Introduction
Cervical cancer is the fourth most prevalent cancer
in women worldwide (1). The main
cause of this disease has been known to be an infection of specific
types of human papillomavirus (HPV), including HPV types 16 and 18
(2,3). Global epidemic studies of cervical
screening with cervical cytology have demonstrated its efficacy.
The greatest decline in cervical cancer-associated mortality was 3%
per year between 1950 and 1970 (4).
Currently, prophylactic HPV16/18 L1-virus-like particle (VLP)
vaccines, which induce neutralizing antibody responses (5,6), have
also been used with great success and show extremely high
preventive effect against HPV16/18 infection (7,8). For
instance, the preventive effect of the HPV16/18 L1-VLP vaccines has
been reported to last up to 8.4 years (9,10).
Nevertheless, only limited information is currently available
regarding the immune responses against HPV16/18 L1, primarily due
to the lack of widely used assays for immune monitoring (11,12).
Several assays, such as pseudovirion-based neutralization assay,
enzyme-linked immunosorbent assay, competitive luminex immunoassay,
and the in situ-purified gluthione-S-transferase L1 fusion
protein-based multiplex immunoassay (GST-L1 MIA), have been
developed to monitor antibody responses to HPV (13–16).
However, no standardized serological assay is currently available
for the assessment of HPV-specific antibody responses, particularly
for large scale examination. Therefore, the biomarkers to precisely
monitor the effects of HPV16/18 L1-VLP vaccines remain to be
identified.
In the present study, B and T cell responses to
HPV16/18 L1 were investigated in healthy females in order to
identify a biomarker for monitoring immune responses subsequent to
the HPV16/18 L1-VLP vaccination.
Materials and methods
Immunization with the bivalent
HPV16/18 L1-VLP vaccine and sample collection
The present study was approved by the Kurume
University Ethical Committee (Kurume, Japan). After a full
explanation of the protocol, a written informed consent was
obtained from 10 healthy females prior to enrollment. The enrolled
females, who were aged between 23 and 33 years with a mean age of
25.5±2.9 years, had a human leukocyte antigen (HLA) -A2 and/or
HLA-A24. They were not subjected to cervical cytology examination
or an HPV DNA test prior to participation to the present study as
titration of neutralizing antibody against HPV is undetectable in
50% of infected women and is at a low level if detected. In
addition, obtaining a cervical sample would cause an unnecessary
burden to these healthy young women. The participants received
intramuscular injection (0.5 ml) of the bivalent HPV16/18 L1-VLP
vaccine (Cervarix®; GlaxoSmithKline, London, UK), which
contained HPV16 L1-VLP (20 µg) and HPV18 L1-VLP (20 µg) mixed with
AS04 adjuvant [aluminum hydroxide (500 µg) and 3-deacylated
monophosphoryl lipid A (50 µg)]. The vaccine was administered in
three doses at 0, 1 and 6 months. In all cases, 20 ml of peripheral
blood was collected prior to immunization and at 1, 2, 7, 12 and 18
months after the first immunization. Plasma was separated from
whole blood and frozen at −80°C until further use. Peripheral blood
mononuclear cells (PBMCs) were obtained by Ficoll-Paque Plus (GE
Healthcare, Uppsala, Sweden) density gradient centrifugation, and
cryopreserved until further use.
Peptides
In total, 10 different HPV16 L1-derived peptides
(20-mer) with binding motifs to both HLA-class I (A2 or A24) and
HLA-class II (DR) were selected using MULTIPRED web software
(antigen.i2r.a-star.edu.sg/multipred/; accessed on 12
Feburary 2012), as reported previously (17), and are listed in Table I. For epitope mapping, 8 different
10-mer overlapping peptides were selected from the amino acid
sequence from positions 295 to 325 of HPV16 L1. The synthetic
peptides were purchased from Greiner Bio-One (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Each peptide was dissolved in
dimethyl sulfoxide, and stored at −80°C until use in further
experiments.
 | Table I.HPV16 L1-derived peptides used in the
present study and their binding motifs to HLA-A2 and HLA-A24. |
Table I.
