Introduction
Leukemia is a group of hematological malignancies
characterized by abnormal proliferation, decreased apoptosis and
blocked differentiation of hematopoietic stem/progenitor cells
(1). To date, the removal or
deactivation of cancerous cells is the primary strategy of treating
leukemia, and this is achieved by methods such as chemotherapy or
bone marrow transplantation (2,3).
However, it has numbers of side effects and high toxicity.
Therefore, apoptosis- and differentiation-inducing therapy seems to
be a promising strategy for the treatment of leukemia, particularly
in patients who cannot tolerate the intensity of chemotherapy and
bone marrow transplantation. Thus, finding effective inducers that
are free of general cytotoxicity and can be used to treat leukemia
have clinical significance (4).
Traditional Chinese medicine (TCM) has been used in
cancer treatment for a long time and its use is supported with
scientific evidence (5). As a
Chinese herbal medicine, panax ginseng is a very important TCM for
‘invigorating qi’. The most effective ingredients of ginseng are
the ginseng polysaccharide (GPS) that prevents and treats tumors.
Previous studies have demonstrated that GPS could induce the
inhibition of proliferation and differentiation of leukemia K562
cells in vitro. In addition, it has reported that ginseng
induced apoptosis in human multiple myeloma cells (6). However, the effectiveness of GPS in a
leukemia cell line remains to be explored and validated.
The human leukemia cell line K562 has been widely
used for studies of leukemia, and is established from a patient
with chronic myelogenous leukemia (7). In the present study, we adopted
experimental hematology technologies to observe the effect of GPS
on the inhibition of proliferation and induction of apoptosis in
K562 cells and explore its underlying mechanisms.
Materials and methods
Drugs and reagents
GPS was purchased from Pude Pharmaceutical Co., Ltd.
(Datong, China). Roswell Park Memorial Institute (RPMI)-1640 medium
was obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Fetal bovine serum (FBS) was purchased from Sijiqing
Corporation (Hangzhou, China). Six-well plates and 96-well plates
were provided by Costar (Cambridge, MA, USA). MTT related reagents
were obtained from Sigma-Aldrich (Merck Millipore, Darmstadt,
Germany). Annexin V-propidium iodide (PI) apoptosis detection kit
was purchased from Zhongshan Biological Co. (Beijing, China).
Rabbit monoclonal antibodies against extracellular signal-regulated
kinase (ERK; sc-292838), phosphorylated (p)-ERK (sc-136521), P38
(sc-4708), p-P38 (sc-101758), c-Jun NH2-terminal kinase (JNK;
sc-571) and p-JNK (sc-135642) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit monoclonal antibodies
against cyclin D1 (EPR2241) and NF-κB p65 (E379) were provided by
Epitomics (Burlingame, CA, USA). Rabbit monoclonal antibody against
β-actin (BA1039) was purchased from Wuhan Boster Biological
Technology, Ltd., (Wuhan, China).
Cell culture
K562 cells were obtained from the Laboratory of the
Faculty of Basic Medical Sciences, Chongqing Medical University
(Chongqing, China). K562 cells were cultured in RPMI-1640 medium
with 10% FBS, penicillin (100 U/ml) and streptomycin (100 µg/ml) at
37°C in a humidified 5% CO2 atmosphere, and were
passaged every three days. GPS was sterilized by filter that was
dissolved by RPMI-1640. Each test was repeated three times.
MTT assay
Viability of K562 cells was determined by MTT assay.
At 90% confluence, K562 cells were adjusted to a density of
7×108 cells/l and divided into the blank control group
and the GPS group. The latter was subdivided into different final
concentration groups with 25, 50, 100, 200, 400, 600 and 800 mg/l
GPS. Cells were cultured in 96-well plates with 200 µl culture
medium for each well and incubated at 37°C. After being cultured
for 24, 48 and 72 h, 5 g/l MTT was added to each well and incubated
at 37°C for 4 h. Then, the cell suspension was centrifuged for 5
min at 1,200 × g and 4°C and the supernatants were removed. The
crystals that had formed were dissolved by adding 150 µl dimethyl
sulfoxide to each well. After mixing, the absorbance of the cells
was measured at 570 nm by a microplate reader. Experiments were
performed in triplicate and the individual mean value was used for
statistical analysis.
Measurement of cell cycle by flow
cytometry (FCM)
The control group was incubated in normal conditions
and the GPS group was incubated with 400 mg/l GPS for 48 h. K562
cells were washed twice with 0.02 M PBS (pH 7.2) and fixed in 70%
ethanol at 4°C. Then, the fixative was removed, and RNase and PI
was added for staining for 30 min. PI-stained samples were analyzed
using an Influx flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). Each sample consisted of 3×104 cells. The data
were analyzed by CellQuest software (BD Biosciences), and the ratio
of each phase of the cell cycle was calculated.
Annexin V assay
The control group was cultured in normal conditions
and the GPS group was cultured with 400 mg/l GPS for 48 h. K562
cells of each group were adjusted to a density of 1×106
cells/ml. Then, 1 ml cell suspension was selected and centrifuged
at 1,000 × g and 4°C for 5 min. Then, the supernatants were
removed, RNase was added, and samples were placed in a water bath
at 37°C for 1 h. Then, samples were placed in ice and incubated
with 0.5 mg/l PI and Annexin V. Finally, the cell apoptosis rate
was detected by FCM.
Wright's staining
At 90% confluence, K562 cells were adjusted to the
density of 7×108 cells/l and divided into the blank
control group and the GPS group. The control group was incubated in
normal conditions and the GPS group was incubated with 400 mg/l
GPS. After being cultivated for 48 h respectively, cells of the two
groups were smeared on slides and treated with Wright's stain.
Then, the morphology of cells was observed in the two groups under
light microscopy.
Transmission electron microscopy
(TEM)
The control group was incubated in normal condition
and the GPS group was incubated with 400 mg/l GPS. After being
cultivated for 48 h, fresh specimens of K562 cells were fixed by
immersing them immediately in 2.5% glutaraldehyde fixative for 24
h. Semi-thin sections (60 nm) were obtained by using an
ultra-microtome. Then, the changes in ultrastructure of K562 cells
were identified under TEM.
Immunofluorescence and confocal laser
scanning microscopy
The control group was cultured in normal conditions
and the GPS group was cultured with 400 mg/l GPS for 48 h. K562
cells were centrifuged at 1,000 × g and 4°C for 5 min and then
placed on slides. Them, slides were fixed with 4% paraformaldehyde
at 4°C for 20 min and washed three times with PBS for 5 min each.
