Introduction
Ischemic stroke is a major threat to health and a
significant cause of human mortality. Thus, understanding the
pathogenesis of ischemic stroke and the development of strategies
that may prevent it is (1). Ischemic
stroke, in which a blood vessel is suddenly occluded by a thrombus
or embolism, accounts for 87% of all cases of stroke (2). Ischemia and reperfusion induce cellular
damage, which triggers a cascade of biochemical processes that
ultimately induce structural and functional changes in organs or
tissues, with transient global brain ischemia causing selective
neuronal injury (3).
As a regular supply of oxygen and glucose is
essential for the maintenance of normal neuronal functions, the
application of oxygen-glucose deprivation (OGD) to cultured
hippocampal tissue can be used to create a cellular model of
ischemic stroke. The lack of a regular supply of oxygen and
glucose, even for a short period, results in
ischemia/re-oxygenation and the production of reactive oxygen
species (ROS), culminating in neuronal cell death and brain damage
(4).
Isoquercetin is a dietary flavonoid present in a
variety of medicinal and dietary plants, including vegetables,
herbs and flowers (5). Isoquercetin
has been shown to have numerous therapeutic properties, including
anti-inflammatory, antioxidant and anti-allergic activities
(6). Morand et al (7) demonstrated that isoquercetin was better
absorbed than quercetin and had higher bioavailability. However, to
the best of our knowledge, whether isoquercetin has the ability to
reduce infarct volumes and behavioral deficits has not been studied
previously. In the present study, the effects of isoquercetin on
transient middle cerebral artery occlusion (MCAO) and primary
hippocampal neurons subjected to OGD followed by reoxygenation
(OGD/R), which are models commonly used for examining the molecular
mechanisms of ischemic stroke injury in vivo and in
vitro, were investigated. In addition, the ability of
isoquercetin to activate the extracellular signal-regulated protein
kinase 1 and 2 (ERK1/2) nuclear factor erythroid 2-related factor 2
(Nrf2) pathway in vivo and in vitro was evaluated in
order to further elucidate the precise protective mechanisms.
Materials and methods
Animals and MCAO surgery
A total of 60 8-week old male Sprague-Dawley (SD)
rats (220–260 g) were obtained from the Experimental Animal Center
of Xinhua Hospital (Shanghai, China). All animals were housed and
handled according to guidelines from the Institutional Animal Care
and Use Committee of Xinhua Hospital and all animal experiments
were approved by the Shanghai Jiaotong University committee. The
rats were randomly allocated to one of four groups: Control, sham,
model and isoquercetin. In the model group, rats were anesthetized
via the intraperitoneal injection of 10% chloral hydrate. The
rectal temperature was monitored and maintained at 37.0±0.5°C by
the use of a feedback-regulated heating system during surgery.
Permanent focal ischemia was induced by occluding the right middle
cerebral artery (MCA) via an intraluminal technique, as previously
described (8). Briefly, a 4–0 nylon
monofilament suture with a slightly enlarged rounded tip was
inserted into the stump of the external carotid artery and advanced
into the lumen of the internal carotid artery until it reached and
occluded the MCA. The distance from the bifurcation of the common
carotid artery (CCA) to the tip of the inserted suture averaged
18–20 mm. Sham operated animals were subjected to the
aforementioned procedures, with the exception of suture insertion.
Rats in the isoquercetin group were subjected to MCAO surgery and
treated with intravenous 50 mg/kg isoquercetin (Dalian Institute of
Chemical Physics, Chinese Academy of Sciences, Dalian, China) once
a day for 7 days. Rats that did not undergo any surgery served as
the control group.
Neurological function and measurement
of infarct volume
Neurological deficits were scored by double-blind
testing based on a modified Longa EZ test (9), on a scale from 0–5 in which 0
represented no deficit, 4 indicated the maximal deficit and 5
indicated mortality 24 h following MCAO. Results were statistically
analyzed using the rank sum test. At 24 h after ischemia, brains
were removed and sliced into 5 coronal sections 2 mm thick
(10). Sections were immediately
stained with 1% tertrazolium chloride at 37°C for 30 min as
previously described (11). The
infarct volume was expressed as a percentage of the volume of the
total brain.
