Introduction
The incidence of hypertension is increasing year by
year and may cause severe organ damage and increase the risk of
patient mortality (1,2). Hypertensive heart disease (HHD) may be
associated with diastolic and systolic heart dysfunction, and
eventually lead to heart failure (3,4).
The primary pathological characteristic of HHD is
left ventricular hypertrophy (LVH), which is independently
associated with a number of cardiovascular endpoints, including
coronary heart disease and stroke (5). Therefore, hypertensive patients with
LVH have an increased risk of experiencing cardiovascular events
compared to hypertensive patients without LVH (6,7).
There is a strong correlation between high blood
pressure and LVH (8,9). However, it has been indicated that
nonhemodynamic factors, including transforming growth factor β1,
the renin-angiotensin system and tumor necrosis factor α may induce
profibrotic effects and proinflammation, thus contributing to LVH
(10,11). Furthermore, studies using animal
models have demonstrated that macrophage, T cell and monocytic
fibroblast precursors serve important roles in angiotensin II
infusion-induced or pathological cardiac remodeling (12–15).
Although studies using animal models have
demonstrated that nonhemodynamic factors, including inflammatory
cells and cytokines, contribute to left ventricular hypertrophy
(LVH) (12,14), there is little clinical data to
confirm this association. Based on the aforementioned results, the
present study aimed to determine whether circulating leukocyte
subtypes are associated with LVH in hypertensive patients treated
with anti-hypertensive drugs.
Patients and methods
Patients
A total of 144 consecutive hypertensive patients
currently taking anti-hypertensive drug therapy were enrolled in
the current study between January 2012 and December 2014 in the
Department of Cardiology, Beijing Friendship Hospital, Capital
Medical University (Beijing, China). All enrolled patients had a
5–20 year history of hypertension and had all previously taken
anti-hypertensive drugs. Exclusion criteria included secondary
hypertension, heart failure symptoms, idiopathic cardiomyopathy,
ischemic heart disease and the presence of other heart diseases.
Blood pressure (BP) was measured at an office at the Beijing
Friendship Hospital on two separate occasions. A calibrated mercury
sphygmomanometer was used while patients were seated following a 10
min rest. Normal BP was defined as systolic BP (SBP) of 90–140 mmHg
or diastolic BP (DBP) of 60–90 mmHg (16,17).
Patients were excluded from the current study if they had secondary
hypertension, heart failure symptoms, idiopathic cardiomyopathy,
ischemic heart disease or other heart diseases. Patient
characteristics are summarized in Table
I. The protocol of the current study was approved by the
Institutional Committee of the Capital Medical University (Beijing,
China) and was performed in accordance with the ethical standards
laid down in the 1964 Declaration of Helsinki and its later
amendments. Written informed consent was given by all patients.
 | Table I.Clinical characteristics of patients
in each group. |
Table I.
Clinical characteristics of patients
in each group.
| Characteristic | Lower LVMI | Higher LVMI | P-value |
|---|
| Age, years | 59.4±12.8 | 61.9±12.6 | 0.301 |
| Proportion of
males, % | 55.7 | 61.5 | 0.526 |
| Systolic blood
pressure, mmHg | 138.2±23.6 | 145.8±23.7 | 0.086 |
| Diastolic blood
pressure, mmHg | 87.0±12.6 | 84.1±14.6 | 0.384 |
| Hyperlipidemia,
% | 12.3 | 15.8 | 0.582 |
| Diabetes mellitus,
% | 23.6 | 13.2 | 0.174 |
| Laboratory
parameters |
|
|
|
| Blood
platelet, 109/l | 218.0±57.4 | 228.3±60.8 | 0.354 |
|
Hemoglobin, g/l | 136.7±14.9 | 131.7±12.4 | 0.065 |
| Serum
creatinine, µmol/l | 83.7±37.6 | 88.6±37.3 | 0.493 |
| Blood
urea nitrogen, mmol/l | 6.29±7.65 | 6.95±4.97 | 0.622 |
| Blood
uric acid, µmol/l | 324.7±105.6 | 355.1±89.1 | 0.116 |
| Medicine |
|
|
|
| ACEI
and/or ARB | 67.0% | 66.7% | 0.945 |
|
Calcium-channel blockers | 51.0% | 51.3% | 0.971 |
|
β-Blocker | 47.2% | 41.0% | 0.510 |
|
Aspirin | 52.8% | 56.4% | 0.701 |
Laboratory analyses
Baseline clinical data was collected for all
patients. Counts for total white blood cells (WBC) and
differentiated subtypes (neutrophils, lymphocytes, monocytes,
eosinophils and basophils) were measured immediately following
presentation using peripheral venous blood in an automated blood
cell counter (ADVIA 2120: Siemens Healthcare Diagnostics,
Camberley, UK). The WBC count was treated as a continuous and
categorical variable and was classified as low
(<6.65×109/l, <33th percentile), intermediate
(6.65–10.11×109/l, 33th-66th percentiles) or high
(>10.11×109/l, >66th percentile) (18,19).
