Introduction
The effects of hospital-acquired infections on the
outcome of critically ill patients have been extensively
investigated. Acinetobacter baumannii (A. baumannii),
a Gram-negative aerobic coccobacillus, has emerged as one of the
leading causes for nosocomial bloodstream infections in intensive
care units (ICUs) (1). A.
baumannii has been demonstrated to cause a number of clinical
infections, including bacteremia, pneumonia, meningitis and
surgical site infections (2,3).
A. baumannii has been involved in infections
with increased mortality rates (4).
The virulence of A. baumannii can be enhanced by the
occurrence of multiple antimicrobial resistance, resulting in
difficulties when determining the therapeutic options to treat the
infection (5,6). It has been reported that
multidrug-resistant A. baumannii induces a fulminant
infection following the treatment of a surgical wound (7). According to the Chinese Meropenem
Susceptibility Surveillance (CMSS) report in 2010 (8), a total of 180 strains of A.
baumannii have been identified in 1259 isolates of
Gram-negative bacilli from 13 hospitals; this makes the infection
second only to Pseudomonas aeruginosa (8). In addition, the susceptibility of A.
baumannii to carbapenems is <37.0% and its susceptibility to
minocycline is 47.8%, while the incidence of extensively
drug-resistant A. baumannii is 60.1% (8). Furthermore, the mortality rates of
patients with A. baumannii infection have been reported to
be 26.0–55.7%, and the attributable mortality rates were 8.4–36.5%
(9).
Currently, there are few studies concerning the
impact of A. baumannii infection on the prognosis of
critically ill patients in China (10,11). In
the present retrospective study, the characteristics of patients
with A. baumannii infections in the ICU are investigated,
and the clinical prognostic factors for A. baumannii
infection are analyzed.
Materials and methods
Study population
The present retrospective study enrolled patients
with an A. baumannii infection who were admitted to the ICU
of the People's Hospital of Xinjiang Uygur Autonomous Region
(Urumqi, China) between January 2013 and December 2013 for the
treatment of infection. According to the Expert Consensus Document
on A. baumannii infection diagnosis, treatment and
prevention in China (12),
multidrug-resistant A. baumannii (MDRAB) were defined as the
strains that were resistant to at least three classes of the
following antimicrobial agents: Cephalosporins, carbapenems,
sulbactam, fluoroquinolones and aminoglycoside drugs. Mixed
infection occurred when other bacteria and/or fungi were detected
in the culture in addition to A. baumannii. Written informed
consent was obtained from patients, and the study was approved by
the ethics committee of the People's Hospital of Xinjiang Uygur
Autonomous Region.
Data collection
The following data were obtained for each patient:
Age, gender, ethnicity, A. baumannii infection status,
concurrent infection status, antibiotics for therapeutic
application, severity of the disease, serum procalcitonin (PCT)
level, the site of infection, shock, sepsis and renal replacement
therapy status. The severity of the disease was evaluated based on
the Acute Physiology and Chronic Health Evaluation II (APACHE II)
and sepsis-related organ failure assessment (SOFA) scores.
The APACHE II scoring system covered 12 routine
physiologic measurements, including age and previous health status,
that provided a general evaluation of the disease severity (range,
0–71). A high score indicated poor prognosis, and this could be
used to predict the mortality of patients (13). The SOFA score (range, 1–4) was used
to assess the course of organ dysfunction/failure in critically ill
patients, including the respiratory system, coagulation system,
liver, cardiovascular system, central nervous system and kidney. A
SOFA score of ≤2 indicated organ dysfunction, and a SOFA score of
≥3 indicated organ failure. High SOFA scores for individual organs
were associated with increased mortality (14). Shock was defined as mean blood
pressure <65 mmHg in spite of an adequate quantity of fluids
(≤1000 ml crystalloids or 500 ml colloids) (15). Sepsis, severe sepsis and septic shock
were diagnosed according to the guidelines of the 2001
SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference
(16).
Statistical analysis
Data are expressed as the mean ± standard deviation
or n (%). SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform statistical analysis. Intergroup comparisons
were performed using the t-test or χ2-test. Logistic
regression analysis was used to determine the factors affecting the
ICU mortality. Receiver operating characteristic (ROC) curve
analysis was performed to determine the cut-off point of the APACHE
II score. P<0.05 was considered to indicate a statistically
significant difference.