Primary hippocampal neuron cell
cultures
All experiments were approved by the Institutional
Animal Care and Use Committee of Xinhua Hospital and according to
guidelines developed by China Council on Animal Care. A total of 20
male newborn SD rats (<24 h old, mean weight, 5±2 g) were
purchased from the SLAC Laboratory Animal Center of Shanghai
(China). Primary hippocampal neuron cultures were established from
the cerebral hippocampi of newborn SD rats. Newborn rats were
sacrificed by cervical dislocation and brains were cleaned of
meninges and blood vessels. The entire cerebral hippocampus was
isolated and cells were dissociated by treatment with 0.25% trypsin
solution for 10 min at 37°C. Subsequently, 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added
and the dissociated cells were forced through a 300 mesh. Following
centrifugation (1,000 × g, 5 min), hippocampal neurons were
resuspended and plated in six-well plates coated with poly-D-lysine
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and grown in
Neurobasal media (Gibco; Thermo Fisher Scientific, Inc.) with 500
µM glutamine and 2% B27 supplement (both Gibco; Thermo Fisher
Scientific, Inc.) in a humidified incubator with 5% CO2
at 37°C. The cell culture medium was replaced with fresh culture
medium on days 3 and 5 in vitro.
OGD/R culture method
Primary hippocampal neurons were placed in an
anaerobic chamber (HERAcell 150 incubator; Thermo Fisher
Scientific, Inc.) and the partial oxygen pressure was maintained at
<2 mmHg). The medium was replaced with a pre-warmed (37°C)
glucose-free balanced salt solution. An anaerobic gas mixture
comprising 95% N2 and 5% CO2 was bubbled
through the solution for 30 min. Cell cultures subjected to OGD
were incubated in the solution at 37°C for different time periods
to produce oxygen deprivation and then re-oxygenated (returned to
the normal aerobic environment). Experimental parameters were
assayed 4 h following re-oxygenation. The primary cultures of rat
hippocampal neurons were pretreated with 25, 50 and 100 µg/ml
isoquercetin for 24 h, followed by exposure to OGD for 4 h, then
reperfusion for 24 h. The hippocampal neurons cultured in plain
medium served as control. At the end of cell treatments, different
tests were carried out as described below. PD98059 (HY-12028; MCE,
Monmouth Junction, NJ, USA) was the inhibitor of the MEK.
Cell viability assay
The viability of the neurons in the five in
vitro treatment groups was determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Hippocampal neurons were seeded onto 96-well plates
(1×104 cells/well). Following treatment, 0.2 mg/ml MTT
salt (Sigma-Aldrich; Merck Millipore) was added to each well and
the cells were further incubated in 5% CO2 at 37°C for 4
h. Dimethyl sulfoxide was then added to dissolve the formazan
crystals for 20 min. The number of viable cells was assessed by
measurement of the absorbance at 490 nm using a Safire 2 microplate
reader (Tecan, San Jose, CA, USA).
Measurement of ROS production
ROS production in the five in vitro treatment
groups was measured using the ROS-sensitive dye
2′,7′-dichlorofluorescein diacetate (H2DCFDA;
Invitrogen; Thermo Fisher Scientific, Inc.) using the protocols
provided by the manufacturer. In the presence of ROS,
H2DCF is rapidly oxidized to form highly fluorescent
DCF. Hippocampal neurons were incubated with H2DCFDA (10
µM) for 30 min at 37°C. Fluorescence images were obtained using a
fluorescence microscope (BX-51; Olympus Corporation, Tokyo, Japan).
The fluorescence intensity was measured using ImageJ software
(National Institutes of Health, Bethesda, MD, USA), averaged and
normalized to the control value.
Lactate dehydrogenase (LDH) release
assay
Cytotoxic activity in the five in vitro
treatment groups was determined using an LDH release assay as
described previously (12). Briefly,
LDH in the culture medium (released LDH) was quantified using an
LDH cytotoxicity detection kit (Nanjing Jiancheng Biotech, Nanjing,
China) according to the protocol provided by the manufacturer. The
relative amount of released LDH was used to reflect the extent of
cell injury.