Alanine transaminase, creatinine, blood urea nitrogen, cholesterol,
triglycerides, uric acid and glucose were measured using an
established immunoassay (Biosite Inc., San Diego, CA, USA).
Quantitative C-reactive protein determination was performed with
the BN II Nephelometer (Siemens Healthcare Diagnostics). Cardiac
troponin T was measured on the Elecsys 10/10 (Roche Diagnostics,
Indianapolis, IN, USA).
Evaluation of cardiac structure and
function
Echocardiographic examination of the patients was
performed by two experienced cardiologists using a VIVID 7
cardiovascular ultrasound system (GE Healthcare Life Sciences,
Uppsala, Sweden) with an M4S 1.5–4.0-MHZ matrix array probe (GE
Healthcare Life Sciences) according to the guidelines of the
American Society of Echocardiography (20). Echocardiographic examination was
performed with patients in the left lateral decubitus position
breathing slowly. The cardiologists were blinded to the patients'
other data. The echocardiograph measurements included left
ventricular end-diastolic diameter (LVEDD), left ventricular
posterior wall thickness in diastole (LVPWT) and inter-ventricular
septal wall thickness in diastole (IVST). Left ventricular systolic
function was assessed using the left ventricular ejection fraction
(LVEF) and left ventricular fractional shortening (LVFS). Left
ventricular mass was calculated using the ASE-recommended formula:
Left ventricular mass (g) = 0.8 × {1.04[(IVST + LVEDD +
LVPWT)3 - (LVEDD)3]} (21). Left ventricular mass was divided by
body surface area to obtain the left ventricular mass index (LVMI).
Body surface (m2) was calculated using (0.0061 × height
+ 0.0124 × weight - 0.0099). LVH was defined as previously
described (22,23). Patients were divided into two
different groups according to LVMI. One group consisted of patients
with lower LVMI (≤100 g/m2) and the other group
consisted of patients with higher LVMI (>100
g/m2).
Statistical analysis
Data analysis was performed using the SPSS
statistical package ver. 16.0 for Windows (SPSS Inc., Chicago, IL,
USA). Differences in the distribution of demographics, laboratory
parameters and medical characteristics among hypertensive patients
with lower LVMI, compared with those who had higher LVMI, were
examined using the χ2 test for categorized variables and
either one-way analysis of variance for continuous variables, or
non-parametric tests if distribution was skewed. Multivariate
logistic regression analyses were performed to examine the
associations of total white blood cell and other potentially
confounding prognostic factors with LVMI. Other factors included in
the multivariate analyses were age, gender, hypercholesterolemia,
diabetes mellitus and medication (aspirin, β-blockers,
calcium-channel blockers, angiotensin converting enzyme inhibitor
and aldosterone receptor blocker). All P-values were the results of
two-tailed tests. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
White blood cells are increased in
patients with LVMI
Clinical characteristics of all patients are
presented in Table I. At baseline,
the 41% of the study population were male and the mean age was 53.7
years [range 44–66 years, standard deviation (SD) 5.8 years]. Out
of all the patients, 10% had diabetes mellitus and 28% had
hyperlipidemia. The mean SBP and DBP (mmHg) were 121.2 (SD 18.8)
and 74.0 (SD 11.3). Blood platelet, hemoglobin, serum creatinine,
urea nitrogen and uric acid levels did not significantly differ
between the two groups. The drug treatments received by patients in
the two groups were not significantly different.