HPV16 L1-derived peptides used in the
present study and their binding motifs to HLA-A2 and HLA-A24.
|
| HLA-DR | HLA-A2 |
| HLA-A24 |
|---|
|
|
|
|
|
|
|---|
| Peptide | Position | Sequence | Position | Sequence | Score | Position | Sequence | Score |
|---|
| Pep 1 | 54–73 |
KPNNNKILVPKVSGLQYRVF | 60–68 |
ILVPKVSGL | 30 | 59–68 |
KILVPKVSGL | 14 |
| Pep 2 | 392–422 |
HSMNSTILEDWNFGLQPPPGG | 398–406 |
ILEDWNFGL | 23 | 397–406 |
TILEDWNFGL | 16 |
| Pep 3 | 62–81 |
VPKVSGLQYRVFRIHLPDPN | 67–75 |
GLQYRVFRI | 22 | 66–75 |
SGLQYRVFRI | 24 |
| Pep 4 | 112–131 |
PLGVGISGHPLLNKLDDTEN | 118–126 |
SGHPLLNKL | 22 | 117–126 |
ISGHPLLNKL | 12 |
| Pep 5 | 243–262 |
GDSLFFYLRREQMFVRHLFN | 249–257 |
YLRREQMFV | 22 | 248–257 |
FYLRREQMFV | 12 |
| Pep 6 | 300–319 |
VTSDAQIFNKPYWLQRAQGH | 305–313 |
QIFNKPYWL | 21 | 305–313 |
QIFNKPYWL | 12 |
| Pep 7 | 144–162 |
RECISMDYKQTQLCLIGCK | 148–156 |
SMDYKQTQL | 20 | 148–156 |
SMDYKQTQL | 11 |
| Pep 8 | 293–312 |
PTPSGSMVTSDAQIFNKPYW | 298–306 |
SMVTSDAQI | 20 | 298–306 |
SMVTSDAQI | 10 |
| Pep 9 | 384–403 |
TADVMTYIHSMNSTILEDWN | 390–399 |
YIHSMNSTIL | 20 | 389–398 |
TYIHSMNSTI | 23 |
| Pep 10 | 152–171 |
KQTQLCLIGCKPPIGEHWG | 157–165 |
CLIGCKPPI | 23 | 156–165 |
LCLIGCKPPI | 12 |
Measurement of immunoglobulin (Ig)
levels reactive to HPV-16 L1-derived peptides
The Ig levels reactive to HPV16 L1-derived peptides
were measured by multiplex bead suspension array using the Luminex
system (Luminex Corp., Austin, TX, USA) as previously described
(18). In order to detect
peptide-specific IgG, IgM and IgE levels, the beads were washed
with wash buffer (0.05% Tween-20 in PBS) and incubated with 100 µl
of biotinylated goat anti-human IgG (1:200; BA-3080), biotinylated
goat anti-human IgM (1:200; BA-3020; both Vector Laboratories Inc.,
Burlingame, CA, USA) or biotinylated goat anti-human IgE (1:200;
AHI0509; BioSource; Thermo Fisher Scientific, Inc.) antibodies for
1 h at 30°C. To detect peptide-specific IgA, the beads were
incubated with 100 µl goat anti-human IgA antibody (1:200;
A80-102A; Bethyl Laboratories, Inc., Montgomery, TX, USA) for 1 h
at 30°C, followed by washing and subsequent incubation with 100 µl
of biotinylated rabbit anti-goat IgG antibody (1:200; 55366;
Cappel, MP Biomedicals, LLC, Solon, OH, USA) for 30 min at 30°C.
Following washing, the beads were incubated with 100 µl of
streptavidin-PE (1:200; S-866; Molecular Probes; Thermo Fisher
Scientific, Inc.) for 30 min at 30°C, followed by washing and
detection of fluorescence intensity units on the beads using the
Luminex system (18).