Apoptosis assay by flow cytometry
Fluorescein isothiocyanate (FITC)-Annexin V and
propidium iodide (PI) double staining was used to examine the
OGD/R-induced apoptosis of the hippocampal neurons in the five
in vitro treatment groups. The assay was conducted using an
FITC-Annexin V/PI apoptosis detection kit according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). Briefly, neurons were cultured in 6-well plates
(1×106 cells/well) and treated as described above. Cells
were collected after OGD/R and flow cytometry was conducted to
assess the apoptosis of the cells.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells from each group
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and treatment of RNA was performed using DNase (Takara
Biotechnology, Co., Ltd., Dalian, China). Reverse transcription was
performed using an Omniscript RT kit (Promega Corporation, Madison,
WI, USA) according to the manufacturer's protocol. qPCR was
subsequently performed to determine gene expression using the SYBR
green Master Mix (Takara Biotechnology, Co., Ltd., Dalian, China).
The primers for Nrf2 were: Forward, 5′-TACTCCCAGGTTGCCCACA-3′ and
reverse, 5′-CATCTACAAACGGGAATGTCTG-3′. The primers for ERK were:
Forward, 5′-GAGGCAGCAGGAACAATGCT-3′ and reverse,
5′-CCAGCTTTCTGCAGAGGGAA-3′. The primers for glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were: Forward,
5′-CATCTTCCAGGAGCGAGACC-3′ and reverse, 5′-CTCGTGGTTCACACCCATCA-3′.
The expression level of each target mRNA relative to GAPDH was
calculated on the basis of the threshold cycle (CT) as r = 2 - Δ
(ΔCT) (13).
Western blot analysis
Hippocampal neurons were washed twice with ice-cold
phosphate-buffered saline and then lysed with ice-cold lysis buffer
(20 mM Tris-HCl (pH=7.5), 150 mM NaCl, 1 mM Na3VO4, 1 mM PMSF, 1 mM
EDTA, 1% NP40, 50 mM NaF) for 30 min. Cell lysates were centrifuged
at 10,000 x g for 15 min at 4°C. Cell lysates were separated
by sodium dodecyl sulfate-polyacrylamide 12% gel electrophoresis
and transferred to polyvinylidene fluoride membranes. Following
incubation with blocking buffer (Tris-buffered saline + 0.1%
Tween-20 (TBST) + 5% non-fat milk), membranes were probed with
rabbit anti-Nrf2 polyclonal antibody, (1:1,000, ab31163 abcam,
Cambridge, UK) mouse anti-ERK1+ERK2 polyclonal antibody (1:1,000,
ab54230, abcam), rabbit anti-ERK1 (phospho T202) + ERK2 (phospho
T185) antibody (1:1,000, ab201015, abcam) and mouse anti-GAPDH
monoclonal antibody (1:2,000, ab8245, abcam) overnight at 4°C. The
membranes were then washed with TBST and incubated with a
horseradish peroxide-conjugated secondary antibody (1:1,000,
00001–2, ProteinTech Group, Inc., Chicago, IL, USA), and signals
were detected with an enhanced chemiluminescent reagent (EMD
Millipore, Billerica, MA, USA). Images were captured using a
Bio-Rad VersaDoc 3000 (Bio-Rad Laboratories, Inc., Gladesville,
NSW, Australia) and protein levels were quantified using Image-pro
Plus 6.0 (Media Cybernetics, MD Rockville, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of at least three independent preparations. Statistical
analysis was conducted by analysis of variance with Tukey's or
Scheffé's post hoc tests using SPSS statistical analysis software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Isoquercetin protects against brain
injury by transient MCAO
The potential ability of isoquercetin to protect
against transient MCAO-induced damage was investigated. Results
showed that infarct volume was 41.05±4.19% in the model group and
21.35±2.03% in the 50 mg/kg isoquercetin group. Statistical
analysis revealed that infarct volume in the 50 mg/kg isoquercetin
group was significantly smaller than that of the model group 24 h
after ischemia (P<0.01; Fig. 1A and
B). Furthermore, 50 mg/kg isoquercetin improved the
neurological function compared with that of the model group at 7
days after ischemia (Fig. 1C).