Cardiac remodeling caused by hypertension is
accepted as an inflammatory response (14,15) in
which inflammatory cells play a critical role. Therefore the
current study measured levels of WBC. The LVMI of the study
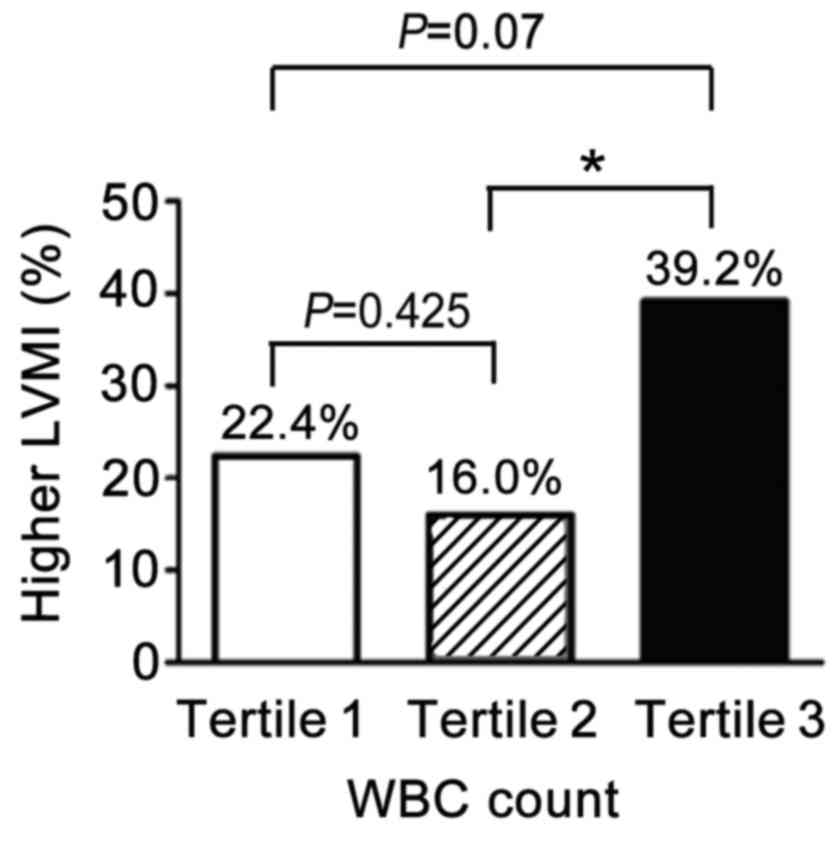
population by tertiles of total WBC level is presented in Fig. 1. In the middle tertile (Tertile 2,
n=44), 16% of patients had higher LVMI, indicative of myocardial
hypertrophy (MH), which was lower than that in the highest tertile
(Tertile 3, n=51; P=0.012). Notably, in the lowest tertile (Tertile
1, n=49) 22.4% of patients had MH, higher than in the middle
tertile (Tertile 2), however, this difference was not statistically
significant (22.4% vs. 39.2%, P=0.425).
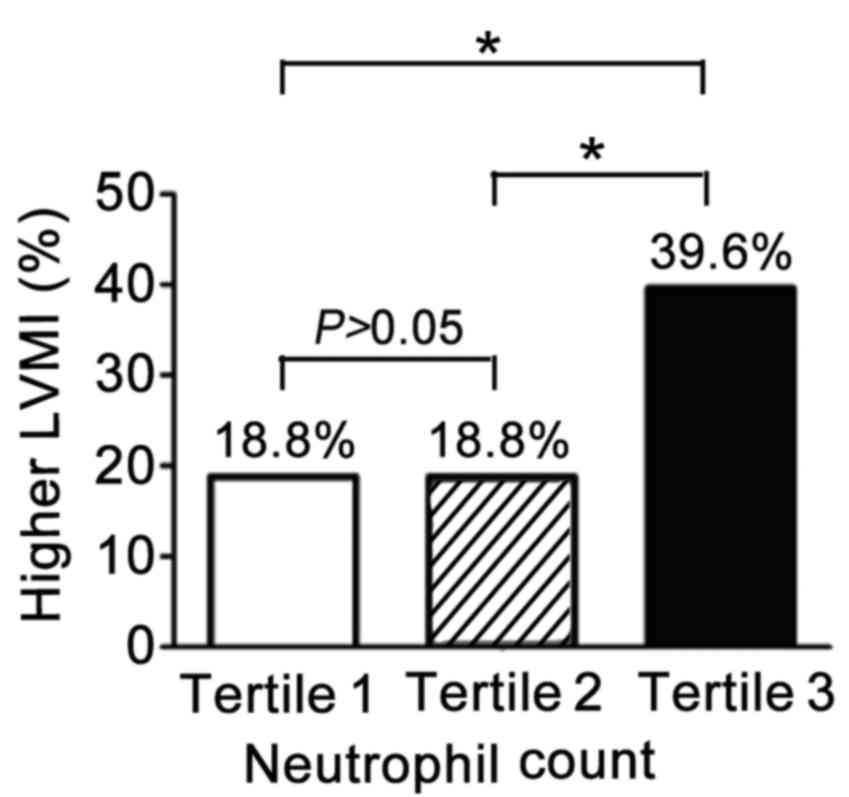
Neutrophil counts correlate with
LVMI
To determine which specific leukocyte types have a
critical role in hypertension-induced MH, different WBC subtypes
were measured. The LVMI of the study population by tertile of
neutrophil counts is presented in Fig.