After blocking with 0.5% bovine serum albumin (Beyotime Institute
of Biotechnology, Haimen, China) at room temperature for 30 min,
slides were incubated with mouse anti-p-ERK, p-P38, p-JNK and
rabbit anti-NF-κB p65 and cyclin D1 (all at 1:150) overnight at
4°C. Then, slides were washed three times with PBS, and treated
with fluorescein isothiocyanate goat anti-mouse (1:100; BA1105) or
rabbit (1:100; BA1101; both Wuhan Boster Biological Technology,
Ltd.) IgG for 40 min at room temperature in the dark. After being
mixed with PI for 1 min, slides were again rinsed with PBS three
times, mounted with 50% glycerol and stored in the dark.
Immunofluorescence was examined with a Leica Sp2 confocal
microscope.
Western blot analysis
Expression of ERK, p-ERK, P38, p-P38, JNK, p-JNK,
NF-κB p65 and cyclin D1 proteins was measured by western blotting.
K562 cells were divided into the blank control group and the
experimental group. In the experimental group, K562 cells were
treated with 400 mg/l GPS at 37°C for 6, 18, 24, 48 and 72 h.
Briefly, cells were washed twice with PBS. Then, cell pellets were
resuspended in lysate buffer, lysed on ice for 20 min, and then
centrifuged at 12,000 × g at 4°C for 15 min to remove nuclei and
unbroken cells. Aliquots of supernatants containing 4 µg/µl of
protein were suspended in SDS sample buffer and boiled for 5 min.
Proteins were separated by 10% SDS-polyacrylamide gel
electrophoresis. The resulting gels were equilibrated in transfer
buffer [25 mmol/l Tris-HCl, 192 mmol/l glycine and 20% methanol (pH
8.3)], and the proteins were transferred electrophoretically to
polyvinylidene difluoride membranes and incubated for 1 h with PBS
containing 5% skimmed milk and 0.05% Tween-20. Then, the membranes
were incubated with antibodies against ERK, P38, JNK (all at
1:800), p-ERK, p-P38, p-JNK (all at 1:300), NF-κB p65 (1:3,000) and
cyclin D1 (1:1,000) overnight at 4°C. Specific biotinylated goat
anti-rabbit antibodies (1:1,000; 7074P2; Cell Signalling
Technologies, Inc., Danvers, MA, USA) were used to detect the
primary antibodies for 1 h at room temperature. Antibodies against
β-actin (1:500) were used for reference. Blots were developed with
an ECL kit (WBKLS0100; EMD Millipore, Billerica, MA, USA) according
to the manufacturer's instructions. The results were analyzed by
Photoshop version 6.0 software (Adobe Systems, Inc., San Jose, CA,
USA).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from K562 cell using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), and quality
was verified by resolving samples using 1% agarose gel
electrophoresis. The following primers were used: ERK forward,
5′-CCCAAATGCTGACTCCAAAG-3′, and reverse 5′-TCGGGTCGTAATACTGCTCC-3′;
P38 forward 5′-ACCGTTTCAGTCCATCATTC-3′, and reverse
5′-GTCAGCTTCTGGCACTTCAC-3′; JNK forward,
5′-CAAGCAGTTAGATGAAAGGGAA-3′, and reverse
5′-CAGACGACGATGATGATGGA-3′; and GAPDH forward
5′-ACAGCCTCAAGATCATCAGCA-3′, and reverse
5′-TGAGTCCTTCCACGATACCAA-3′. The PCR conditions were as follows:
Denaturing at 94°C for 5 min, 94°C for 30 sec, 58°C for 30 sec and
72°C for 20 sec; target genes underwent 34 cycles and GAPDH
underwent 28 cycles at 72°C for 10 min. PCR products were resolved
by using 2% agarose gel electrophoresis for 40 min. The results
were observed under an ultraviolet lamp, photos were captured and
the optical densities of bands were quantified and analyzed using
UVI-Map V.99 software (UVItec, Ltd., Cambridge, UK).
Statistical analysis
The data are presented as the mean ± standard
deviation. Data were analyzed using SPSS version 13.0 statistical
package (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance and Student's t-test were used with the Bonferroni method
to determine statistically significant differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
Growth inhibition and cell cycle
perturbation were induced by GPS
The results indicated that exposure of K562 cells to
GPS at different concentrations (25, 50, 100, 200, 400, 600 and 800
mg/l) for 24, 48 and 72 h caused significant inhibition to the
proliferation of cells in a dose-dependent manner (P=0.03; Fig. 1A). Exposure to 400 mg/l GPS for 48 h
resulted in IC50. Therefore, 400 mg/l GPS treatment for
48 h was used in the following experiments of K562 cells. In
addition, it was identified that the G0/G1 phase of the cell cycle
increased significantly (P=0.02), while the G2+M and S phases of
the cell cycle decreased significantly (P=0.02) after GPS (400
mg/l) treatment, in a time-dependent manner (Fig. 1B). These results demonstrated that
GPS could inhibit K562 cell proliferation and arrest the cell cycle
in the G0/G1 phase.