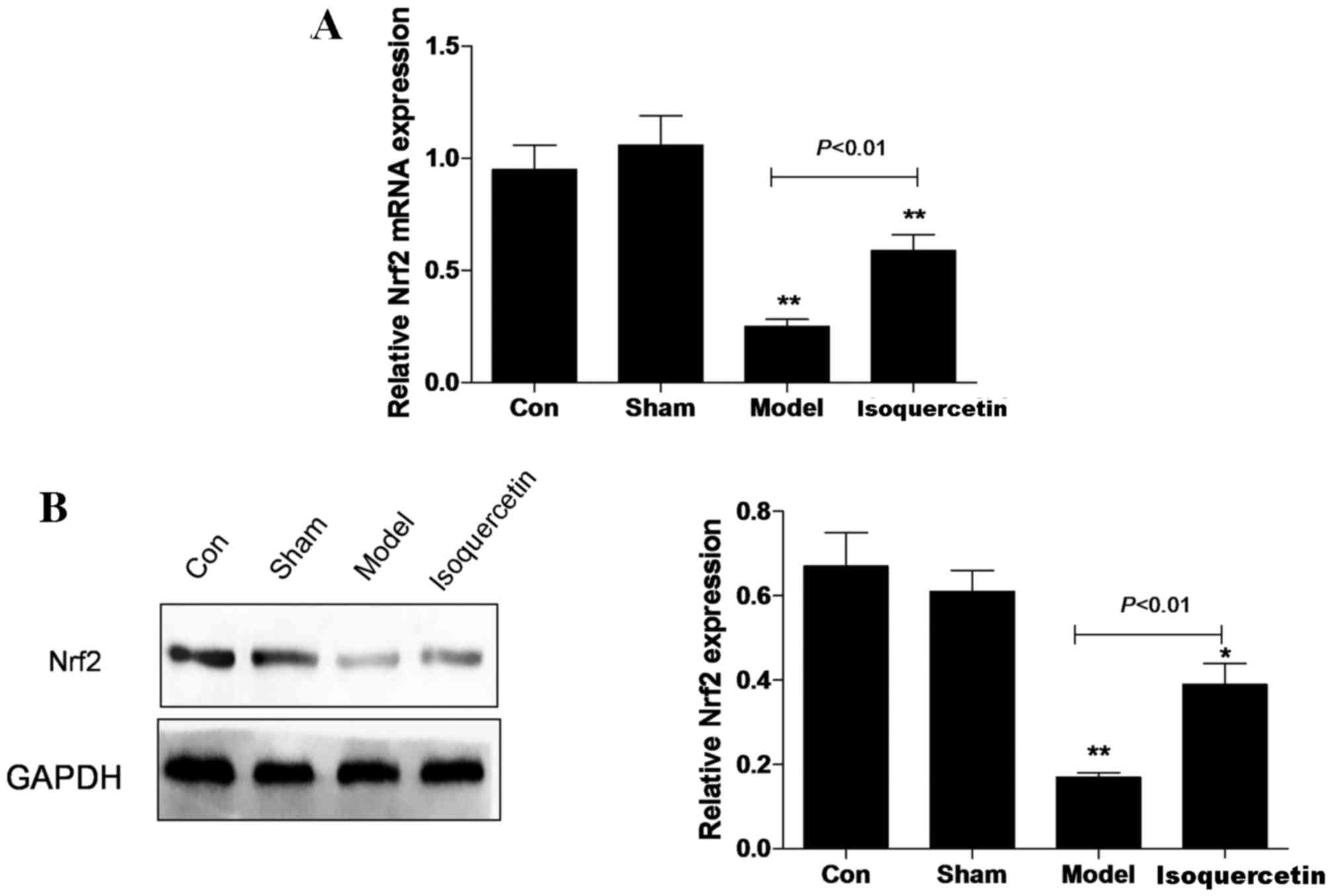
Isoquercetin increases Nrf2 expression
in the hippocampus in vivo
In the hippocampal tissue, the expression of Nrf2 at
the mRNA and protein levels was decreased in the model group 7 days
after ischemia, and the reduction in expression levels was
significantly attenuated in the isoquercetin treated-group
(Fig. 2).