2. A high LVMI indicates the presence of MH. The proportion of
patients with MH in both the lowest (n=48) and middle tertile
(n=48) was 18.8%. However, a larger proportion (39.6%) of patients
in the highest tertile had MH (18.8% vs. 39.6%, P=0.012) compared
with the middle and lowest tertiles.
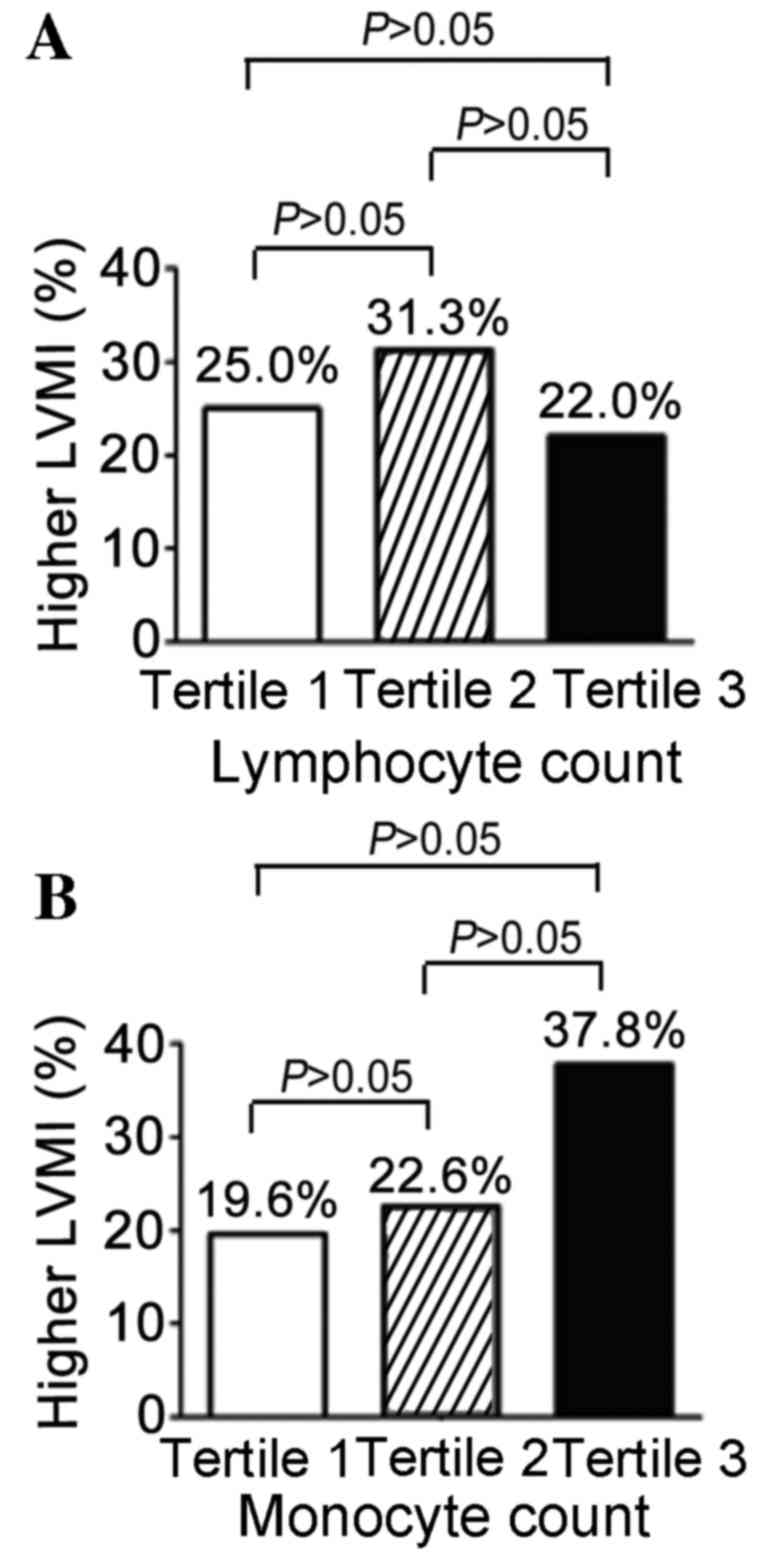
As presented in Fig.