Measurement of T cell responses to an
HPV-16 L1-derived peptide
T cell responses specific to HPV16 L1-derived
peptides were evaluated by IFN-γ ELISPOT assay (8223; Medical &
Biological Laboratories Co., Ltd., Nagoya, Japan). Briefly, PBMCs
(2×105 cells/well) isolated from whole peripheral blood
samples of the participants were cultured with 10 µg/ml of
synthetic peptides in 96 round well plates (Nunc; Thermo Fisher
Scientific, Inc.) in 200 µl culture medium containing 45% AIM-V
(Thermo Fisher Scientific, Inc.), 45% RPMI-1640 (Thermo Fisher
Scientific, Inc.), 10% FBS (MP Biomedicals, Solon, OH, USA), 20
IU/ml interleukin-2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and 0.1 mM MEM non-essential amino acid solution (Thermo Fisher
Scientific, Inc.). Half of the medium was replaced with new medium
containing the peptide (20 µM) at every 3 or 4 days. After 5
stimulations, T cells were stimulated with peptide-pulsed T2 cells
(ATCC, Manassas, VA, USA) or C1R-A24 cells (kindly provided by Dr
Masafumi Takiguchi, Kumamoto University, Kumamoto, Japan), and
IFN-γ production in response to the specific peptide was determined
in comparison with the response to irrelevant peptide. Cells were
also tested for IFN-γ production in response to a negative control
peptide, HLA-A2 rescticted peptide (SLYNTVATL, synthesized; Greiner
Bio-One, Kremsmünster, Austria) from human immunodeficiency virus
(HIV) or HLA-A24 restricted HIV peptide (RYLRQQLLGI, synthesized;
Greiner Bio-One). HIV peptides were used as negative control
peptides since these peptides were irrelevant to HPV. Spot numbers
of IFN-γ-secreting cells after 18-h incubation were determined by
ELISPOT assay with an ELISPOT reader (ImmunoSpot S5 Versa Analyzer;
Cellular Technology Ltd., Shaker Heights, OH, USA). All assays were
performed in duplicate. Peptide-specific T cell responses were
shown as the differences between the spot numbers per
1×105 PBMCs in response to the specific peptide and
those in response to the control peptide. When the PBMCs from
females with both HLA-A2 and HLA-A24 were used, mean values of spot
numbers from the culture with peptide-pulsed T2 cells and those
with peptide-pulsed C1R-A24 cells were used for calculation. PBMCs
were available for this analysis in only 9 of 10 individuals at 2
months after vaccination, but were available in all 10 individuals
at other time points prior to and following vaccination.
Statistical analysis
The levels of IgG, IgM, IgE and IgA specific to
HPV-16 L1-derived peptides were compared at the indicated
time-points prior to and following the vaccination using Wilcoxon
signed-rank test. All reported P-values were two-sided and
statistically significant differences were indicated by P<0.05.
Comparison of IgG and IgA response was compared by analysis of
variance. Analyses were performed with the use of JMP version 11
(SAS Institute Inc., Cary, NC, USA).
Results
IgG responses to HPV-16 L1-derived
peptides subsequent to the HPV16/18 L1-VLP vaccination
Plasma samples obtained from the healthy females
(n=10) were examined for the levels of IgG reactive to each of the
10 different HPV16 L1-derived peptides before immunization and at
1, 2, 7, 12 and 18 months after the first immunization with the
HPV16/18 L1-VLP vaccine. As shown in Fig. 1, the IgG levels specific to Peptide 6
and Peptide 8 increased significantly at 2, 7 and 12 months after
the first immunization with the HPV16/18 L1-VLP vaccine (Peptide 6:
P=0.002, 0.004 and 0.002, respectively; Peptide 8: P=0.002, 0.002
and 0.020, respectively). A significant increase was also observed
in the IgG levels specific to Peptide 4 after 2 and 7 months from
the first immunization (P=0.002 and 0.002, respectively; Fig. 1). The peptide-specific IgG responses
peaked at 7 months after the first immunization (i.e. 1 month after
the third immunization) and declined thereafter.
Comparison of IgM, IgE and IgA levels
prior to and following vaccination
The IgM, IgE, and IgA levels were then measured
against HPV16 L1-derived peptides in plasma obtained from the
healthy females (n=10) before immunization and at 1, 2, 7, 12 and
18 months after the first immunization. As shown in Fig. 2, the IgE and IgA levels against
Peptide 8 showed significant increase after 2 and 7 months from the
first vaccination (IgE: P=0.007 and 0.029, respectively; IgA:
P=0.001 and 0.007, respectively). In addition, IgA levels against
Peptide 6 at 2 months after the first vaccination also demonstrated
an increasing trend (P=0.080). IgE and IgA levels against Peptide 4
did not change significantly after any time of vaccination.
However, there were no significant changes in the IgM levels
against any peptides. Furthermore, the increase of IgG and IgA
levels against Peptide 8 at 2 and 7 months after the first
vaccination showed a positive correlation (Fig. 3). According to these findings, it is
hypothesized that the overlapped position of Peptides 6 and 8 may
be a B cell epitope.
