange EC: Emerging
insights for translational pharmacokinetic and pharmacokinetic
pharmacodynamic studies: Towards prediction of nose-to-brain
transport in humans. AAPS J. 17:493–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Lange EC: The mastermind approach to
CNS drug therapy: Translational prediction of human brain
distribution, target site kinetics, and therapeutic effects. Fluids
Barriers CNS. 10:122013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Djupesland PG, Messina JC and Mahmoud RA:
The nasal approach to delivering treatment for brain diseases: An
anatomic, physiologic, and delivery technology Overview. Ther
Deliv. 5:709–733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lochhead JJ and Thorne RG: Intranasal
delivery of biologics to the central nervous system. Adv Drug Deliv
Rev. 64:614–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gambaryan PY, Kondrasheva IG, Severin ES,
Guseva AA and Kamensky AA: Increasing the efficiency of Parkinson's
disease treatment using a poly (lactic-co-glycolic acid) (PLGA)
based L-DOPA delivery system. Exp Neurobiol. 23:246–252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji JF, He BP, Dheen ST and Tay SS:
Interactions of chemokines and chemokine receptors mediate the
migration of mesenchymal stem cells to the impaired site in the
brain after hypoglossal nerve injury. Stem Cells. 22:415–427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarkar S, Thomas B, Muralikrishnan D and
Mohanakumar KP: Effects of serotoninergic drugs on tremor induced
by physostigmine in rats. Behav Brain Res. 109:187–193. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haobam R, Sindhu KM, Chandra G and
Mohanakumar KP: Swim-test as a function of motor impairment in MPTP
model of Parkinson's disease: A comparative study in two mouse
strains. Behav Brain Res. 163:159–167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlsson T, Winkler C, Lundblad M, Cenci
MA, Bjorklund A and Kirik D: Graft placement and uneven pattern of
reinnervation in the striatum is important for development of
graft-induced dyskinesia. Neurobiol Dis. 21:657–668. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blandini F, Cova L, Armentero MT, Zennaro
E, Levandis G, Bossolasco P, Calzarossa C, Mellone M, Giuseppe B,
Deliliers GL, et al: Transplantation of undifferentiated human
mesenchymal stem cells protects against 6-hydroxydopamine
neurotoxicity in the rat. Cell Transplant. 19:203–217. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goya R Laguna, Tyers P and Barker RA: The
search for a curative cell therapy in Parkinson's disease. J Neurol
Sci. 265:32–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paul G: Cell transplantation for patients
with Parkinson's disease. Handb Exp Pharmacol. 174:361–388. 2006.
View Article : Google Scholar
|
|
24
|
Inden M, Kim DH, Qi M, Kitamura Y,
Yanagisawa D, Nishimura K, Tsuchiya D, Takata K, Hayashi K,
Taniguchi T, et al: Transplantation of mouse embryonic stem
cell-derived neurons into the striatum, subthalamic nucleus and
substantia nigra, and behavioral recovery in hemiparkinsonian rats.
Neurosci Lett. 387:151–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sayles M, Jain M and Barker RA: The
cellular repair of the brain in Parkinson's disease-past, present
and future. Transpl Immunol. 12:321–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao YX, He BP, Tay SS and Tay W:
Mesenchymal stem cell transplantation attenuates blood brain
barrier damage and neuroinflammation and protects dopaminergic
neurons against MPTP toxicity in the substantia nigra in a model of
Parkinson's disease. J Neuroimmunol. 216:39–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhanda DS, Frey WH II, Leopold D and
Kompella UB: Approaches for drug deposition in the human olfactory
epithelium. Drug Deliv Technol. 5:64–72. 2005.
|
|
28
|
Thorne RG and Frey WH II: Delivery of
neurotrophic factors to the central nervous system: Pharmacokinetic
considerations. Clin Pharmacokinet. 40:907–946. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bossolasco P, Cova L, Levandis G, Diana V,
Cerri S, Deliliers G Lambertenghi, Polli E, Silani V, Blandini F
and Armentero T: Noninvasive near infrared live imaging of human
adult mesenchymal stem cells transplanted in a rodent model of
Parkinson's disease. Int J Nanomedicine. 7:435–447. 2012.PubMed/NCBI
|
|
30
|
Danielyan L, Hammer S Beer, Stolzing A,
Schäfer R, Siegel G, Fabian C, Kahle P, Biedermann T, Lourhmati A,
Buadze M, et al: Intranasal delivery of bone marrow derived
mesenchymal stem cells, macrophages and microglia to the brain in
mouse models of Alzheimer's and Parkinson's disease. Cell
Transplant. 23:(Suppl 1). S123–S139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Y, Zhu J, Xu G and Liu X: Intranasal
delivery of stem cells to the brain. Expert Opin Drug Deliv.
8:623–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dawson T, Mandir A and Lee M: Animal
models of PD: Pieces of the same puzzle? Neuron. 35:219–222. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Emborg ME: Evaluation of animal models of
Parkinson's disease for neuroprotective strategies. J Neurosci
Methods. 139:121–143. 2004. View Article : Google Scholar : PubMed/NCBI
|