3, other subtypes of WBC were detected. A slightly higher
proportion of patients had MH (31.3%) in the middle tertile (n=48)
compared with the other tertiles; the percentages in the lowest
tertile (n=46) and highest tertile (n=50) were 25.0 and 22.0%
respectively (P=0.582; Fig. 3A). As
presented in Fig. 3B for the
monocyte count, a marked increase (37.8%) of MH in the highest
tertile (n=45) was observed, while the proportion of patients with
MH in the other two tertiles were 19.6% and 22.6% respectively.
However, none of these differences were statistically
significant.
Table II presents
the results from the logistic analysis. Total WBC counts differed
significantly between the two LVMI groups (the lowest and the
highest; P=0.013). Additionally, accompanied by an increase in
total WBCs particularly over the middle tertile, the percentage of
patients with higher LVMI (or MH) was significantly increased
(P=0.008). Furthermore, Table II
indicated that older patients (>65 years old) had higher LVMI
than those ≤65 years (P=0.042). However, there were no significant
differences in gender, DM, hypercholesterolemia and cardiovascular
drug treatment between the groups.
 | Table II.Multivariate logistic regression
analyses for left ventricular mass index in hypertensive
patients. |
Table II.
Multivariate logistic regression
analyses for left ventricular mass index in hypertensive
patients.
|
|
|
| 95.0% C.I. for EXP
(B) |
|
|---|
|
|
|
|
|
|
|---|
| Variable | B | OR | Lower LVMI | Higher LVMI | P-value |
|---|
| Total WBC
count |
|
|
|
| 0.013a |
| WBC Tertile
(lowest) | 0.364 | 1.439 | 0.478 | 4.338 | 0.518 |
| WBC Tertile
(highest) | 1.415 | 4.117 | 1.452 | 11.693 | 0.008a |
| Old age (>65
years) | 0.865 | 2.374 | 1.037 | 5.473 | 0.042a |
| Male (%) | −0.314 | 0.731 | 0.312 | 1.716 | 0.469 |
|
Hypercholesterolemia | 0.414 | 1.512 | 0.493 | 4.636 | 0.469 |
| DM | −0.934 | 0.395 | 0.126 | 1.237 | 0.111 |
| ACEI or ARB | −0.047 | 0.955 | 0.507 | 1.796 | 0.885 |
| CCB | −0.011 | 0.989 | 0.444 | 2.203 | 0.979 |
| Aspirin | 0.165 | 1.179 | 0.517 | 2.693 | 0.695 |
| β-blocker | −0.277 | 0.758 | 0.339 | 1.695 | 0.511 |
Discussion
In the present study, a correlation was detected
between white blood cell count and LVMI in hypertensive patients
undergoing anti-hypertensive drug therapy. Hypertension is an
important risk factor for cardiovascular diseases including
atherosclerosis and myocardial hypertrophy, and is independent of
age, gender and ethnicity (24,25). The
incidence of hypertension is an important basis for the progress of
cardiovascular diseases, which itself is a result of many
interacting factors. There is evidence that nonhemodynamic factors,
which possibly lead to profibrotic effects and proinflammation, may
influence LVH (10,11).
Hypertension is a chronic disease (26,27).
Active drug therapy may reduce and delay the organ damage caused by
hypertension (27). Excluding the
impact of other factors, there are significant differences in LVH
among patients receiving the same drug therapy (28,29). LVH
is closely associated with the plasma levels of white blood cells
in patients, which gives an indication of hypertension (17,19).