Results
Univariate analysis
A total of 37 patients in the ICU (male, 28; female,
9; age range, 34–70 years) were included in the present study. The
sample culture was primarily derived from the sputum, peritoneal
drainage fluid, urine and wound secretion (Table I). Of the 37 patients, 8 patients did
not survive, resulting in an ICU mortality rate of 21.6%.
 | Table I.Acinetobacter baumannii culture
sources. |
Table I.
Acinetobacter baumannii culture
sources.
| Culture source | Cases, n (%) |
|---|
| Sputum | 34 (91.9) |
| Peritoneal drainage
fluid | 1
(2.7) |
| Urine | 1
(2.7) |
| Wound secretion | 1
(2.7) |
As presented in Table
II, univariate analysis indicated that, in comparison with the
survival group, the APACHE II and SOFA scores were significantly
higher in the mortality group (P<0.002 and P<0.001,
respectively). In addition, a great number of patients with septic
shock were observed in the mortality group in comparison with the
survival group (n=4 and n=3, respectively), and a greater number of
patients were infected with MDRAB in the survival group compared
with those in the mortality group (51.9 and 41.3%, respectively).
However, there were no statistically significant differences in the
age, gender, ethnicity or PCT level between the two groups.
Furthermore, the prognosis was not significantly influenced by the
various antibiotics used for therapeutic applications, the presence
of mixed infection or the site of infection.
 | Table II.Univariate analysis of intensive care
unit mortality in patients with A. baumannii infection. |
Table II.
Univariate analysis of intensive care
unit mortality in patients with A. baumannii infection.
| Clinical
variable | Survival group
(n=29) | Mortality group
(n=8) | t-test/2 | P-value |
|---|
| Age, years | 50.17±19.25 | 60.62±15.16 | −1.414 | 0.166 |
| Males, n (%) | 23 (79.3%) | 5 (62.5%) |
0.963 | 0.327 |
| Females, n (%) | 6 (20.7%) | 3 (37.5%) |
|
|
| Multidrug-resistant
A. baumannii, n (%) | 14/27 (51.9%) | 1/7 (14.3%) |
3.182 | 0.074 |
| Antibiotics, n
(%) |
|
|
|
|
|