Isoquercetin protects primary culture
of rat hippocampus neurons against OGD/R-induced cytotoxicity with
attenuation of LDH release and ROS content
An MTT assay, in which MTT was reduced by
mitochondrial reductase in viable cells, was used to determine cell
viability. Cultured hippocampal neurons were pre-conditioned with
isoquercetin for 24 h prior to 4 h OGD and 24 h normoxia with
isoquercetin for recovery. As shown in Fig. 3A, isoquercetin at concentrations of
25, 50 and 100 µg/ml significantly promoted the cell viability of
cultured hippocampus neurons. The protective activity of
isoquercetin against OGD/R-induced injury in cultured hippocampus
neurons was further investigated using ROS and LDH assays. In these
assays, 25, 50 or 100 µg/ml isoquercetin was added to the culture
medium from 24 h prior to OGD until the end of recovery. The
results shown in Fig. 3B and C
reveal that OGD/R significantly increased the ROS content of and
LDH release by hippocampal neurons compared with those in the
control group. Furthermore, LDH release and ROS content were
significantly higher in the group treated with 100 µg/ml
isoquercetin compared with the other treatment groups.
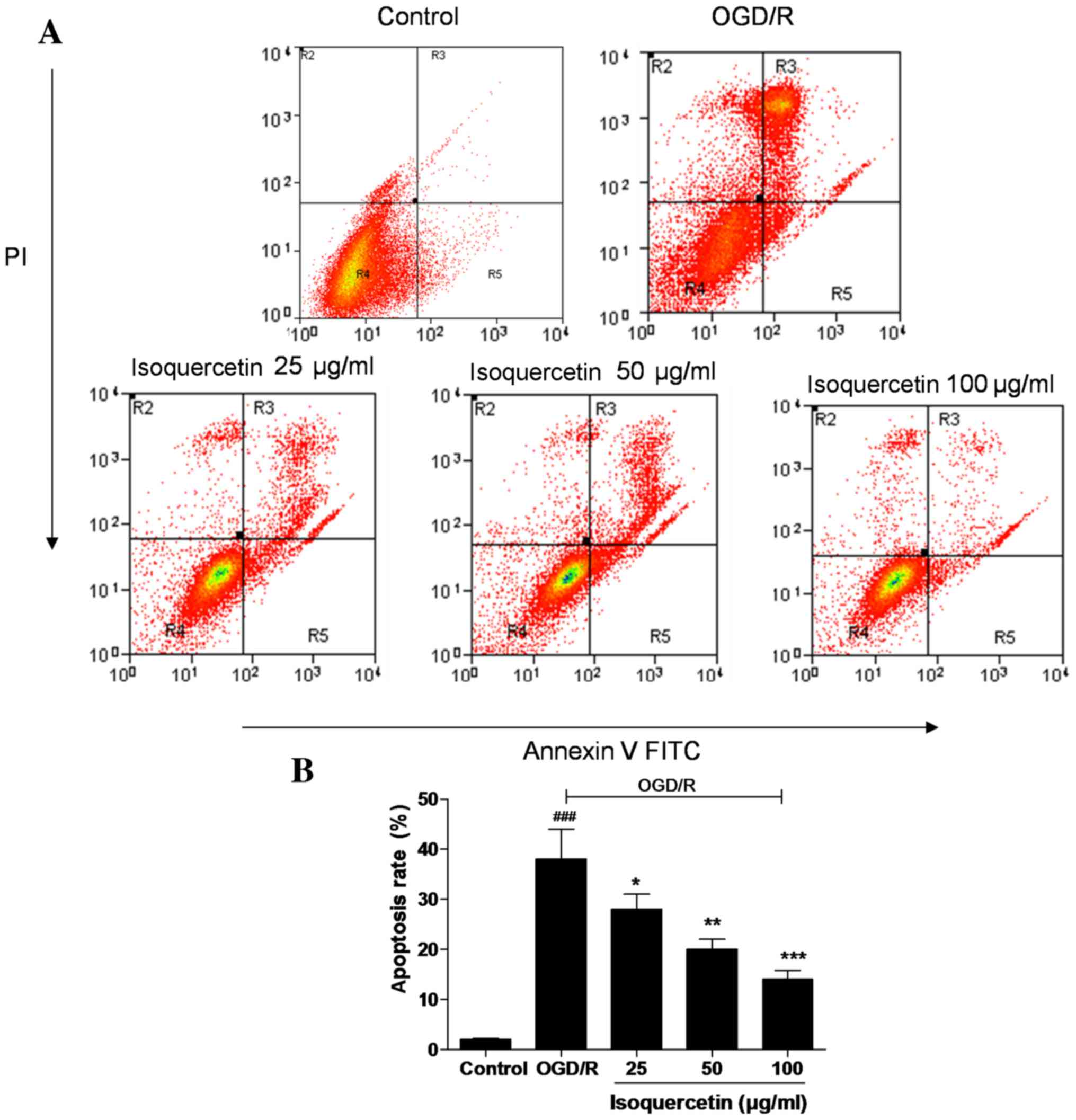
FITC-Annexin V/PI double staining
As shown in Fig. 4,
almost no apoptotic cells were detected in the control group, but a
large number were present in the OGD/R-treated group. Treatment of
the cells with isoquercetin at doses of 25, 50 and 100 µg/ml
significantly reduced the proportion of apoptotic cells in a
concentration-dependent manner, indicating the ability of
isoquercetin to inhibit an early event of the apoptotic
process.
Isoquercetin upregulates the gene and
protein expression of Nrf2 and triggers ERK1/2 phosphorylation
As shown in Fig. 5A and
B, the expression of Nrf2 at the mRNA and protein levels
following treatment with 25, 50 or 100 µg/ml isoquercetin for 24 h
in cells that had undergone OGD/R was evaluated using RT-qPCR and
western blotting, respectively. The protein expression of Nrf2 in
hippocampal neurons in the 100 µg/ml group increased by 200%
compared with the OGD/R group. This increase in Nrf2 protein
expression was attenuated in the presence of PD98059, which
suggests that the Nrf2-related neuroprotective effect of
isoquercetin may be mediated via the ERK1/2 signaling pathway