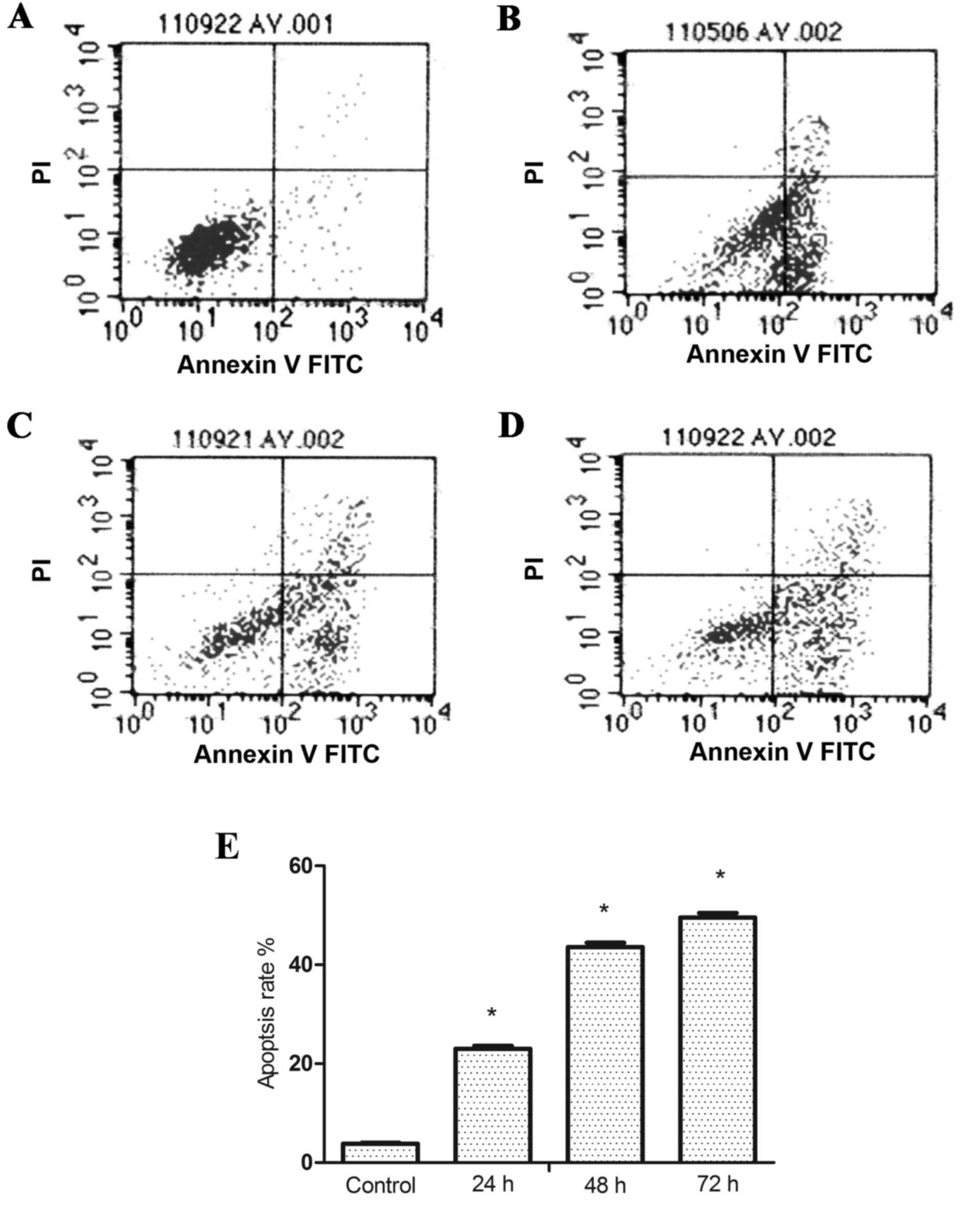
GPS triggers apoptosis in K562
cells
The percentage of apoptotic cells following
treatment with GPS at 400 mg/l for 24, 48 and 72 h in K562 cells
were analyzed by flow cytometry. Results of Annexin V/PI double
staining confirmed that after adding 400 mg/l GPS for 24
(23.00±0.65%), 48 (43.85±0.87%) and 72 h (49.56±0.93%) the
apoptosis rates of K562 cells were significantly increased compared
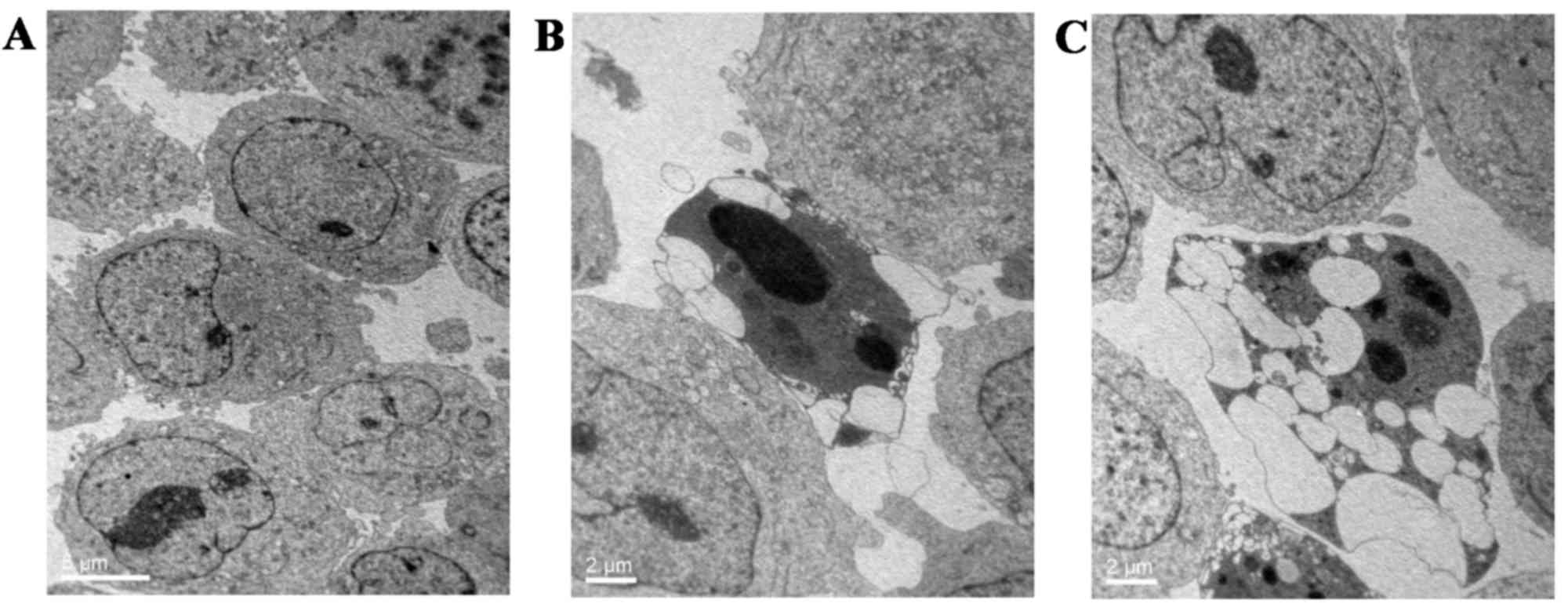
with the control group (3.82±0.23%; P<0.05; Fig. 2). Furthermore, TEM was used to
observe the changes in ultrastructure morphology in K562 cells upon
GPS treatment (400 mg/l for 48 h). The results showed that
increased blebbing, cell shrinkage, nuclear fragmentation,
chromatin condensation and the formation of apoptotic bodies were
present in GPS-treated K562 cells in comparison with untreated
control cells (Fig. 3).
Morphological changes
In the control group, the nuclear volume was
relatively high. The nucleoli were clearly visible and the
nuclei:cytoplasm ratio was high. As can be observed in Fig. 4, the cytoplasm showed strong
basophilia with specialized granules (Fig. 4A). Compared with the control group,
the cell volume and nuclear diameter in the GPS group were smaller
after 48 h (Fig. 4B; black arrows).
The cytoplasm was abundant and the nuclei:cytoplasm ratio was
decreased. Interestingly, in the GPS group a number of cells showed
nuclear division, and the basophilia in the cytoplasm was weakened
(Fig. 4B; red arrows).