Sulbactam | 13 (44.8%) | 3 (37.5%) |
0.137 | 0.711 |
|
Cephalosporins | 10 (34.5%) | 4 (50.0%) |
0.642 | 0.423 |
|
Carbapenems | 8 (27.6%) | 2 (25.0%) |
0.021 | 0.884 |
|
Minocycline | 7 (24.1%) | 2 (25.0%) |
0.003 | 0.960 |
| Mixed infection, n
(%) |
|
|
3.117 | 0.210 |
| None | 9 (31.0%) | 3 (37.5%) |
|
|
| Same
site | 16 (55.2%) | 2 (25.0%) |
|
|
| Different
sites | 4 (13.8%) | 3 (37.5%) |
|
|
| APACHE II scores | 11.31±5.46 | 18.25±4.23 | −3.319 | 0.002 |
| SOFA scores | 5.14±2.30 | 8.75±3.37 | −3.352 | 0.001 |
| Procalcitonin level,
pg/l | 6.25±15.66 | 25.03±37.34 | −1.209 | 0.277 |
| Shock, n (%) | 6 (6.9%) | 5 (62.5%) |
5.247 | 0.022 |
| Renal replacement
therapy, n (%) | 2 (6.9%) | 1 (12.5%) |
0.264 | 0.607 |
| Disease severity, n
(%) |
|
|
6.778 | 0.034 |
|
Sepsis | 23 (79.3) | 4
(50.0) |
|
|
| Severe
Sepsis | 3 (10.3) | 0 (0.0) |
|
|
| Septic
shock | 3 (10.3) | 4
(50.0) |
|
|
| Infection sites, n
(%) |
|
|
0.264 | 0.607 |
| Lung | 27 (93.1%) | 7 (87.5%) |
|
|
|
Others | 2 (6.9%) | 1 (12.5%) |
|
|
| Length of intensive
case unit stay, days | 25.90±22.97 | 16.12±6.40 |
1.180 | 0.246 |
| Length of hospital
stay, days | 40.45±23.18 | 20.88±8.63 |
3.711 | 0.001 |
Multivariate analysis
The results of multivariate analysis demonstrated
that the APACHE II score of the patients at diagnosis is the only
independent factor that can indicate the disease prognosis
(Table III). The SOFA score,
septic shock and hospitalization duration were related with the
disease prognosis; however, they were not independent prognostic
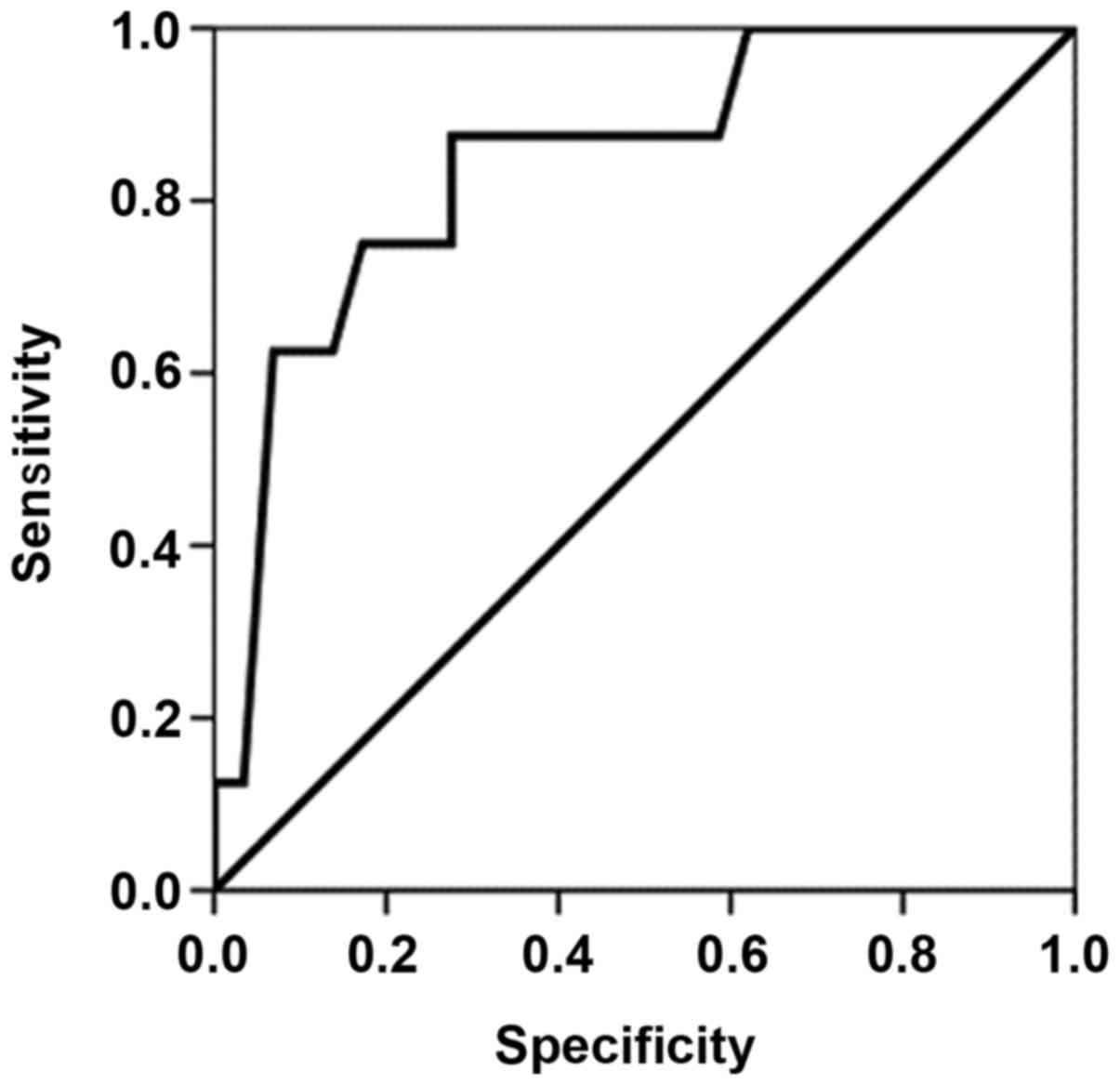
factors. ROC curve analysis was performed in order to determine the
cut-off point for the APACHE II score, and the area under the ROC
curve (Fig. 1) was found to be
0.845±0.078 (95% confidence interval, 0.692–0.998). In patients
with an APACHE II score of 15, the sensitivity and specificity for
the prediction of mortality were 87.5 and 72.4%, respectively.
 | Table III.Multivariate analysis of intensive
care unit mortality in patients with A. baumannii infection. |
Table III.