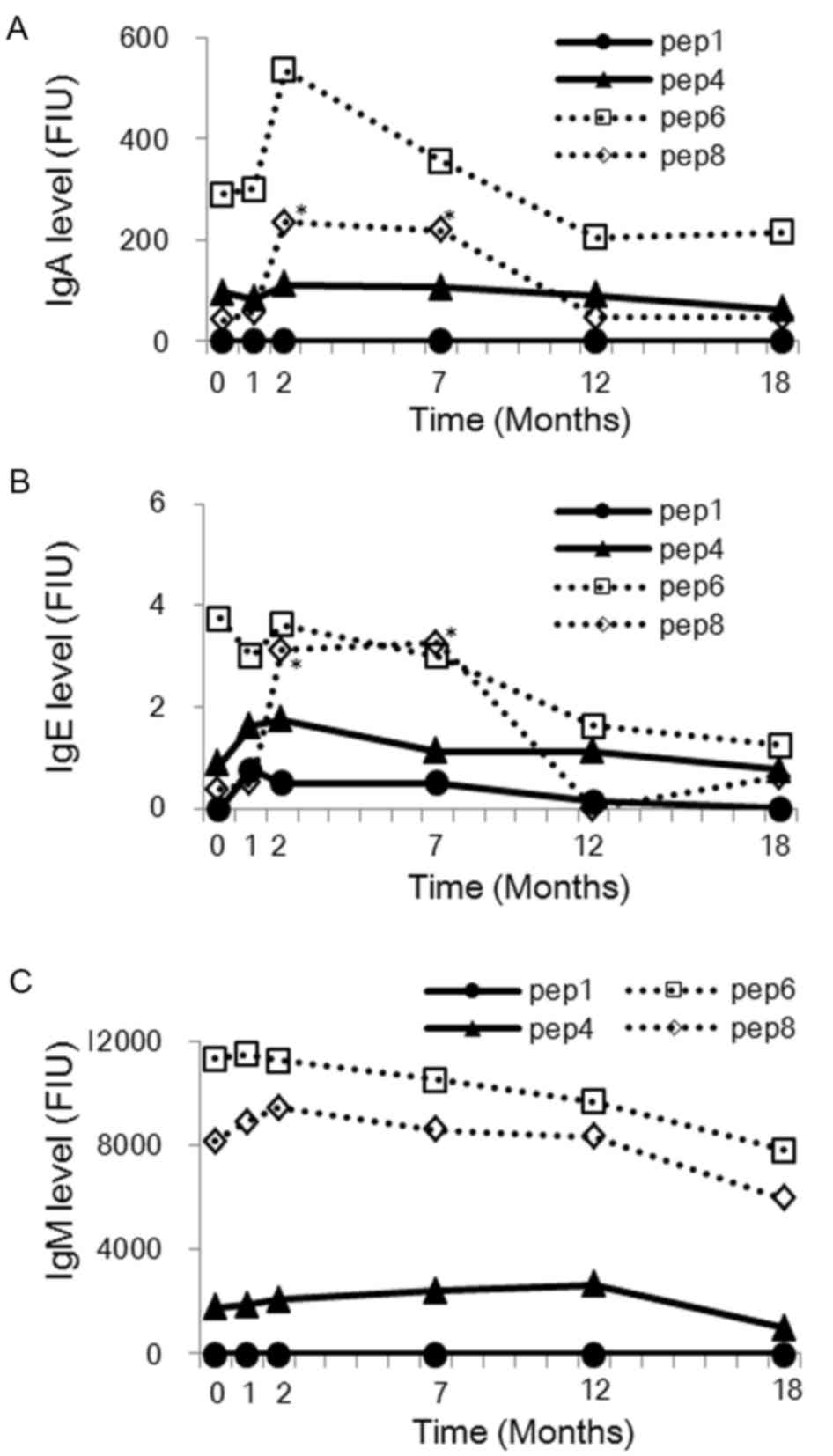 | Figure 2.(A) IgA, (B) IgE and (C) IgM responses
(levels in FIU) to HPV-16 L1-derived 20-mer peptides subsequent to
the HPV16/18 L1-VLP vaccination. The levels in the plasma (100-fold
dilution) were examined in 10 healthy females prior to immunization
and at 1, 2, 7, 12 and 18 months after the first immunization.