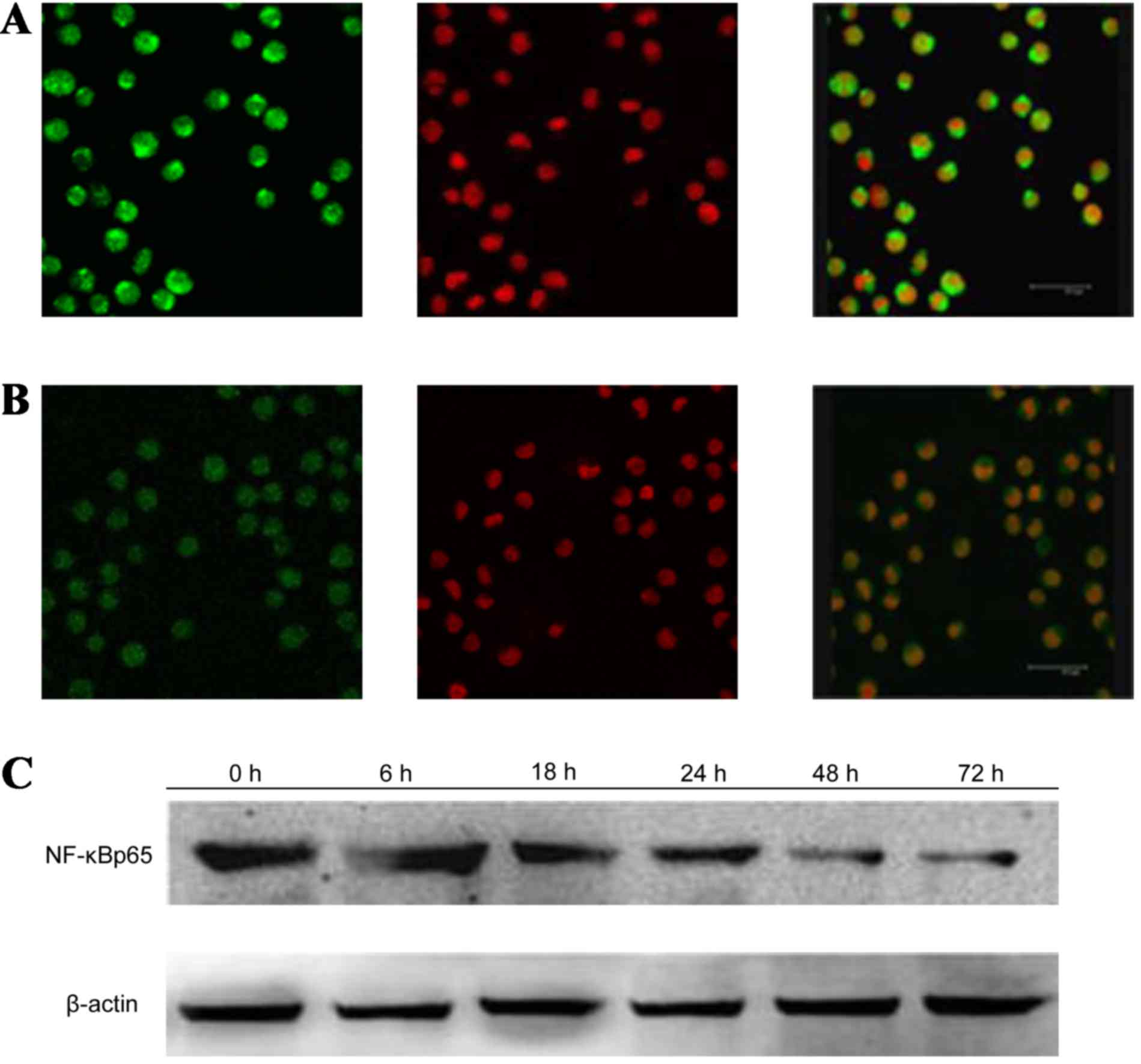
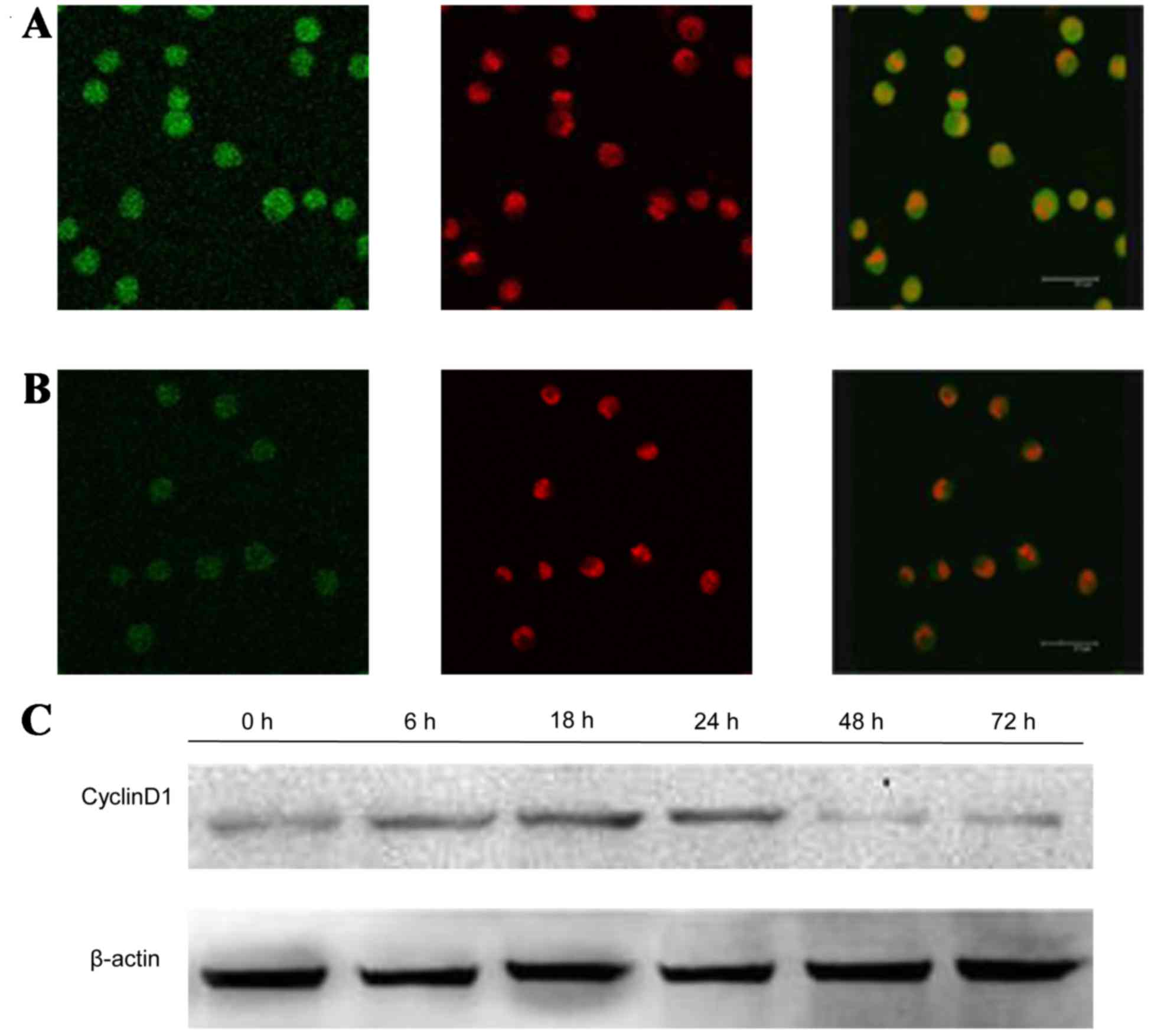
GPS reduces the expression of p-ERK,
NF-Bp65 and CyclinD1, while increase the expression of P-P38 and
P-JNK in K562 cells
After treatment with 400 mg/l GPS for 48 h, the
expression of p-ERK (Fig. 5), NF-κB
p65 (Fig. 6) and cyclin D1 (Fig. 7) protein evidently decreased, while
the expression of p-P38 (Fig. 8) and
P-JNK (Fig. 9) protein increased,
particularly the protein in the nucleus. Moreover, as shown in
Fig. 6, the expression of NF-κB p65
decreased markedly and was transferred from the nucleus to the
cytoplasm and cell membrane in K562 cells. After being incubated
with 400 mg/l GPS for 0, 6, 18, 24, 48 and 72 h, it was observed
that the variation of p-ERK, NF-κB p65, cyclin D1, p-P38 and p-JNK
protein were expressed in a time-dependent manner. However, the
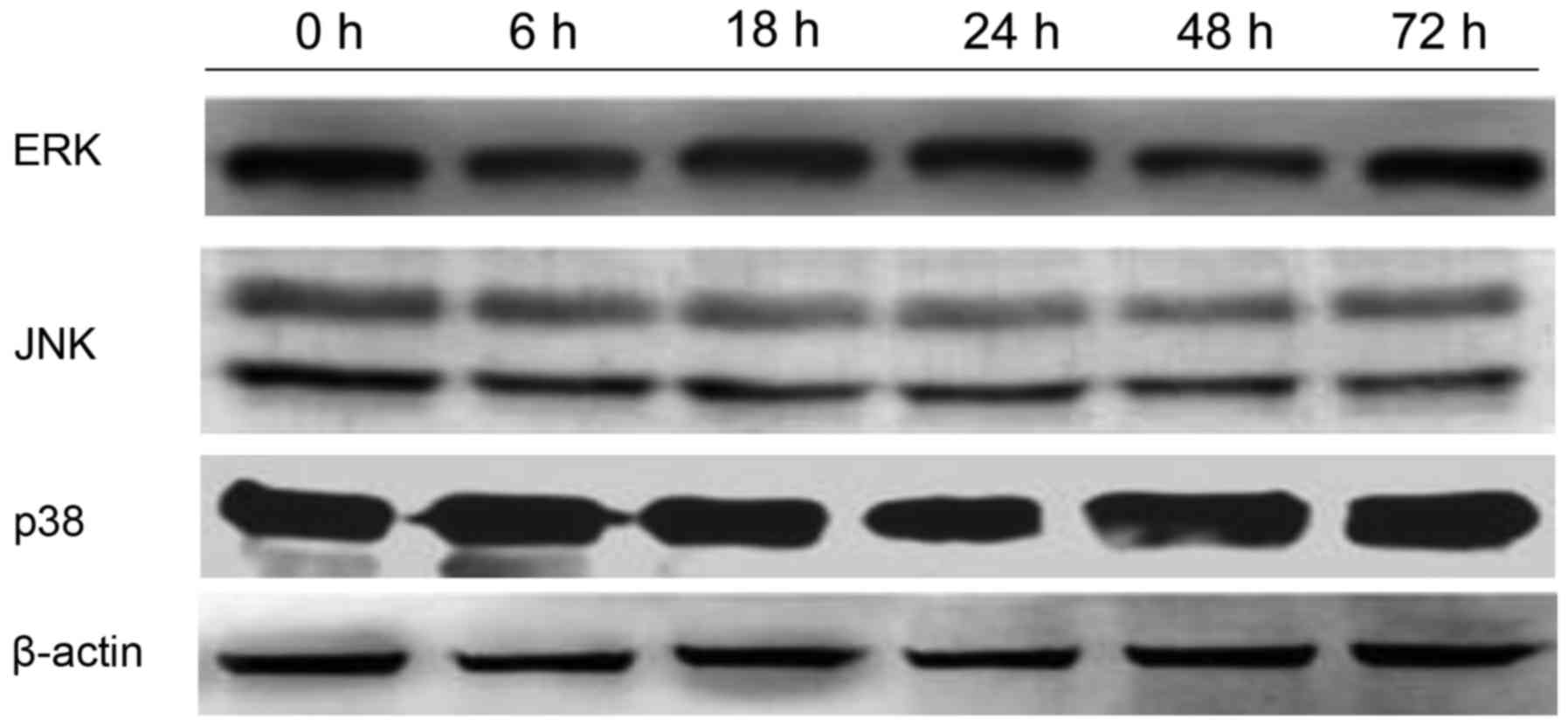
expression of ERK, P38 and JNK did not change, as evidenced by
western blotting analysis (Fig.
10).
GPS upregulated the transcription of
P38 and JNK mRNA, and downregulated the transcription of ERK
mRNA
After treatment with 400 mg/l GPS for 48 h, the
transcription of ERK mRNA was significantly decreased (P<0.05),
while the transcription of P38 and JNK mRNA were significantly
increased (P<0.05; Fig. 11). In
conclusion, these results indicate that GPS-mediated
MAPK/NF-κB/Cyclin D1 signaling pathway played a crucial role in the
cell cycle arrest and apoptosis of K562 cells.
Discussion
In the present study, human erythroleukemia cell
line K562 cells were cultured from chronic myelogenous leukemia
patients' pleural fluid. Since drugs can affect erythroid,
granulocyte-monophyletic and megakaryocytic cell differentiation,
it is an ideal model for the study of cell proliferation and
differentiation, since its biological features are similar to the
normal hematopoietic stem cell (CFU-Mix) (8). In the current study, the primary
ingredient of ginseng, GPS, was demonstrated to inhibit
proliferation and induce cell apoptosis of K562 cells. Furthermore,
it was observed that GPS induces apoptosis and differentiation of
K562 cells by influencing the MAPK signaling pathway.