Multivariate analysis of intensive
care unit mortality in patients with A. baumannii infection.
| Clinical
variable | Regression
coefficient | Standard error | Wald value | P-value | Relative risk (95%
CI) |
|---|
| APACHE II
score |
0.275 | 0.116 | 5.570 | 0.018 | 1.316
(1.048–1.654) |
| Constant | −6.146 | 2.101 | 8.560 | 0.003 | N/A |
Discussion
In a previous study, A. baumannii infections
were found to account for 33.94% of all Gram-negative bacilli
infections (17). The resistant rate
of A. baumannii to sulbactam is 30–60%, and its resistance
rate to carbapenems is 60–80% (17).
In the present study, it was observed that the incidence of MDRAB
was 44.1% (15/34), which is in accordance with the results of
previous studies. In addition, a greater number of patients
infected with MDRAB were detected in the survival group. This may
be due to A. baumannii not being the dominant species in
certain microbial cultures that were recognized as MDRAB, and thus
the infection would not result in mortality. A study by Qiao et
al (18) also indicated that the
percentage of MDRAB was significantly higher in the survival group
in comparison with that in the mortality group (P<0.05).
It has been observed that the genotype of A.
baumannii contributes towards its susceptibility to
antibiotics. ST75 and ST138 A. baumannii containing
OXA-23-like genes were shown to be resistant to carbapenem drugs
(19). However, A. baumannii
with drug-resistant and non-resistant genotypes could not be
distinguished by routine laboratory tests currently performed in
clinical practice (12). This may
explain why a number of researchers reported weak virulence of
A. baumannii in the clinic, and did not detct any
association between A. baumannii infection and disease
prognosis (11,20).
A. baumannii is an opportunistic pathogen
that is prone to be located at sites such as the skin, conjunctiva,
and oral, respiratory, rectal and genitourinary tracts (12,21,22).
When a specimen culture detects only A. baumannii, the
prediction of whether an infection will occur may be determined by
evaluating a number of clinical manifestations, auxiliary
examinations and laboratory examinations. However, if other
bacteria and/or fungi are detected in the same culture or at other
infection sites along with A. baumannii, further clinical
tests are required in order to confirm the diagnosis. In the
present study, only 33.3% (12/36) of patients were infected by
A. baumannii alone, while 66.7% (24/36) of patients
presented mixed infection at the same or different sites.
Comprehensive judgment regarding the type of antibiotics prescribed
to patients should, therefore, be applied when treating A.
baumannii and other pathogenic microorganisms. The results from
the present study demonstrated that the APACHE II score at
diagnosis was the only independent prognostic factor in patients
with A. baumannii infection, and this is in accordance with
the results of Qiao et al (18). However, the present study is
single-centered with a limited number of subjects. Further in-depth
multi-center studies with larger sample sizes are required in order
to elucidate the prognostic factors involved in an A.
baumannii infection.
In conclusion, the results of the present study
demonstrated that the APACHE II score at diagnosis is an
independent factor for the disease prognosis of patients in the ICU
with an A. baumannii infection. This prognosis is not
significantly influenced by the antibiotics administered for
therapeutic applications, mixed infections, MDRAB or the site of
infection in these patients. The findings of the present study
contribute towards the prognostic evaluation of patients in ICUs
with an A. baumannii infection.
Acknowledgements
The present study was supported by funding from the
Key Laboratory of Xinjiang Uygur Autonomous Region (grant no.
xjys0906-2014-03).
References
|
1
|
García-Garmendia JL, Ortiz-Leyba C,
Garnacho-Montero J, Jiménez-Jiménez FJ, Pérez-Paredes C,
Barrero-Almodóvar AE and Gili-Miner M: Risk factors for
Acinetobacter baumannii nosocomial bacteremia in critically ill
patients: A cohort study. Clin Infect Dis. 33:939–946. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaggi N, Sissodia P and Sharma L: Control
of multidrug resistant bacteria in a tertiary care hospital in
India. Antimicrob Resist Infect Control. 1:232012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falagas ME and Rafailidis PI: Attributable
mortality of Acinetobacter baumannii: No longer a controversial
issue. Crit Care. 11:1342007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemos EV, de la Hoz FP, Einarson TR,
McGhan WF, Quevedo E and Castañeda C and Kawai K: Carbapenem
resistance and mortality in patients with Acinetobacter baumannii
infection: Systematic review and meta-analysis. Clin Microbiol
Infect. 20:416–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grupper M, Sprecher H, Mashiach T and
Finkelstein R: Attributable mortality of nosocomial Acinetobacter
bacteremia. Infect Control Hosp Epidemiol. 28:293–298. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aydemir H, Celebi G, Piskin N, Oztoprak N,
Keskin AS, Aktas E, Sumbuloglu V and Akduman D: Mortality
attributable to carbapenem-resistant nosocomial Acinetobacter
baumannii infections in a Turkish university hospital. Jpn J Infect
Dis. 65:66–71. 2012.PubMed/NCBI
|
|
7
|
Maragakis LL, Cosgrove SE, Song X, Kim D,
Rosenbaum P, Ciesla N, Srinivasan A, Ross T, Carroll K and Perl TM:
An outbreak of multidrug-resistant Acinetobacter baumannii
associated with pulsatile lavage wound treatment. JAMA.