(Fig. 5C and D). Western blotting
results show that OGD/R slightly induced ERK1/2 phosphorylation.
Isoquercetin increased ERK1/2 phosphorylation and Nrf2 expression
levels in OGD/R-treated cells by 29 and 91% compared with those in
cells treated with OGD/R alone. The isoquercetin-induced increases
in ERK1/2 phosphorylation and Nrf2 levels were completely abrogated
in the presence of PD98059.
Discussion
Diets rich in colorful, flavonoid-rich fruits,
vegetables and nuts have been shown to improve the health of the
brain by reducing oxidative stress, and improving neuronal
signaling, behavior and cognitive functions (14). The results of the present study
demonstrated for the first time that intravenous post-treatment
with isoquercetin had a protective effect against transient
MCAO-induced brain damage in rats, with positive effects on
neurobehavioral function, infarct volume and neuronal loss without
any adverse effects on physiological parameters.
Oxidative stress has been shown to be a key cause of
neurodegenerative and vascular diseases pathologies affecting the
brain (15,16). Consistent with this, the present
study found that isoquercetin could downregulate oxidative damage
in primary hippocampal neurons subjected to OGD/reoxygenation in
vitro. Also, isoquercetin reduced the amount of cell death
induced by OGD/R in a dose-dependent manner. The results of the
present indicate that isoquercetin acts as a regulator of cerebral
ischemic injury and thus may exhibit potential in the development
of an interventional therapy for cerebral ischemic stroke.
When cerebral ischemia and reperfusion occur, ROS
are generated, and can cause the brain to become damaged (17). A previous study has demonstrated that
isoquercetin acts via an antioxidative mechanism to protect against
cerebral ischemic damage in vivo (18). The protective effects of isoquercetin
may involve the direct scavenging of free radicals and/or other
endogenous antioxidant mechanisms. The results of the present study
demonstrate that isoquercetin decreased the levels of ROS and LDH
release. Previous studies have shown that the neuroprotective
effects of isoquercetin are mediated by an anti-apoptotic signaling
pathway involving the deactivation of ERK, an increase in the
protein expression of Nrf2 and the inhibition of apoptosis
(19,20).