Ras-MAPK signal transduction pathway is a type of
serine/threonine protein kinase in cells, and serves a key role in
the pathogenesis of chronic myeloid leukemia (9). The activation of MAPK by means of
conservative 3-level kinase cascade, the kinase of MAPK kinase
(MAPKKK, MAP3K, MEKK or MKKK) activates MAPK kinase (MAPKK, MAP2K,
MEK or MKK), then MAPK kinase activates MAPK, and the performance
of this activation reaction is through successive phosphorylation
(10). The activated MAPK
participates in a number of cell responses, such as maintaining
cell survival and inducing apoptosis (11). The MAPK family primarily includes
three groups, including ERK1/2, P38 mitogen-activated protein
kinase, P38 and JNK. Among them, the ERK1/2's primary response is
the stimulation of growth factors and the mediating of the cell
proliferation signal (12). If it
remains activated, it can cause cells to undergo malignant
transformation. One study showed that the anti-apoptotic features
of the K562 cell line associated with activity of ERK were more
pronounced than in control cells, and that inhibition of the
activity of ERK can cause K562 cell apoptosis (13). In addition, JNK and P38 primarily
mediate cells apoptosis, differentiation and inflammation caused by
emergency stimuli (14,15). This demonstrates that the MAPK system
controls the cell proliferation, differentiation and apoptosis
processes, and that a disruption of any one of them can cause
excessive cell growth and result in cancer.
In previous studies, it was demonstrated that GPS
can promote the proliferation and differentiation of pluripotent
hemopoietic stem cells (CFU-Mix) (16–19).
Meanwhile, increasing attention has been paid to the role of
ginseng in cancer therapies. The primary strategy of the treatment
for leukemia is to inhibit the proliferation of abnormal
differentiated cells. In this study, different concentrations of
GPS were used to examine K562 cells in vitro, and the effect
of GPS was observed on the proliferation of K562 cells. MTT assay
showed that GPS significantly inhibited the proliferation of K562
cells in a time- and dose-dependent manner. By analyzing the
experimental results, it was concluded that GPS promoted normal
hematopoiesis, and inhibited the proliferation of leukemic cells.
It is hoped that the conclusion will provide theoretical basis and
positive prospects for clinical applications.
Apoptosis is the process of programmed cell death
that may occur in multicellular organisms. Biochemical events lead
to characteristic cell changes and death. Unlike necrosis,
apoptosis produces cell fragments called apoptotic bodies that
phagocytic cells are able to engulf and quickly remove before the
contents of the cell can spill out onto surrounding cells and cause
damage. Apoptosis is a multi-step, multi-pathway cell-death program
that is inherent in every cell of the body. In cancer, the
apoptosis cell division ratio is altered. Cancer treatment by
chemotherapy and irradiation kills target cells primarily by
inducing apoptosis. In the present study, a large number of
apoptotic cells were observed by TEM in the 400 mg/l GPS group
after 48 h. These changes included blebbing, cell shrinkage,
nuclear fragmentation, chromatin condensation and the formation of
apoptotic bodies. Therefore, it was demonstrated that GPS can
induce apoptosis of K562 cells and arrest tumor progression.
MAPK signaling pathway regulates the survival,
growth and apoptosis of cells (20).
The abnormal activation of ERK in tumor cells can be observed.
Therefore, blocking the ERK signaling pathway is an important
method for cancer treatment. P38 and JNK serve a pivotal role in
apoptosis (21). In the present
study, results of immunofluorescence demonstrated that the protein
expression of p-ERK was located in the nucleus and cytoplasm in the
control group. Compared with the control group, expression of p-ERK
protein, particularly protein in the nucleus, decreased markedly in
the 400 mg/l GPS group at 48 h. The protein expression of p-P38 was
located in the nucleus and cytoplasm in the control group. Compared
with the control group, the protein expression of p-P38,
particularly protein in the nucleus, increased markedly in the 400
mg/l GPS group at 48 h. The protein expression of p-JNK was located
primarily in the cytoplasm in the control group. Compared with the
control group, protein expression of p-JNK, particularly in the
nucleus, increased significantly in the 400 mg/l GPS group at 48
h.
Results of RT-PCR confirmed that the expression of
ERK mRNA in the 400 mg/l GPS group after 48 h was lower than that
in the blank control group, while the expression of P38 and JNK
mRNA was higher than that in the blank control group. Results of
western blotting demonstrated that the expression of ERK, P38 and
JNK has not changed markedly, while p-ERK decreased, and p-P38 and
p-JNK were increased, compared with control group, following the
treatment of cells with 400 mg/l GPS for different time periods. A
number of studies have shown that activated MAPK, namely p-ERK,
p-P38 and p-JNK, participate in the processes of gene transcription
and the induction of apoptosis (20,22).
Hence, the effect of GPS on the apoptosis of K562 cells may be
caused by the phosphorylation of MAPK and the activation of
downstream pro-apoptotic proteins, followed by changes in the
location of p-ERK, p-P38 and p-JNK.
The NF-κB signaling pathway is a key pathway in the
occurrence and progression of tumors. Anomalous activation of the
NF-κB signaling pathway can lead to abnormal expression of a wide
range of tumor-associated genes, regulation of the proliferation
and apoptosis of cells, transformation of normal cells, and the
formation and transfer of tumor vessels, which have direct impacts
on the occurrence and progression of malignancies (23). Therefore, NF-κB may be a key target
for the prevention of human cancer. Baldwin (24) demonstrated that MAPK and NF-κB
signaling pathways are closely associated, and that the activation
of NF-κB is indispensable for the oncogenes BCR-ABL and Ras
transforming. Each molecule in the MAPK signaling pathway serves an
important role in the activation of NF-κB. Studies have shown that
phosphorylation of ERK can promote the phosphorylation of IK kinase
(IKK), which promotes the activation of NF-κB; this is called the
ERK-IKKs-IκB-NF-κB signaling cascade (25,26). The
rapid and sustained activation of P38 caused by vitamin C inhibits
the activation of IKK caused by tumor necrosis factor (TNF), while
the use of a P38 inhibitor causes the increase of NF-κB (27). Baldwin (24) showed that the inactivation of cyclin
D1/cyclin-dependent kinase 4 caused by P38 reduces the nuclear
translocation of NF-κB in colorectal cancer. The inhibition of P38,
JNK and NF-κB signaling pathways caused by disulfiram inhibits the
performance of breast cancer stem cells (28). Baud and Karin (29) discovered that NF-κB and JNK signaling
pathways are negatively associated. In gliomas, the inhibition of
matrix metalloproteinase 2 reduces the activation of NF-κB mediated
by TNF-α, and then causes the cell death mediated by JNK (30). The inhibition of NF-κB requires the
activation of JNK, which is one of the mechanisms for apoptosis
(31–33). A number of researchers have
maintained that one of the possible mechanisms of cell apoptosis
mediated by JNK3 may be that the transcription of NF-κB is
arrested, followed by the phosphorylation of p65 protein by JNK3
(34,35). In the present study, the expression
of p-ERK and NF-κB decreased in a time-dependent manner, while
p-P38 and p-JNK increased in a time-dependent manner in the 400
mg/l GPS group, which suggests that the apoptosis of K562 cells is
caused by the inhibition of the MAPK/NF-κB signaling pathway.