Thus practitioners can focus on the level of white blood cell
intervention required, further reducing the patient's long-term
target organ damage.
The current study detected a strong correlation
between white blood cell counts (particularly neutrophil counts)
and LVMI in hypertensive patients undergoing anti-hypertensive drug
therapy. It demonstrates that modulating neutrophil number to a
moderate level for hypertensive patients alongside
anti-hypertensive drug therapy may benefit the long-term prognosis
of patients. Inflammation is an indicator for the progression of
myocardial remodeling underlying the pathogenesis of LVH (30–34).
Inflammatory factors are typically derived from white blood cells,
particularly neutrophils (35–39).
Myocardial expression of inflammatory mediators, including monocyte
chemoattractant protein-1 or fractalkine, is significantly
increased in experimental myocarditis or dilated cardiomyopathy
(40,41). The infiltrating inflammatory cells
and/or cardiomyocytes may account for enhanced LVH. Inflammatory
cells and cytokines participate in the pathological process of
cardiovascular remodeling and are a double-edged sword; they can
clear necrotic cells and foreign antigens and promote angiogenesis
and scar repair (42). However,
excessive inflammatory cell infiltration may seriously upset the
balance of the body microenvironment, causing organ damage
(42–44).
To the best of our knowledge, the present study is
the first to demonstrate that circulating specific types of
leukocyte may be associated with LVH in hypertensive patients
currently taking anti-hypertensive drugs and thus may provide a
novel preventative strategy for LVH by modulating myocardial
inflammation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81300209, 81400263,
81230007 and 81200147) and Basic-Clinical Cooperation Project of
Chinese Capital Medical University (no. 13JL59).
References
|
1
|
Westerlund E, Brandt L, Hovatta O, Wallén
H, Ekbom A and Henriksson P: Incidence of hypertension, stroke,
coronary heart disease, and diabetes in women who have delivered
after in vitro fertilization: A population-based cohort study from
Sweden. Fertil Steril. 102:1096–1102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veloso HH: Incidence of sudden cardiac
death in congestive heart failure: Chagas disease versus systemic
arterial hypertension. Int J Cardiol. 175:175–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim W, Park CS, Kim HJ, Kim KH, An HM, Kim
YH, Lim CH, Kang WY, Hwang SH and Kim W: Hypertensive heart failure
associated with middle aortic syndrome reversed dramatically by
endovascular management. J Cardiovasc Ultrasound. 19:144–147. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maskali F, Poussier S, Louis H, Boutley H,
Lhuillier M, Thornton SN, Karcher G, Lacolley P and Marie PY:
Assessment of the early stage of cardiac remodeling of
spontaneously hypertensive heart failure rats using the
quantitative 3-dimensional analysis provided by acipimox-enhanced
FDG-PET. Int J Cardiovasc Imaging. 30:449–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sosner P, Cabasson S, Hulin-Delmotte C,
Saulnier PJ, Gand E, Torremocha F, Piguel X, Miot A, Maréchaud R,
Herpin D, et al: Effect of Cornell product and other ECG left
ventricular hypertrophy criteria on various cardiovascular
endpoints in type 2 diabetic patients. Int J Cardiol. 175:193–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seto S: Left ventricular hypertrophy,
ischemic heart disease and the incidence of cardiovascular events
in Japanese high-risk hypertensive patients. Circ J. 73:1014–1015.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ibsen H, Olsen MH, Wachtell K,
Borch-Johnsen K, Lindholm LH and Mogensen CE: Reduction in
albuminuria translates to reduction in cardiovascular events in
hypertensive patients with left ventricular hypertrophy and
diabetes. J Nephrol. 21:566–569. 2008.PubMed/NCBI
|
|
8
|
Pai AU, Chakrapani M, Bhaskaran U and
Kamath P: Study of home-monitored night blood pressure and its
correlation with left ventricular hypertrophy in treatment-naive
hypertensive patients. Singapore Med J. 53:95–98. 2012.PubMed/NCBI
|
|
9
|
Nathwani D, Reeves RA, Marquez-Julio A and
Leenen FH: Left ventricular hypertrophy in mild hypertension:
correlation with exercise blood pressure. Am Heart J. 109:386–387.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Simone G, Pasanisi F and Contaldo F:
Link of nonhemodynamic factors to hemodynamic determinantsof left
ventricular hypertrophy. Hypertension. 38:13–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauwens FR, Duprez DA, De Buyzere ML, De
Backer TL, Kaufman JM, Van Hoecke J, Vermeulen A and Clement DL:
Influence of the arterial blood pressure and nonhemodynamic factors
on left ventricular hypertrophy in moderate essential hypertension.