Marked changes in ERK are associated with the
pathophysiology of focal cerebral ischemia. Previous studies have
shown that ischemia and the subsequent neuronal damage are
associated with inhibition of ERK1/2, and the activation of ERK1/2
has neuroprotective effects (21,22).
Furthermore, it has been demonstrated that OGD/R treatment of
hippocampal cultures leads to the activation of ERK1/2 (23). Under oxidative stress conditions,
Nrf2 is liberated from repression by Kelchlike ECH-associated
protein 1, and then translocates to the nucleus, where it interacts
with small Maf proteins to form a heterodimer, identifies and binds
to a cis-acting antioxidant response element, and, finally,
assembles the whole transcription machinery for transcription of
its target genes (24). When
activated, for example by pharmacological agents including
resveratrol, curcumin and retinoic acid, Nrf2 signaling protects
tissue from oxidative and cytotoxic injury, and is a key pathway
for protecting tissue against endogenous and exogenous stress
(25). The results of the present
study reveal that isoquercetin notably increases the expression of
Nrf2 in OGD/R-treated neurons. It has previously been shown that
Nrf2 overexpression is positively regulated via ERK1/2, and that
blocking ERK1/2 activation inhibits hyperoxia-induced
Nrf2-activation (22).
In the present study, neurotoxicity was found to be
reduced by isoquercetin in an ERK1/2-dependent manner, and the
isoquercetin-induced activation of ERK1/2 was blocked by the ERK1/2
inhibitor PD98059. It remains to be determined whether the finding
of an ERK1/2-dependent and -independent component of isoquercetin
inducible Nrf2 is an artifact of blocking an isoquercetin preferred
signaling pathway. The present study also suggested that
isoquercetin suppressed ROS and LDH release in hippocampal neurons
via the regulation of ERK1/2-mediated Nrf2 expression, which may
provide a useful strategy for the moderation of neurodegenerative
diseases, including Parkinson's disease and Alzheimer's
disease.
In conclusion, the present study has demonstrated
that isoquercetin is an effective antioxidant that protects against
OGD/R, induces reductions in ROS and LDH levels, and increases Nrf2
expression via the activation of ERK1/2, leading to a significant
reduction in cell death. However, additional studies exploring the
biological activities of isoquercetin in vivo are required
to support the use of isoquercetin as a potential drug for
MCAO.
Acknowledgements
The present study was supported by the following
three projects: (1) the 2013–2014
National Clinical Key Specialty Construction Project, supported by
the National Health and Family Planning Commission, China;
(2) The Early Management and Process
Optimization Study of Acute Ischemic Stroke in ER, supported by
Shanghai Health and Family Planning Commission, China (grant no.
201440496) and (3) the Shanghai
Advanced Integrated Traditional Chinese And Western Medicine
Personnel Training Project, supported by Shanghai Health and Family
Planning Commission, China (grant no. ZY3 RCPY 4 2009).
References
|
1
|
Nabel EG and Braunwald E: A tale of
coronary artery disease and myocardial infarction. N Engl J Med.
366:54–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Heart disease and stroke statistics-2014 update: A report from the
American Heart Association. Circulation. 129:e28–e292. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH, Shin HK, Park SY, Kim CD, Lee WS
and Hong KW: Cilostazol preserves CA1 hippocampus and enhances
generation of immature neuroblasts in dentate gyrus after transient
forebrain ischemia in rats. Exp Neurol. 215:87–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou J, Yoshitomi H, Liu T, Zhou B, Sun W,
Qin L, Guo X, Huang L, Wu L and Gao M: Isoquercitrin activates the
AMP-activated protein kinase (AMPK) signal pathway in rat H4IIE
cells. BMC Complement Altern Med. 14:422014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Zhang A, Guo Y and Dong C:
Electrochemical sensor for ultrasensitive determination of
isoquercitrin and baicalin based on DM-β-cyclodextrin
functionalized graphene nanosheets. Biosens Bioelectron.