Peptides 4, 6 and 8 were tested, since these induced IgG responses
after immunization as shown Fig. 1,
while Peptide 1 was used as the control. Median levels of IgM, IgE,
and IgA from the 10 healthy females are shown. *P<0.05, as
determined by the Wilcoxon rank signed-rank test. Ig,
immunoglobulin; FIU, fluorescent intensity units; HPV, human
papillomavirus; VLP, virus-like particle; Pep, peptide. |
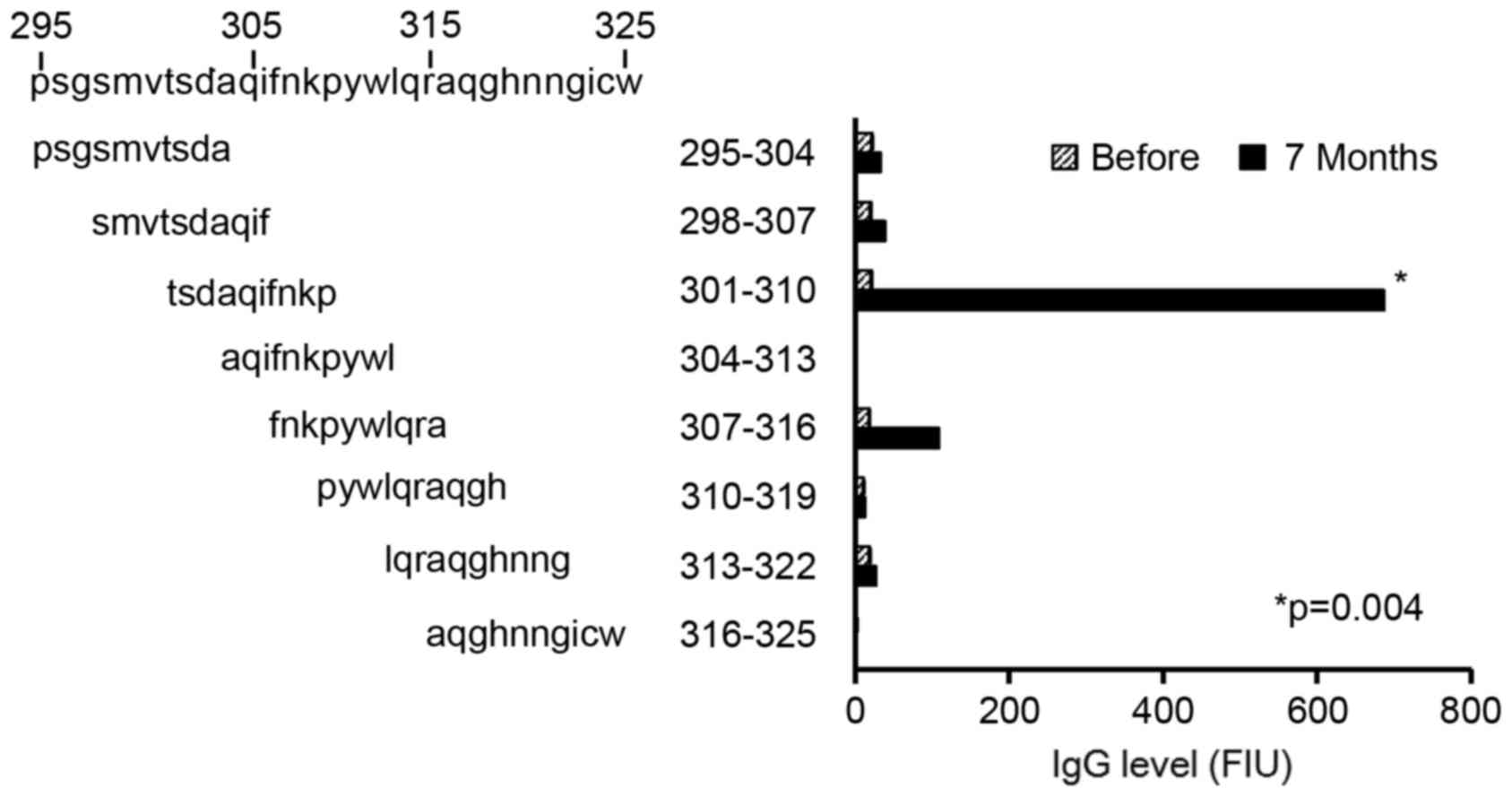
Detailed B cell epitope mapping
To obtain further detailed information on
immunogenic epitopes, the levels of IgG reactive to each of the 8
different 10-mer peptides derived from the amino acid sequence
between positions 295 and 325 of HPV16 L1 were determined, which
contained the overlapped position of Peptide 6 and Peptide 8
sequences (position 300–312: VTSDAQIFNKPYW). As shown in Fig. 4, the IgG levels specific to the
peptide at position 301–310 (TSDAQIFNKP) of HPV16 L1, but not
specific to other 10-mer peptides, were significantly increased at
7 months after first vaccination compared with before vaccination
(P=0.004). Notably, this amino acid sequence was shared by the
immunogenic 20-mer Peptides 6 and 8.