Am J Cardiol. 68:925–929. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rateri DL, Howatt DA, Moorleghen JJ,
Charnigo R, Cassis LA and Daugherty A: Prolonged infusion of
angiotensin II in apoE(−/−)mice promotes macrophage recruitment
with continued expansion of abdominal aortic aneurysm. Am J Pathol.
179:1542–1548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ismahil MA and Prabhu SD: Cardiac immune
cell remodeling after myocardial infarction. J Mol Cell Cardiol.
62:142–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee AA and McCulloch AD: Multiaxial
myocardial mechanics and extracellular matrix remodeling:
Mechanochemical regulation of cardiac fibroblast function. Adv Exp
Med Biol. 430:227–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ngu JM, Teng G, Meijndert HC, Mewhort HE,
Turnbull JD, Stetler-Stevenson WG and Fedak PW: Human cardiac
fibroblast extracellular matrix remodeling: dual effects of tissue
inhibitor of metalloproteinase-2. Cardiovasc Pathol. 23:335–343.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orme S, Ralph SG, Birchall A,
Lawson-Matthew P, McLean K and Channer KS: The normal range for
inter-arm differences in blood pressure. Age Ageing. 28:537–542.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chue CD, Edwards NC, Ferro CJ, Steeds RP
and Townend JN: Reduction of blood pressure already in the normal
range further regresses left ventricular mass. Heart. 96:10802010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colquitt JL and D'Orazio JA: Intracranial
hemorrhage and a white blood cell count of almost 1 million
cells/muL. J Pediatr. 162:2142013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Twig G, Afek A, Shamiss A, Derazne E, Tzur
D, Gordon B and Tirosh A: White blood cells count and incidence of
type 2 diabetes in young men. Diabetes Care. 36:276–282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Troianos CA, Hartman GS, Glas KE, Skubas
NJ, Eberhardt RT, Walker JD and Reeves ST: Councils on
Intraoperative Echocardiography and Vascular Ultrasound of the
American Society of Echocardiography: Guidelines for performing
ultrasound guided vascular cannulation: Recommendations of the
american society of echocardiography and the society of
cardiovascular anesthesiologists. J Am Soc Echocardiogr.
24:1291–1318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lang RM, Bierig M, Devereux RB,
Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward
J, Shanewise JS, et al: Recommendations for chamber quantification:
A report from the American Society of Echocardiography's Guidelines
and Standards Committee and the Chamber Quantification Writing
Group, developed in conjunction with the European Association of
Echocardiography, a branch of the European Society of Cardiology. J
Am Soc Echocardiogr. 18:1440–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganau A, Devereux RB, Roman MJ, de Simone
G, Pickering TG, Saba PS, Vargiu P, Simongini I and Laragh JH:
Patterns of left ventricular hypertrophy and geometric remodeling
in essential hypertension. J Am Coll Cardiol. 19:1550–1558. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koren MJ, Devereux RB, Casale PN, Savage
DD and Laragh JH: Relation of left ventricular mass and geometry to
morbidity and mortality in uncomplicated essential hypertension.
Ann Intern Med. 114:345–352. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin KC, Tsao HM, Chen CH and Chou P:
Hypertension was the major risk factor leading to development of
cardiovascular diseases among men with hyperuricemia. J Rheumatol.
31:1152–1158. 2004.PubMed/NCBI
|
|
25
|
Kováčová M and Kiňová S: Arterial
hypertension in gravidity-a risk factor for cardiovascular
diseases. Vnitr Lek. 58:922–927. 2012.(In Czech). PubMed/NCBI
|
|
26
|
Azancot MA, Ramos N, Moreso FJ, Ibernon M,
Espinel E, Torres IB, Fort J and Seron D: Hypertension in chronic
kidney disease: the influence of renal transplantation.
Transplantation. 98:537–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barreto MS, Reiners AA and Marcon SS:
Knowledge about hypertension and factors associated with the
non-adherence to drug therapy. Rev Lat Am Enfermagem. 22:491–498.