58:242–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morand C, Manach C, Crespy V and Remesy C:
Quercetin 3-O-beta-glucoside is better absorbed than other
quercetin forms and is not present in rat plasma. Free Radic Res.
33:667–676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu XK, Yang WZ, Wang CH, Shi SS, Zhang YL,
Chen CM, Yang YK, Jin CD and Wen S: Zileuton reduces inflammatory
reaction and brain damage following permanent cerebral ischemia in
rats. Inflammation. 33:344–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu XM, Qian ZM, Zhu L, Du F, Yung WH, Gong
Q and Ke Y: Neuroprotective effect of ligustilide against
ischemia-reperfusion injury via up-regulation of erythropoietin and
down-regulation of RTP801. Br J Pharmacol. 164:332–343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joshi CN, Jain SK and Murthy PS: An
optimized triphenyltetrazolium chloride method for identification
of cerebral infarcts. Brain Res Brain Res Protoc. 13:11–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alrob OA, Sankaralingam S, Ma C, Wagg CS,
Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED,
et al: Obesity-induced lysine acetylation increases cardiac fatty
acid oxidation and impairs insulin signalling. Cardiovasc Res.
103:485–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hell RC Rodrigues, Costa MM Silva, Goes AM
and Oliveira AL: Local injection of BDNF producing mesenchymal stem
cells increases neuronal survival and ynaptic stability following
ventral root avulsion. Neurobiol Dis. 33:290–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poulose SM, Carey AN and Shukitt-Hale B:
Improving brain signaling in aging: Could berriesbe the answer?
Expert Rev Neurother. 12:887–889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dajas F, Rivera F, Blasina F, Arredondo F,
Echeverry C, Lafon L, Morquio A and Heinzen H: Cell culture
protection and in vivo neuroprotective capacity of flavonoids.
Neurotox Res. 5:425–432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ossola B, Kääriäinen TM and Männistö PT:
The multiple faces of quercetin in neuroprotection. Expert Opin
Drug Saf. 8:397–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen CL and Bayraktutan U: Oxidative
stress and it srole in the pathogenesis of ischaemic stroke. Int J
Stroke. 4:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilic U, Kilic E, Matter CM, Bassetti CL
and Hermann DM: TLR-4 deficiency protects against focal cerebral
ischemia and axotomy-inducedneurodegeneration. Neurobiol Dis.
31:33–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loniewski KJ, Patial S and Parameswaran N:
Sensitivity of TLR4- and −7-induced NF kappa B1 p105-TPL2-ERK
pathway to TNF-receptor-associated factor6 revealed by RNAi in
mouse macrophages. Mol Immunol. 44:3715–3723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu HH, Li SJ, Wang P, Yan HC, Cao X, Hou
FQ, Fang YY, Zhu XH and Gao TM: An L-type calcium channel agonist,
bay K8644, extends the window of intervention against ischemic
neuronal injury. Mol Neurobiol. 47:280–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu RL, Xiong QJ, Shu Q, Wu WN, Cheng J,
Fu H, Wang F, Chen JG and Hu ZL: Hyperoside protects cortical
neurons from oxygen-glucose deprivation-reperfusion induced injury
via nitric oxide signal pathway. Brain Res. 1469:164–173. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motohashi H and Yamamoto M: Nrf2-Keap1
defines a physiologically important stress response mechanism.
Trends Mol Med. 10:549–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen G, Hebbar V, Nair S, Xu C, Li W, Lin
W, Keum YS, Han J, Gallo MA and Kong AN: Regulation of Nrf2
transactivation domain activity. The differential effects of
mitogen-activated protein kinase cascades and synergistic
stimulatory effect of Raf and CREB-binding protein. J Biol Chem.
279:23052–23060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pitha-Rowe I, Liby K, Royce D and Sporn M:
Synthetic triterpenoids attenuate cytotoxic retinal injury:
Cross-talk between Nrf2 and PI3K/AKT signaling through inhibition
of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci.
50:5339–5347. 2009. View Article : Google Scholar : PubMed/NCBI
|