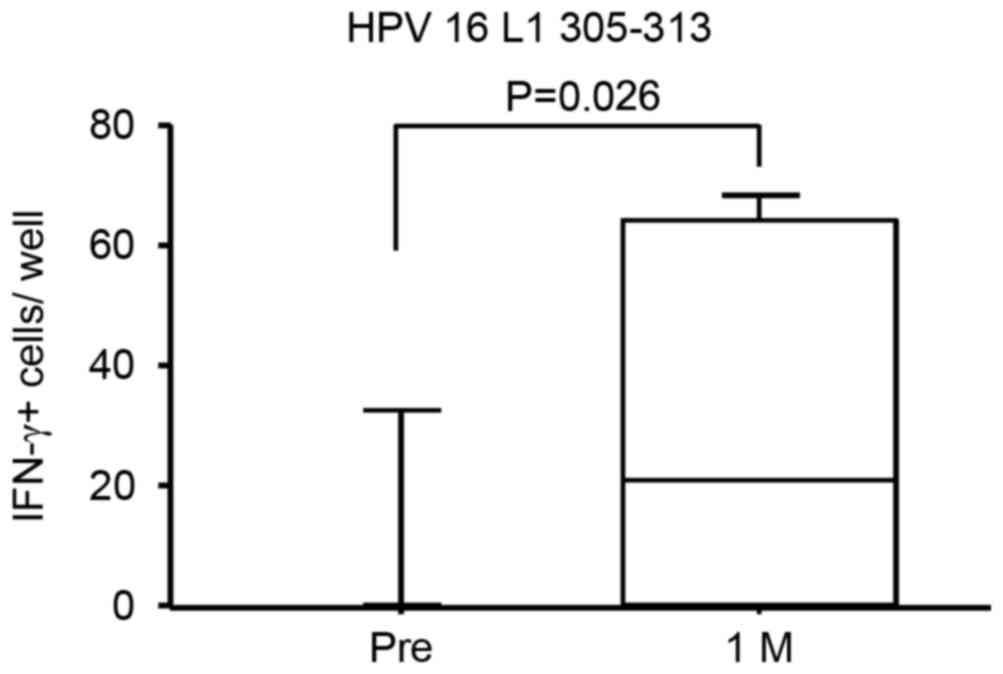
T cell responses specific to an HPV-16
L1-derived peptide in PBMCs subsequent to vaccination
T cell responses to an HPV16 L1-derived peptide at
position 305–313 (QIFNKPYWL) of HPV16 L1 in the PBMC cultures
before and after vaccinations were further assessed. The peptide at
position 305–313 (QIFNKPYWL) of HPV16 L1 (was used in the current
experiment since it was previously demonstrated that it binds well
to both HLA-A*0201 and HLA-A*2402 (17). PBMCs were stimulated in vitro
with specific or control peptides, and then their IFN-γ production
in response to the peptide-pulsed T2 (HLA-A2+) or
C1R-A24 (HLA-A24+) cells was examined by ELISPOT assay.
As shown in Fig. 5, the
peptide-specific T cell responses at 1 month after the first
immunization demonstrated significant increase compared with the
response prior to vaccination (P=0.026). These results suggest that
the HPV16/18 L1-VLP vaccination was able to induce specific immune
responses in T and B cells simultaneously.
Discussion
In the present study, the humoral and cellular
immune responses to HPV16 L1-derived peptides were analyzed in
healthy females subsequent to immunization with the HPV16/18 L1-VLP
vaccine. When B cell responses to 10 different 20-mer peptides were
screened, the levels of IgG of Peptides 6 and 8 were significantly
elevated in the plasma at 2, 7 and 12 months after first
vaccination compared with before vaccination, and the levels of IgG
of Peptide 4 were significantly elevated in the plasma at 2 and 7
months after first vaccination compared with before vaccination.
Notably, the levels of IgE and IgA against Peptide 8 also revealed
a significant increase at 2 and 7 months after the first
vaccination, suggesting an isotype switch to IgE and IgA.