292:3006–3011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Zhao CJ, Wang ZW, Ni YX, Chen MJ,
Xu YC, Yu YS, Zhang LY, Mei YN, Chu YZ, et al: Report from Chinese
Meropenem Susceptibility Surveillance in 2010 Antimicrobial
resistance among nosocomial Gram negative bacilli. Zhong Hua Jian
Yan Yi Xue Za Zhi. 34:897–904. 2011.(In Chinese).
|
|
9
|
Falagas ME and Rafailidis PI: Attributable
mortality of Acinetobacter baumannii: No longer a controversial
issue. Crit Care. 11:1342007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y, Xu YC, Liu Y, Du W, Rui X and Wang
Y: Cefoperazone-sulbactam plus minocycline in the treatment of
extentively drug resistant Acinetobacter infections. Zhong Hua Yi
Xue Za Zhi. 92:2847–2850. 2012.(In Chinese).
|
|
11
|
Yu Z, Zhang D, Yan JJ and Zhou QS:
Acinetobacter baumannii infection in intensive care unit: A
retrospective analysis. Wu Han Da Xue Xue Bao (Yi Xue Ban).
34:895–898. 2013.(In Chinese).
|
|
12
|
Chen B, He LX, Hu B, Ni YX, Qiu H, Shi Y,
Shi Y, Wang H, Wang M, Yang Y, et al: Expert Consensus Document on
Acinetobacter baumannii infection diagnosis, treatment and
prevention in China. Zhong Hua Yi Xue Za Zhi. 92:76–85. 2012.(In
Chinese).
|
|
13
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vincent JL, Moreno R, Takala J, Willatts
S, De M, endonça A, Bruining H, Reinhart CK, Suter PM and Thijs LG:
The SOFA (Sepsis-related organ failure assessment) score to
describe organ dysfunction/failure. On behalf of the working group
on sepsis-related problems of the European society of intensive
care medicine. Intensive Care Med. 22:707–710. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing XZ, Wang HJ, Huang CL, Yang QH, Qu
SN, Zhang H, Wang H, Gao Y, Xiao QL and Sun KL: Prognosis of
patients with shock receiving vasopressors. World J Emerg Med.
4:59–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G:
SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM/ESICM/ACCP/ ATS/SIS
International Sepsis Definitions Conference. Crit Care Med.
31:1250–1256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Shi XH and Xiao D: Drug resistance
of Acinetobacter baumannii infection in SICU. Xinjiang Medicine.
43:141–142. 2013.
|
|
18
|
Qiao L, Zhang JS, Mei YN, Zhang HZ and Su
CL: Analysis of risk factors for the prognosis of Acinetobacter
baumannii bloodstream infection. Zhonghua Wei Zhong Bing Ji Jiu Yi
Xue. 25:471–474. 2013.(In Chinese). PubMed/NCBI
|
|
19
|
Park YK, Jung SI, Park KH, Kim DH, Choi
JY, Kim SH and Ko KS: Changes in antimicrobial susceptibility and
major clones of Acinetobacter calcoaceticus-baumannii complex
isolates from a single hospital in Korea over 7 years. J Med
Microbiol. 61:71–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Liu D, Chen P, Wang Z and Cheng Y:
A survey of risk factors of Acinetobacter baumannii infection in
trauma patients and their mortality. Chong Qing Yi Xue.
40:3665–3667. 2011.(In Chinese).
|
|
21
|
Ma MY, Xu J, Yu N and Huang GM: Analysis
of drug resistance of Acinetobacter baumannii and its related
factors in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 25:686–689.
2013.(In Chinese). PubMed/NCBI
|
|
22
|
Bacakoğlu F, Ekren P Korkmaz, Taşbakan MS,
Başarik B, Pullukçu H, Aydemir S, Gürgün A and Başoğlu OK:
Multidrug-resistant Acinetobacter baumannii infection in
respiratory intensive care unit. Mikrobiyol Bul. 43:575–55.
2009.(In Turkish). PubMed/NCBI
|