However, the question whether all of the molecules in the MAPK
signaling pathway are involved in the process of NF-κB activation
or not requires further investigation.
The cell cycle consists the G1, S, G2 and M phases.
Cell cycle checkpoints are used by the cell to monitor and regulate
the progress of the cycle. Checkpoints prevent cell cycle
progression at specific points, allowing verification of necessary
phase processes and repair of DNA damage. The cell is not able to
proceed to the next phase until checkpoint requirements have been
met. Several checkpoints are designed to ensure that damaged or
incomplete DNA is not passed on to daughter cells. Two primary
checkpoints exist, including the G1/S checkpoint and the G2/M
checkpoint. G1/S transition is a restriction point and a
rate-limiting step in the cell cycle. An alternative model of the
cell cycle's response to DNA damage has previously been proposed,
and it is known as the post-replication checkpoint. In this
response, two key classes of regulatory molecules, cyclins and
CDKs, determine a cell's progress through the cell cycle (36). Cyclin D1 has the function of
regulating cells entering the G1 phase. When cycling D1 is
expressed at low levels, cells are arrested in the G1 phase.
Regulation of cell cycle is one of the most important biological
processes in cells, and disorders in the cycle can lead to
malignancies. Cell cycle arrest may be a new target for the
exploitation of novel drugs. However, the mechanism of effect of
GPS on K562 cell cycle arrest remains unknown and further research
is required. In the present study article, GPS was used to affect
the growth of K562 cells, and it was discovered that the cell cycle
was arrested in the G0/G1 phase, which was likely associated with
the differentiation and apoptosis of leukemia cells.
Cyclin D1 is a member of the cyclin protein family
that is involved in regulating cell cycle progression. The
synthesis of cyclin D1 is initiated during the G1 phase, and its
synthesis drives the G1/S phase transition. Overexpression of
cyclin D1 enables persistent proliferation of cells and serves an
important role in the process of carcinogenesis (37,38). In
particular, overexpression of cyclin D1 can be found in a variety
of malignant tumors. Polynucleotide chains of cyclin D1 are adopted
in the treatment of lung cancer (39). It has been found that cyclin D1 and
p53 are the downstream target genes of NF-κB. NF-κB regulates the
expression of cyclin D1, and p53 arrests the cell cycle and
inhibits the proliferation of tumor cells (40). Cyclin D1 promoter contains two
binding sites for NF-κB. The activation of NF-κB has the function
of promoting cyclin D1 expression and driving the G1/S phase, so
that normal cells change to malignant cells (41). In this study, it was observed that
NF-κB and cyclin D1 decreased in a time-dependent manner following
treatment with GPS. It can be speculated that one of the probable
mechanisms of the effect of GPS on K562 apoptosis may be that the
inhibition of the MAPK/NF-κB-mediated signaling transduction
pathway can inhibit the expression of cyclin D1, followed by the
arrest if the cell cycle in the G0/G1 phase, which ultimately
induces the apoptosis of K562 cells.
In conclusion, GPS can inhibit proliferation and
induce apoptosis of K562 cells by arresting cell cycle at the G0/G1
phase. In addition, the results demonstrated that MAPK/NF-κB/cyclin
D1 serves a crucial role in cell cycle arrest and the induction of
apoptosis of K562 cells. Consequently, the MAPK/NF-κB/cyclin D1
signaling pathway is a potential molecular target for the treatment
of leukemia and has promising prospects.
Acknowledgements
The present study was supported by projects from the
National Science Foundation of China (grant no. 81171929).
References
|
1
|
Levis M: Quizartinib in acute myeloid
leukemia. Clin Adv Hematol Oncol. 11:586–588. 2013.PubMed/NCBI
|
|
2
|
Cesaro S, De Filippi P, Di Meglio A, Leszl
A, Donska S, Zaccaron A, Cagioni C, Galavotti R, Danesino C, Aprili
F, et al: Different outcomes of allogeneic hematopoietic stem cell
transplant in a pair of twins affected by juvenile myelomonocytic
leukemia. Int J Hematol. 99:208–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu J, Huang X, Bao L, Jiang H, Zhu H and
Jiang B: Treatment outcomes in relapsed acute promyelocytic
leukemia patients initially treated with all-trans retinoic acid
and arsenic compound-based combined therapies. Oncol Lett.
7:177–182. 2014.PubMed/NCBI
|
|
4
|
Hait WN, Choudhury S, Srimatkandada S and
Murren JR: Sensitivity of K562 human chronic myelogenous leukemia
blast cells transfected with a human multidrug resistance cDNA to
cytotoxic drugs and differentiating agents. J Clin Invest.
91:2207–2215. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang G, Li X, Li X, Wang L, Li J, Song X,
Chen J, Guo Y, Sun X, Wang S, et al: Traditional Chinese medicine
in cancer care: A review of case series published in the chinese
literature. Evid Based Complement Alternat Med. 2012:7510462012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Zhang P, Zeng HQ, Lou SF and Wang
DX: Ginsenoside Rg3 induces apoptosis in human multiple myeloma
cells via the activation of Bcl-2-associated X protein. Mol Med
Rep. 12:3557–3562. 2015.PubMed/NCBI
|
|
7
|
Lozzio CB and Lozzio BB: Human chronic
myelogenous leukemia cell-line with positive Philadelphia
chromosome. Blood. 45:321–334. 1975.PubMed/NCBI
|
|
8
|
Gambari R and Fibach E: Medicinal
chemistry of fetal hemoglobin inducers for treatment of
beta-thalassemia. Curr Med Chem. 14:199–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schnetzke U, Fischer M, Frietsch JJ,
Finkensieper A, Clement JH, Hochhaus A and La Rosée P: Paradoxical
MAPK-activation in response to treatment with tyrosine kinase
inhibitors in CML: Flow cytometry loses track. Cytometry B Clin
Cytom. 86:229–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118:(Pt 16). 3569–3572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang CD, Yoo SD, Hwang BW, Kim KW, Kim DW,
Kim CM, Kim SH and Chung BS: The inhibition of ERK/MAPK not the
activation of JNK/SAPK is primarily required to induce apoptosis in
chronic myelogenous leukemic K562 cells. Leuk Res. 24:527–534.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao LY, Zhang J, Guo B, Yang J, Han J,
Zhao XG, Wang XF, Liu LY, Li ZF, Song TS and Huang C: MECP2
promotes cell proliferation by activating ERK1/2 and inhibiting p38
activity in human hepatocellular carcinoma HEPG2 cells. Cell Mol
Biol (Noisy-le-grand) Suppl. 59:OL1876–OL1881. 2013.
|
|
13
|
Pal P, Kanaujiya JK, Lochab S, Tripathi
SB, Bhatt ML, Singh PK, Sanyal S and Trivedi AK: 2-D gel
electrophoresis-based proteomic analysis reveals that ormeloxifen
induces G0-G1 growth arrest and ERK-mediated apoptosis in chronic
myeloid leukemia cells K562OL. Proteomics. 11:1517–1529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obata T, Brown GE and Yaffe MB: MAP kinase
pathways activated by stress: The p38 MAPK pathway. Crit Care Med.