2014.(Article in English, Portuguese, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hachamovitch R, Sonnenblick EH, Strom JA
and Frishman WH: Left ventricular hypertrophy in hypertension and
the effects of antihypertensive drug therapy. Curr Probl Cardiol.
13:375–421. 1988.PubMed/NCBI
|
|
29
|
Eselin JA and Carter BL: Hypertension and
left ventricular hypertrophy: is drug therapy beneficial?
Pharmacotherapy. 14:60–88. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mehta SK, Rame JE, Khera A, Murphy SA,
Canham RM, Peshock RM, de Lemos JA and Drazner MH: Left ventricular
hypertrophy, subclinical atherosclerosis and inflammation.
Hypertension. 49:1385–1391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai WC, Lin CC, Huang YY, Chen JY and
Chen JH: Association of increased arterial stiffness and
inflammation with proteinuria and left ventricular hypertrophy in
non-diabetic hypertensive patients. Blood Press. 16:270–275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Y, Chen Y, Li D, Li J, Liu X, Cui C and
Yu C: Hypertension, fluid overload and micro inflammation are
associated with left ventricular hypertrophy in maintenance
hemodialysis patients. Ren Fail. 35:1204–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salles GF, Fiszman R, Cardoso CR and
Muxfeldt ES: Relation of left ventricular hypertrophy with systemic
inflammation and endothelial damage in resistant hypertension.
Hypertension. 50:723–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cottone S, Nardi E, Mulè G, Vadalà A,
Lorito MC, Riccobene R, Palermo A, Arsena R, Guarneri M and
Cerasola G: Association between biomarkers of inflammation and left
ventricular hypertrophy in moderate chronic kidney disease. Clin
Nephrol. 67:209–216. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fanning NF, Kell MR, Shorten GD, Kirwan
WO, Bouchier-Hayes D, Cotter TG and Redmond HP: Circulating
granulocyte macrophage colony-stimulating factor in plasma of
patients with the systemic inflammatory response syndrome delays
neutrophil apoptosis through inhibition of spontaneous reactive
oxygen species generation. Shock. 11:167–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scapini P, Morini M, Tecchio C, Minghelli
S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM
and Cassatella MA: CXCL1/macrophage inflammatory protein-2-induced
angiogenesis in vivo is mediated by neutrophil-derived vascular
endothelial growth factor-A. J Immunol. 172:5034–5040. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Droemann D, Hansen F, Aries SP, Braun J,
Zabel P, Dalhoff K and Schaaf B: Neutrophil apoptosis, activation
and anti-inflammatory cytokine response in granulocyte
colony-stimulating factor-treated patients with community-acquired
pneumonia. Respiration. 73:340–346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanabe J, Watanabe M, Mue S and Ohuchi K:
Leukocyte-derived neutrophil chemotactic factor-2 produced by
infiltrated leukocytes in allergic inflammation model in rats is
macrophage inflammatory protein-2. Immunol Invest. 24:757–764.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tavares-Murta BM, Lefort J, Cunha FQ,
Ferreira SH and Vargaftig BB: Interference of a neutrophil
recruitment inhibitory factor upon the accumulation of inflammatory
cells and airway hyperreactivity in sensitized guinea-pigs after
intranasal antigen challenge. Br J Pharmacol. 108:538–543. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen Y, Zhang FQ and Wei X: Truncated
monocyte chemoattractant protein-1 can alleviate cardiac injury in
mice with viral myocarditis via infiltration of mononuclear cells.
Microbiol Immunol. 58:195–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ning J, Li YH and Zhang CB: Expression of
monocyte chemoattractant protein-1 in sudden death due to viral
myocarditis and its medicolegal significance. Fa Yi Xue Za Zhi.
25:334–336. 2009.(In Chinese). PubMed/NCBI
|
|
42
|
Halaris A: Inflammation, heart disease,
and depression. Curr Psychiatry Rep. 15:4002013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huston JM and Tracey KJ: The pulse of
inflammation: heart rate variability, the cholinergic
anti-inflammatory pathway and implications for therapy. J Intern
Med. 269:45–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luttmann-Gibson H, Suh HH, Coull BA,
Dockery DW, Sarnat SE, Schwartz J, Stone PH and Gold DR: Systemic
inflammation, heart rate variability and air pollution in a cohort
of senior adults. Occup Environ Med. 67:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|