Similarly, the levels of IgA against Peptide 6 showed an increasing
trend at 2 months after the first immunization. Although it is
reported that the dominant antibody isotype in the cervical mucosa
after immunization with HPV 16/18 L1-VLP is IgG, IgA is known to be
the dominant antibody isotype in the mucosal immune system. Hence,
IgA against the Peptide 6 and 8 may transudate and/or exudate from
the systemic circulation to the cervical mucosa, and thus can be
detectable in the mucosa after HPV16/18 L1-VLP vaccination.
Previously, we identified the B cell epitopes
derived from HPV16 L1 in BALB/c and C57BL/6 N mice (17). Similar to the results obtained in
humans in the present study, Peptide 6 was the major B cell epitope
shared by female Balb/c (H-2d) and C57BL/6 N (H-2b) mice in our
previous study (17). Nevertheless,
detailed mapping analysis of B cell epitopes in humans indicated
that a 10-mer peptide (TSDAQIFNKP) at the position 301–310 of HPV16
L1 was immunogenic, whereas another 10-mer peptide (AQIFNKPYWL) at
position 304–313, which was identified as the B cell epitope in
mice (17), was not immunogenic.
This finding suggested that immunogenic B cell epitopes may be
slightly different between mice and humans, although the reason for
this discrepancy remains to be determined.
The present study also analyzed the T cell responses
to an HPV16 L1-derived peptide (QIFNKPYWL), which was demonstrated
to efficiently bind HLA-A2 and HLA-A24 (17), in females with HLA-A2 and/or HLA-A24
following immunization with the HPV16/18 L1-VLP vaccine. The
results suggested that the HPV16/18 L1-VLP vaccination was able to
induce specific immune responses in T and B cells
simultaneously.
In the present study, the epitope recognized by B
cells (TSDAQIFNKP) was not completely identical to that recognized
by T cells (QIFNKPYWL); however, 6 out of 9 or 10 amino acids were
shared by these two epitopes. Since it has been well recognized
that cellular and humoral immune responses are crucial in the
induction of effective immunity in animal models (19–21),
simultaneous induction of both B and T cell responses against HPV16
L1 may be important for efficient prevention of HPV infection and
subsequent HPV-associated neoplasia.
In conclusion, a 10-mer amino acid sequence
(TSDAQIFNKP) derived from HPV16 L1 was identified in the present
study as an immunogenic B cell epitope in the females after
HPV16/18 L1-VLP immunization. In addition, T cell responses to
another HLA-A2- and HLA-A24-restricted epitope (QIFNKPYWL) derived
from HPV16 L1 in the vaccinated individuals were detected. The
identified B and T cell epitopes may be useful for monitoring the
immune responses to HPV16/18 L1-VLP subsequent to vaccination.
Whether the vaccine-induced IgG and/or IgA response to the
identified B cell epitope possesses the biological activity to
neutralize the HPV infection and/or facilitate the prophylactic
effect of the HPV 16/18 L1 vaccine remains to be clarified in the
near future. However, the present study may provide novel
information for better understanding the immune responses to HPV 16
L1-VLP vaccines.
Acknowledgements
The present research was supported in part by The
Fukuoka Obgyn Researcher's Charity Foundation Fund, Japan.
Glossary
Abbreviations
Abbreviations:
|
HPV
|
human papillomavirus
|
|
VLP
|
virus-like particle
|
|
HLA
|
human leukocyte antigen
|
|
FIU
|
fluorescent intensity units
|
References
|
1
|
International Agency for Research on
Cancer, . 2012 Globocan 2012: Estimated cancer incidence, mortality
and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx27–November;2016.
|
|
2
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ:
International agency for research on cancer multicenter cervical
cancer study group. Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li N, Franceschi S, Howell-Jones R,
Snijders PJ and Clifford GM: Human papillomavirus type distribution
in 30,848 invasive cervical cancers worldwide: Variation by
geographical region, histological type and year of publication. Int
J Cancer. 128:927–935. 2010. View Article : Google Scholar
|
|
4
|
Wingo PA, Cardinez CJ, Landis SH, Greenlee
RT, Ries LA, Anderson RN and Thun MJ: Long-term trends in cancer
mortality in the United States, 1930–1998. Cancer. 97:3133–3275.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villa LL, Costa RL, Petta CA, Andrade RP,
Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M,
et al: Prophylactic quadrivalent human papillomavirus (types 6, 11,
16, and 18) L1 virus-like particle vaccine in young women: A
randomised double-blind placebo-controlled multicentre phase II
efficacy trial. Lancet Oncol. 6:271–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harper DM, Franco EL, Wheeler C, Ferris
DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS,
et al: Efficacy of a bivalent L1 virus-like particle vaccine in
prevention of infection with human papillomavirus types 16 and 18
in young women: A randomised controlled trial. Lancet.