28:(Suppl 4). N67–N77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park EJ, Kiselev E, Conda-Sheridan M,
Cushman M and Pezzuto JM: Induction of apoptosis by
3-amino-6-(3-aminopropyl)-5,6-dihydro-5,11-dioxo-11H-indeno
[1,2-c]isoquinoline via modulation of MAPKs (p38 and c-Jun
N-terminal kinase) and c-Myc in HL-60 human leukemia cells. J Nat
Prod. 75:378–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Wang HF, Liu Y, Huang LJ, Wang ZF
and Li Y: The haematopoietic effect of Panax japonicus on blood
deficiency model mice. J Ethnopharmacol. 154:818–824. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li SS, Jin YP, Yao CL and Wang YP:
Research achievements on structures and activities of
polysaccharides from Panax ginseng. Zhongguo Zhong Yao Za Zhi.
39:4709–4715. 2014.(In Chinese). PubMed/NCBI
|
|
18
|
Wang Z, Meng J, Xia Y, Meng Y, Du L, Zhang
Z, Wang E and Shan F: Maturation of murine bone marrow dendritic
cells induced by acidic Ginseng polysaccharides. Int J Biol
Macromol. 53:93–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HJ, Kim MH, Byon YY, Park JW, Jee Y
and Joo HG: Radioprotective effects of an acidic polysaccharide of
Panax ginseng on bone marrow cells. J Vet Sci. 8:39–44. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhattacharyya S, Ghosh J and Sil PC: Iron
induces hepatocytes death via MAPK activation and
mitochondria-dependent apoptotic pathway: Beneficial role of
glycine. Free Radic Res. 46:1296–1307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho PJ, Chou CK and Yeh SF: Role of JNK and
p38 MAPK in Taiwanin A-induced cell death. Life Sci. 91:1358–1365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Filomeni G, Piccirillo S, Rotilio G and
Ciriolo MR: p38(MAPK) and ERK1/2 dictate cell death/survival
response to different pro-oxidant stimuli via p53 and Nrf2 in
neuroblastoma cells SH-SY5Y. Biochem Pharmacol. 83:1349–1357. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reikvam H, Olsnes AM, Gjertsen BT, Ersvar
E and Bruserud Ø: Nuclear Factor-kappaB Signaling: A contributor in
leukemogenesis and a target for pharmacological intervention in
human acute myelogenous leukemia. Crit Rev Oncog. 15:1–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu CJ, Wang YH, Lin CJ, Chen HH and Chen
YJ: Tetrandrine down-regulates ERK/NF-κB signaling and inhibits
activation of mesangial cells. Toxicol In Vitro. 25:1834–1840.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dia VP and de Mejia E Gonzalez: Lunasin
potentiates the effect of oxaliplatin preventing outgrowth of colon
cancer metastasis, binds to α5β1 integrin and suppresses
FAK/ERK/NF-κB signaling. Cancer Lett. 313:167–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kato T Jr, Delhase M, Hoffmann A and Karin
M: CK2 is a C-Terminal IkappaB kinase responsible for NF-kappaB
activation during the UV response. Mol Cell. 12:829–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bowie AG and O'Neill LA: Vitamin C
inhibits NF-kappa B activation by TNF via the activation of p38
mitogen-activated protein kinase. J Immunol. 165:7180–7188. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baud V and Karin M: Signal transduction by
tumor necrosis factor and its relatives. Trends Cell Biol.
11:372–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kesanakurti D, Chetty C, Bhoopathi P,
Lakka SS, Gorantla B, Tsung AJ and Rao JS: Suppression of MMP-2
attenuates TNF-α induced NF-κB activation and leads to JNK mediated
cell death in glioma. PloS One. 6:e193412011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Lin ZN, Yang CF, Shi X, Ong CN
and Shen HM: Suppressed NF-kappaB and sustained JNK activation
contribute to the sensitization effect of parthenolide to
TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis.
25:2191–2199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin A: Activation of the JNK signaling
pathway: Breaking the brake on apoptosis. Bioessays. 25:17–24.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Smaele E, Zazzeroni F, Papa S, Nguyen
DU, Jin R, Jones J, Cong R and Franzoso G: Induction of gadd45beta
by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature.
414:308–313. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boonyarat C, Yenjai C, Vajragupta O and
Waiwut P: Heptaphylline induces apoptosis in human colon
adenocarcinoma cells through bid and Akt/NF-κB (p65) pathways.
Asian Pac J Cancer Prev. 15:10483–10487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y: Attenuation of berberine on
lipopolysaccharide-induced inflammatory and apoptosis responses in
β-cells via TLR4-independent JNK/NF-κB pathway. Pharm Biol.
2013.(Epub ahead of print).
|
|
36
|
Wang CZ, Xie JT, Fishbein A, Aung HH, He
H, Mehendale SR, He TC, Du W and Yuan CS: Antiproliferative effects
of different plant parts of Panax notoginseng on SW480 human
colorectal cancer cells. Phytother Res. 23:6–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozuysal S, Oztürk H, Bilgin T and Filiz G:
Expression of cyclin D1 in normal, hyperplastic and neoplastic
endometrium and its correlation with Ki-67 and clinicopathological
variables. Arch Gynecol Obstet. 271:123–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan KX, Liu BC, Shi XL, You BR, Xu M, Kang
N and Zhao CY: Role of cyclin D1 in carcinogenesis of human cells
induced by quartz. Zhonghua Yu Fang Yi Xue Za Zhi. 38:396–399.
2004.(In Chinese). PubMed/NCBI
|
|
39
|
Li ZL, Shao SH, Xie SY, Yue Z and Ma Y:
Anti-sense nucleic acid of CyclinD1 induces apoptosis of lung
adenocarcinoma cancer cell A549. Sheng Li Xue Bao. 63:261–266.
2011.(In Chinese). PubMed/NCBI
|
|
40
|
Harlozińska A, Bar J and Montenarh M:
Analysis of the immunoreactivity of three anti-p53 antibodies and
estimation of the relations between p53 status and MDM2 protein
expression in ovarian carcinomas. Anticancer Res. 20:1049–1056.
2000.PubMed/NCBI
|
|
41
|
Manna SK and Aggarwal BB:
All-trans-retinoic acid upregulates TNF receptors and potentiates
TNF-induced activation of nuclear factors-kappaB, activated
protein-1 and apoptosis in human lung cancer cells. Oncogene.
19:2110–2119. 2000. View Article : Google Scholar : PubMed/NCBI
|