364:1757–1765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garland SM, Hernandez-Avila M, Wheeler CM,
Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M,
Bryan J, et al: Quadrivalent vaccine against human papillomavirus
to prevent anogenital diseases. N Engl J Med. 356:1928–1943. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC,
Skinner SR, et al: Efficacy of human papillomavirus (HPV)-16/18
AS04-adjuvanted vaccine against cervical infection and precancer
caused by oncogenic HPV types (PATRICIA): Final analysis of a
double-blind, randomised study in young women. Lancet. 374:301–314.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roteli-Martins CM, Naud P, De Borba P,
Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B and
Descamps D: Sustained immunogenicity and efficacy of the HPV-16/18
AS04-adjuvanted vaccine: Up to 8.4 years of follow-up. Hum Vaccin
Immunother. 8:390–397. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowhani-Rahbar A, Alvarez FB, Bryan JT,
Hughes JP, Hawes SE, Weiss NS and Koutsky LA: Evidence of immune
memory 8.5 years following administration of a prophylactic human
papillomavirus type 16 vaccine. J Clin Virol. 53:239–243. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joura EA, Kjaer SK, Wheeler CM, Sigurdsson
K, Iverson OE, Hernandez-Ailta M, Perez G, Brown DR, Koutsky LA,
Tay EH, et al: HPV antibody levels and clinical efficacy following
administration of a prophylactic quadrivalent HPV vaccine. Vaccine.
26:6844–6851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryding J, Dahlberg L, Wallen-Ohman M and
Dilner J: Deletion of a major epitope of human papillomavirus
tyle16 virus-like particles. J Gen Virol. 88:792–802. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirnbauer R, Hubbert NL, Wheeler CM,
Becker TM, Lowy DR and Schiller JT: A virus-like particle
enzyme-linked immunosorbent assay detects serum antibodies in a
majority of women infected with human papillomavirus type 16. J
Natl Cancer Inst. 86:494–499. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pastrana DV, Buck CB, Pang YY, Thompson
CD, Castle PE, FitzGerald PC, Kjaer S Kruger, Lowy DR and Schiller
JT: Reactivity of human sera in a sensitive, high-throughput
pseudovirus-based papillomavirus neutralization assay for HPV16 and
HPV18. Virology. 321:205–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dias D, Van Doren JV, Schlottmann S, Kelly
S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green
T, et al: Optimization and validation of a multiplexed luminex
assay to quantify antibodies to neutralizing epitopes on human
papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol.
12:959–969. 2005.PubMed/NCBI
|
|
16
|
Waterboer T, Sehr P, Michael KM,
Franceschi S, Nieland JD, Joos TO, Templin MF and Pawlita M:
Multiplex human papillomavirus serology based on in situ-purified
glutathione S-transferase fusion proteins. Clin Chem. 51:1845–1853.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukui A, Matsueda S, Kawano K, Tsuda N,
Komatsu N, Shichijo S, Sasada T, Hattori S, Ushijima K, Itoh K and
Kamura T: Identification of B cell epitopes reactive to human
papillomavirus type-16 L1-derived peptides. Virol J. 9:1992012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: A novel tool for monitoring immune responses to peptides
used for immunization. Scand J Clin Lab Invest. 64:535–546. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Avogadri F, Merghoub T, Maughan MF,
Hirschhorn-Cymerman D, Morris J, Ritter E, Olmsted R, Houghton AN
and Wolchok JD: Alphavirus replicon particles expressing TRP-2
provide potent therapeutic effect on melanoma through activation of
humoral and cellular immunity. PLoS One. 5:e126702010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong S, Qian J, Li H, Yang J, Lu Y, Zheng
Y and Yi Q: CpG or IFN-α are more potent adjuvants than GM-CSF to
promote anti-tumor immunity following idiotype vaccine in multiple
myeloma. Cancer Immunol Immunother. 6:561–571. 2012. View Article : Google Scholar
|
|
21
|
Cubillos C, de la Torre BG, Bárcena J,
Andreu D, Sobrino F and Blanco E: Inclusion of a specific T cell
epitope increases the protection conferred against foot-and-mouth
disease virus in pigs by a linear peptide containing an
immunodominant B cell site. Virol J. 9:662012. View Article : Google Scholar : PubMed/NCBI
|